Abstract
Breakdown of the blood–brain barrier (BBB) is a key step associated with ischemic stroke and its increased permeability causes extravasation of plasma proteins and circulating leukocytes. Polymorphonuclear neutrophil (PMN) proteases may participate in BBB breakdown. We investigated the role of PMNs in ischemic conditions by testing their effects on a model of BBB in vitro, under oxygen-glucose deprivation (OGD) to mimic ischemia, supplemented or not with high-density lipoproteins (HDLs) to assess their potential protective effects. Human cerebral endothelial cells cultured on transwells were incubated for 4 hours under OGD conditions with or without PMNs and supplemented or not with HDLs or alpha-1 antitrypsin (AAT, an elastase inhibitor). The integrity of the BBB was then assessed and the effect of HDLs on PMN-induced proteolysis of extracellular matrix proteins was evaluated. The release of myeloperoxidase and matrix metalloproteinase 9 (MMP-9) by PMNs was quantified. Polymorphonuclear neutrophils significantly increased BBB permeability under OGD conditions via proteolysis of extracellular matrix proteins. This was associated with PMN degranulation. Addition of HDLs or AAT limited the proteolysis and associated increased permeability by inhibiting PMN activation. Our results suggest a deleterious, elastase-mediated role of activated PMNs under OGD conditions leading to BBB disruption that could be inhibited by HDLs.
Keywords: blood–brain barrier, high-density lipoproteins, ischemia, neutrophil elastase, stroke
Introduction
The blood–brain barrier (BBB) is a biologic filter designed to segregate the vascular compartment from the central nervous system, in which endothelial cells have a pivotal role.1 The major determinant of this barrier is the low permeability to all types of molecules including proteins, peptides, and metabolites (>400 Da) across the endothelial layer that lines cerebral microvessels. Breakdown or dysfunction of the BBB is a key step associated with both vascular and degenerative diseases of the central nervous system (ischemic stroke, tumors, multiple sclerosis, Alzheimer disease, etc.).2 In ischemic conditions, BBB permeability is increased causing extravasation of leukocytes and plasma proteins such as plasminogen that may be deleterious for the parenchymal compartment of the brain.3
In humans, polymorphonuclear neutrophils (PMNs) represent the most abundant class of leukocyte (70% of white blood cells) and are rapidly activated during acute ischemia.4 Similarly, activation of cerebral endothelial cells leads to increased expression of adhesion molecules leading to transmigration of PMNs through the endothelial barrier into the tissue.5 Polymorphonuclear neutrophils have a key role in acute ischemic cerebral injury and in ischemia-induced BBB disruption, where their proteases such as matrix metalloproteinase 9 (MMP-9) participate in BBB breakdown.6, 7, 8, 9 In particular, PMN depletion was shown to limit edema and infarct size area in a rat model of middle cerebral artery occlusion,10 and also to reduce BBB breakdown and inflammation subsequent to intracerebral hemorrhage.11 Furthermore, the role of PMNs in cardiac ischemia/reperfusion is well documented.12
In addition to reverse transport of excess cholesterol from the tissues back to the liver, high-density lipoprotein (HDL) particles exert anti-inflammatory, antioxidant, anti-elastase, and antithrombotic effects that may protect endothelial cells from acute injury.13, 14 It has been reported in vivo that HDLs inhibit the expression of adhesion molecules by endothelial cells and hence reduce PMN adhesion and transmigration.15 We have previously reported that intravenous injection of HDLs was neuroprotective in an embolic stroke model, in particular by modulating PMN extravasation.16 The role of PMNs on BBB permeability remains unclear. Inglis et al17 reported that only N-formyl-methionyl-leucyl-phenylalanine-activated PMNs led to increased permeability, associated with transmigration through the endothelium. Other authors have suggested that elastase was responsible for the PMN-induced increased permeability of the endothelial layer.18 We recently reported that anti-elastase activity of HDLs14 may represent a novel protective effect of these particles in pathologic conditions involving PMN activation and subsequent elastase release. In addition to their well-documented functions in endothelial stabilization, HDLs may thus represent a therapeutic option for limiting BBB permeability under ischemic conditions.
In this study, we aimed at investigating the role of PMNs in ischemic conditions by testing their effects on a simplified model of BBB using cerebral endothelial cells in vitro, under glucose and oxygen deprivation to mimic ischemia, supplemented or not with HDLs to assess their potential protective effects.
Materials and methods
Cell Cultures
The human brain endothelial cell line hCMEC/D3 were kindly provided by Dr. Couraud. Cells were cultured on uncoated plastic plates in complete EBM-2 medium (Endothelial Basal Medium; Lonza, Basel, Switzerland), supplemented with 2.5% fetal calf serum, hydrocortisone, and growth factors (Promocell, Heidelberg, Germany) and used at passages 29 to 35. The medium was replaced twice a week, and cells reached confluence after 7 to 10 days. Cells were passaged using trypsin-EDTA 10X (PAA, Yeovil, Somerset, UK).
Polymorphonuclear Neutrophil Isolation
Human PMNs were isolated from blood of healthy volunteers with informed consent, by elimination of mononuclear and red blood cells. Briefly, blood was collected in EDTA tubes (BD Vacutainer, Plymouth, UK) and centrifuged at 2,000 g for 10 minutes at 20°C to obtain plasma, which was taken to isolate HDLs and LDLs (see below). The plasma was replaced by RPMI and an equal volume of 2% Dextran was added before gentle mixing for red blood cell agglutination. After 20 minutes of sedimentation at room temperature, the supernatant containing the leukocytes was slowly layered on 12 mL Ficoll (PAA), d=1.077, at a 2:1 ratio, and then centrifuged for 25 minutes at 616 g at 20°C without active braking. The ring formed by mononuclear cells (monocytes and lymphocytes) was discarded. The pellet consisted of polymorphonuclear cells and some residual red blood cells that were then eliminated by hypotonic lysis (5 mL H2O for 30 seconds). The osmolarity was restored by adding 5 mL of 1.8% NaCl, and the pH corrected to 7.4 by addition of 10 mL phosphate buffered saline (PBS, NaCl 8 g/L, KCl 0.2 g/L, Na2HPO4·7H2O 1.44 g/L, KH2PO4 0.24 g/L, pH 7.4). Cells were then centrifuged (800 g, 5 minutes, 20°C) and washed with PBS before resuspension in RPMI. An aliquot was used for counting PMNs and for assessment of their purity.
Isolation of High-Density Lipoproteins
Lipoproteins were isolated from plasma of healthy volunteers by ultracentrifugation.14 Plasma density was adjusted to d=1.22 with KBr and the resulting solution was overlaid with KBr saline solution (d=1.063). Ultracentrifugation was performed at 100 000 g for 5 hours at 10°C. The density of the bottom fraction containing HDLs was adjusted to 1.25 with KBr and this solution was overlaid with KBr saline solution (d=1.21). After this step, the HDL fraction (orange top layer) was recovered as a single band, and was then extensively rinsed with PBS (at least 5 times the initial volume) and concentrated using a centrifugal concentrating device (cutoff 10 kDa; Vivascience, Stonehouse, UK).
Endothelial Cell Treatments
Before each experiment, endothelial cells were washed three times with PBS. They were then incubated with 100 nℳ elastase or 1 × 106 PMNs/mL, supplemented or not with 400 μg/mL HDL preparation and/or 1 μM of alpha-1-antitrypsin (AAT, Alfalastin, LFB Biomédicaments, Courtaboeuf, France). Oxygen-glucose deprivation (OGD) conditions were achieved by using a hypoxia chamber (Billups-Rothenberg, Del Mar, CA, USA) where atmospheric air was replaced by a mixture of gas (0% O2, 5% CO2, 95% N2, Air Product, Allantown, PA, USA) and cells were maintained in DMEM without glucose (Life Technologies Corporation, Paisley, UK) equilibrated with the same mixture. Non-OGD conditions were obtained by maintaining the cells in DMEM medium 1 g/L glucose with 5% CO2.
In Vitro Permeability Measurements
For permeability experiments, hCMEC/D3 were seeded at 5 × 105 cells/cm2 onto collagen-coated inserts (PCF filters, 0.4 μm pore size, Millicell, Millipore, Billevica, MA, USA) in complete EBM-2. Cells were grown 14 days post confluence before use. Permeability was calculated from the kinetics of clearance of fluorescein isothiocyanate (FITC)-labeled dextran (70 kDa). This marker of BBB permeability has been previously validated in vivo.19 We also checked the validity of this marker in a mouse model of middle cerebral artery occlusion (4 hours with a monofilament; Supplementary Figure 1). Animal care and experimental protocols were approved by the Animal Ethics Committee of the INSERM-University Paris 7, authorization 2010/13/698-0002.
Inserts were placed in wells of 24-well plates. To measure the permeability, FITC-dextran (0.385 mg/mL) was added to the inserts, which were transferred every 15 minutes over 45 minutes to collect wells containing 600 μL of fresh medium. Samples (200 μL) from the collecting wells were placed in a 96-well plate, and the fluorescence was determined using a microplate reader at 485 nm (excitation) and 538 nm (emission). Fluorescence was converted to dextran concentration using a standard curve. The volume cleared was calculated from the ratio of dextran concentration in each sample to the applied concentration (0.385 mg/mL). The incremental cleared volume was plotted against time and the slope of the regression line used to calculate a permeability surface area value (PS). The PS for the endothelial monolayer (PS) was calculated from the following equation: 1/PS=1/PSe−1/PSf, where PSe is the regression slope across a test insert and PSf is the regression slope across a blank insert (cell free). The PS values were divided by the surface area of the insert (0.7 cm2) to obtain the endothelial permeability coefficient Pe (cm/min). Alternatively, human umbilical vein endothelial cells were used in the same conditions as hCMEC/D3, before passage 5 (Promocell).
SDS-PAGE and Western-Blot Analysis
Proteins contained in culture supernatants from OGD and non-OGD experiments were separated by SDS-7.5% PAGE for detection of fibronectin. After electrophoresis, proteins were transferred onto nitrocellulose membranes, blocked with 5% milk powder in Tris-buffer saline, pH 7.4/0.1% Tween-20, and then probed with rabbit polyclonal anti-human fibronectin (dilution 1:1 000; Sigma-Aldrich Corp., St. Louis, MO, USA). An anti-rabbit peroxidase-conjugated secondary antibody was used (dilution 1:10 000; Jackson ImmunoResearch Laboratories, Newmarket, Suffolk, UK) followed by ECL detection. Densitometry analysis of fibronectin fragments (<100 kDa) was performed using a calibrated scanner (GS800; Bio-Rad, Hercules, CA, USA).
Immunocytofluorescence
For these experiments, hCMEC/D3 were seeded at 5 × 105 cells/cm2 onto collagen coated EZ-slide (Millipore) in complete EBM-2. Cells were grown to confluence over 7 days before use. After treatment, cells were fixed in 3.7% paraformaldehyde for 30 minutes and stored in PBS at 4°C. Rabbit polyclonal antibody to human VE-Cadherin (1:200; Bender Med Systems, Vienna, Austria) was applied, followed by a secondary antibody conjugated with Alexa 488 (Life Technologies Corporation). Nuclei were stained with 4′,6-diamidino-2-phenylindole (0.1 μg/mL). Negative controls using the corresponding nonimmune IgGs were included in each set of experiments to check for nonspecific staining. Images are provided using whole-slide scanner NanoZoomer (Hamamatsu Photonics, Massy, France) and are representative of three independent experiments.
Determination of Elastase Activity
Elastase activity was determined in cell culture supernatants for each condition. In all cases, 125 μL of culture supernatants were incubated with 0.1 mℳ of an elastase fluorogenic substrate: MeO-Suc-Ala-Ala-Pro-Val-AMC (Millipore). Human PMN elastase (range 50 to 1.5 nℳ; Elastin Products, Owensville, MO, USA) was used to make a standard curve. Substrate hydrolysis was monitored for 2 hours at 37°C by (spectro)fluorometry at 390 nm (excitation) and 460 nm (emission).
Gelatin Zymography
Zymography for determination of gelatinolytic activity of MMP-9 was performed on cell supernatants. Equal volumes of samples were mixed 1:1 with loading buffer (80 mM Tris-HCl (pH 6.8), 4% SDS, 10% glycerol, 0.01% bromophenol blue). Proteins were separated by electrophoresis on SDS-polyacrylamide gels containing 0.1% gelatin (Sigma, Sigma-Aldrich Corp.) at 100 V for 1 hour. After electrophoresis, gels were washed with 2.5% Triton X-100 for 1 hour to remove SDS and then incubated for 19 hours at 37°C with shaking in a buffer containing 50 mℳ Tris-HCl (pH 7.8) and 10 mℳ CaCl2. Bands of lysis were visualized after staining for 1 hour with 0.45% Coomassie Blue (30% propanol, 10% acetic acid, 0.45% Coomassie blue) and destained for 30 minutes with a solution of 30% (v/v) ethanol and 10% (v/v) glacial acetic acid. To compare gelatinase activity, gels were analyzed by densitometry using a calibrated scanner (GS800, Bio-Rad), and the intensity of the bands (arbitrary units) was normalized to the control sample (supernatant of PMNs+hCMEC/D3 in non-OGD conditions was set at 100) in each set of experiments.
Statistics
Statistical analyses were performed using GraphPad Prism 5 software (La Jolla, CA, USA). Results are expressed as means±s.d. Comparison between multiple groups were performed by one-way analysis of variance (ANOVA, Kruskall Wallis), followed by Mann–Whitney tests. A value of P<0.05 was considered statistically significant.
Results
Polymorphonuclear Neutrophils Increase Blood–Brain Barrier Permeability in Ischemic Conditions
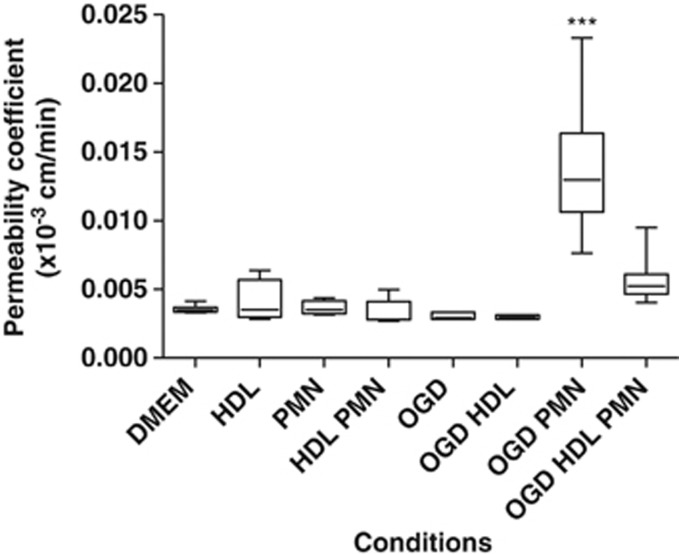
To determine the role of ischemia and PMNs on BBB permeability, the well-established model of hCMEC/D3 cells20 was used in coculture with PMNs under OGD conditions. The permeability of the BBB obtained under control conditions (with EBM-2 medium or DMEM 1 g/L glucose) was comparable to that reported by Weksler et al20 (0.0045±0.00026 × 10−3 versus 0.013 × 10−3 cm/min) and significantly lower as compared with another endothelial cell type used in the same conditions (human umbilical veni endothelial cell permeability: 0.374 × 10−3 cm/min) (Supplementary Figure 2). This result indicated that the BBB model used in our study is relevant in terms of permeability. The permeability was then measured after 4 hours of treatment (OGD conditions±PMNs). As shown in Figure 1, neither PMNs nor OGD conditions induced significant changes in permeability. An increase in permeability was observed only when the BBB was incubated with PMNs under OGD conditions (P<0.0001, compared with control conditions with DMEM, respectively, 0.0035±0.00025 × 10−3 versus 0.014±0.0048 × 10−3 cm/min).
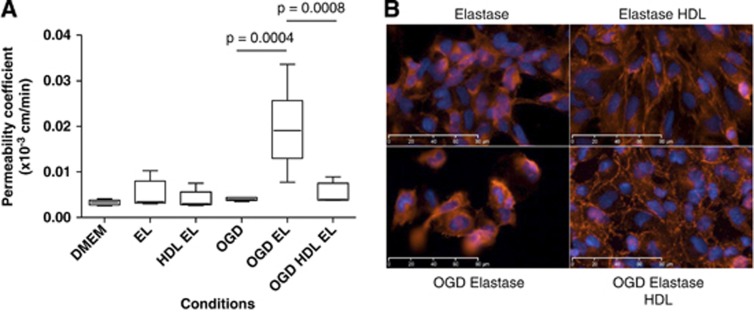
Figure 1.
In vitro effects of polymorphonuclear neutrophils (PMNs) on the permeability in a blood–brain barrier (BBB) model (hCMEC/D3 cells) under normoxic or oxygen-glucose deprivation (OGD) conditions. Cells were incubated with high-density lipoproteins (HDLs) (0.4 g/L)±PMNs (1 × 106 cells/mL) for 4 hours (n⩾9 for each condition). Changes in permeability were only observed with PMNs in OGD conditions. Results are presented as box plots in which the median is shown (***P<0.0001 comparing DMEM versus OGD PMNs, P<0.0001 comparing OGD PMNs±HDLs).
High-Density Lipoproteins Limit Polymorphonuclear Neutrophil-Induced Permeability of the Blood–Brain Barrier in Ischemic Conditions
Since HDLs have been shown, in vivo, to reduce cerebral infarct volume in a rat model of embolic stroke that was associated with a limited BBB leakage16 and given the documented effects of HDLs on the endothelium,21 we therefore tested the potential protective effects of HDLs on the BBB. Coincubation with HDLs decreased significantly the permeability induced by PMNs under OGD conditions (Figure 1, P<0.0001 versus without HDLs).
Ischemic Conditions Induce Elastase, Matrix Metalloproteinase 9, and Myeloperoxidase Release by Polymorphonuclear Neutrophils in Coincubation with Endothelial Cells
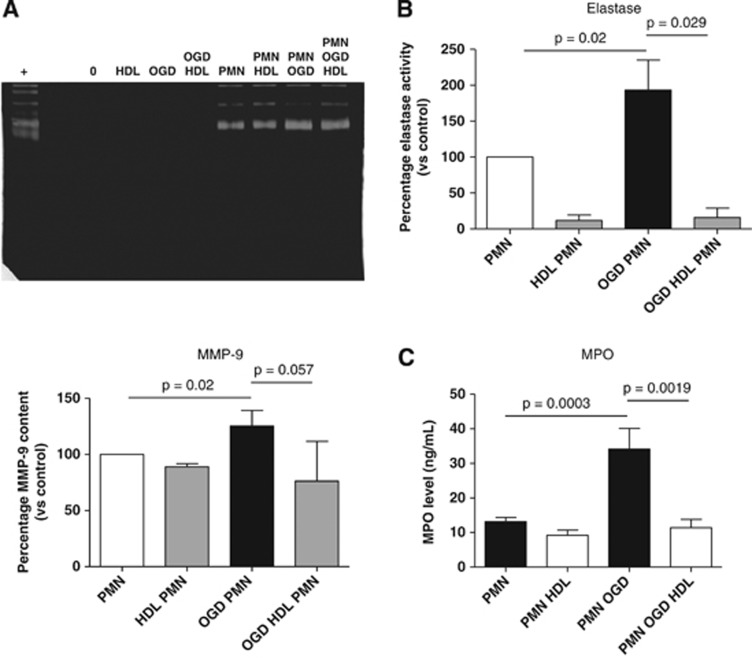
We hypothesized that OGD may stimulate PMN activation and the release of proteases, leading to endothelial damage. Supernatants of each condition were collected to measure elastase and MMP-9 activities (endothelial cells in control or OGD conditions±PMNs). Oxygen-glucose deprivation significantly increased MMP-9 release by PMNs (P<0.05) and elastase activity (P<0.0001), as assessed respectively by gelatin zymography and by fluorimetry using a selective substrate for elastase (Figures 2A and 2B). No activity could be detected for these proteases in the supernatant of endothelial cells alone. Myeloperoxidase (MPO) levels were quantified in the same conditions. Similarly, MPO release by PMNs was induced under OGD conditions (Figure 2C). We also tested PMNs without endothelial cells under OGD conditions, supplemented or not with HDLs. In these conditions, OGD did not activate PMNs (no changes could be observed in elastase and MMP-9 activities; Supplementary Figure 3, and MPO levels).
Figure 2.
(A) Determination of gelatinolytic activity of matrix metalloproteinase 9 (MMP-9) in the supernatant of the blood–brain barrier (BBB) (n=4) after treatment with high-density lipoproteins (HDLs) (0.4 g/L)± polymorphonuclear neutrophils (PMNs) (1 × 106 cells/mL) for 4 hours under control (normoxic and normoglycemic conditions) or oxygen-glucose deprivation (OGD) conditions. The lower panel represents the quantification by densitometry of gels corresponding to four separate experiments. (B) Determination of elastase activity (n⩾6) under similar conditions. (C) Quantification of myeloperoxidase (MPO) levels by ELISA (n⩾6) under similar conditions. (Results are expressed as mean values±s.d., P values are indicated on the diagrams).
High-Density Lipoproteins Inhibit Polymorphonuclear Neutrophil Activation Induced by Oxygen-Glucose Deprivation Conditions in the Presence of Endothelial Cells
Incubation with HDLs significantly decreased elastase activity in both OGD and non-OGD conditions and the release of MPO in ischemic conditions only (Figure 2). In these conditions, addition of HDLs did not significantly decrease MMP-9 levels detected in the supernatant. Since HDLs display an important anti-elastase activity14 but have no documented action on MMP-9, it is possible that they inhibit elastase after its release from activated PMNs. However, it cannot be excluded that HDLs may modulate PMN activation in coincubation with endothelial cells since MPO levels are decreased in the supernatants of PMN-endothelial cells in OGD versus non-OGD conditions. Surprisingly, addition of HDLs to isolated PMNs (without endothelial cells) increased MMP-9 release (Supplementary Figure 3), suggesting a partial activation of PMNs (no changes in elastase were observed, possibly because of the anti-elastase activity of HDLs). Of note, in coincubation with endothelial cells, PMN release of MMP-9 and MPO was similar in OGD and non-OGD conditions.
High-Density Lipoproteins Inhibit VE-Cadherin Disorganization and Limit Fibronectin Proteolysis Induced by Polymorphonuclear Neutrophils in Oxygen-Glucose Deprivation Conditions
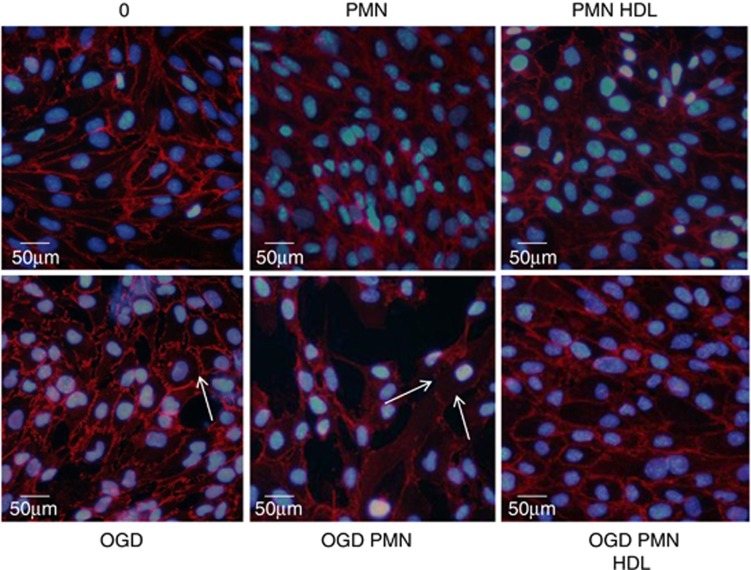
Immunocytofluorescence analysis of VE-cadherin showed the typical sharp membrane staining delimitating endothelial cells under control conditions (DMEM). No change occurred after incubation with PMNs, but a strong disorganization of VE-cadherin was observed under OGD conditions with PMNs. Oxygen-glucose deprivation without PMNs only produced a moderate effect on VE-cadherin distribution, as illustrated by a patchy staining (arrows, Figure 3). Coincubation with HDLs prevented VE-cadherin disorganization induced by OGD±PMNs, showing a pattern similar to that observed in control conditions (Figure 3).
Figure 3.
Immunofluorescent staining of VE-Cadherin (red) after treatment with high-density lipoproteins (HDLs) (0.4 g/L)±polymorphonuclear neutrophils (PMNs) (1 × 106 cells/mL) for 4 hours under normoxic or oxygen-glucose deprivation (OGD) conditions. Nuclei are stained with DAPI (blue). Results are representative of three independent experiments. The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online.
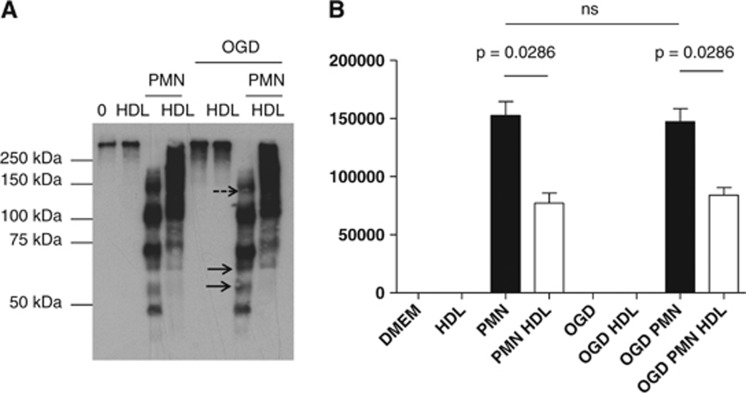
In addition, western-blot analysis of the supernatants showed an important proteolysis of fibronectin by PMNs in non-OGD and even more in OGD conditions, as shown by the presence of degradation fragments in these conditions (more intense bands corresponding to small fragments were observed in the OGD+PMN condition; Figure 4 and Supplementary Figure 4B, plain arrows). High-density lipoproteins partially prevented the proteolysis of fibronectin.
Figure 4.
(A) Western blot for detection of soluble fibronectin fragments in the supernatant of cells after treatment with high-density lipoproteins (HDL) (0.4 g/L)±polymorphonuclear neutrophils (PMNs) (1 × 106 cells/mL) for 4 hours under normoxic or oxygen-glucose deprivation (OGD) conditions. Results are representative of three independent experiments. Plain arrows show more intense degradation fragments in OGD versus non-OGD conditions. Dashed arrow shows a decreased amount of a high molecular weight fragment of fibronectin. (B) Densitometric quantification of fragments <100 kDa (n=3, results are expressed as mean values±s.d., P values are indicated on the diagrams).
High-Density Lipoproteins Prevent Deleterious Effects of Purified Elastase on the Blood–Brain Barrier
To evaluate the role of elastase in PMN-induced BBB destabilization, we tested the effect of purified elastase in our model of BBB. Endothelial cells were exposed to 100 nM elastase under normal or OGD conditions±HDLs (0.4 g/L). After 4 hours of treatment, the integrity of the BBB was assessed in each condition. Surprisingly, elastase induced a loss of integrity of the BBB only under OGD conditions (P=0.0004, 0.019±0.0053 × 10−3 versus 0.0033±0.00053 × 10−3 cm/min in DMEM). Coincubation with HDLs returned permeability to baseline (Figure 5A). Immunocytofluorescence detection of VE-cadherin showed that the deleterious effects of elastase were inhibited by HDLs in both non-OGD and OGD conditions (Figure 5B). It should be noted that disorganization of VE-cadherin by elastase was more important when cells were subjected to OGD. For immunostaining, cells were cultured on Labtek chambers at a lower confluence than for permeability experiments. This could be an explanation for the discrepancy between the observed disorganization of the endothelial layer after treatment with elastase in non-OGD conditions and the unmodified permeability under these conditions (Figures 5B versus 5A). Western-blot analysis showed increased proteolysis of fibronectin by elastase in OGD versus non-OGD conditions as previously observed for PMNs, which was only partially prevented by addition of HDLs (Supplementary Figure 4A).
Figure 5.
(A) In vitro effects of elastase (EL) on permeability of hCMEC/D3 cells under oxygen-glucose deprivation (OGD) versus non-OGD conditions. Cells were incubated with high-density lipoproteins (HDLs) (0.4 g/L)±EL (100 nM) for 4 hours (n⩾6 for each condition). Changes in permeability were only observed with EL in OGD conditions. (B) Immunofluorescent staining of VE-Cadherin (red) after treatment with HDLs (0.4 g/L)±EL (100 nM) for 4 hours under OGD or non-OGD conditions. Results are representative of three independent experiments. The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online.
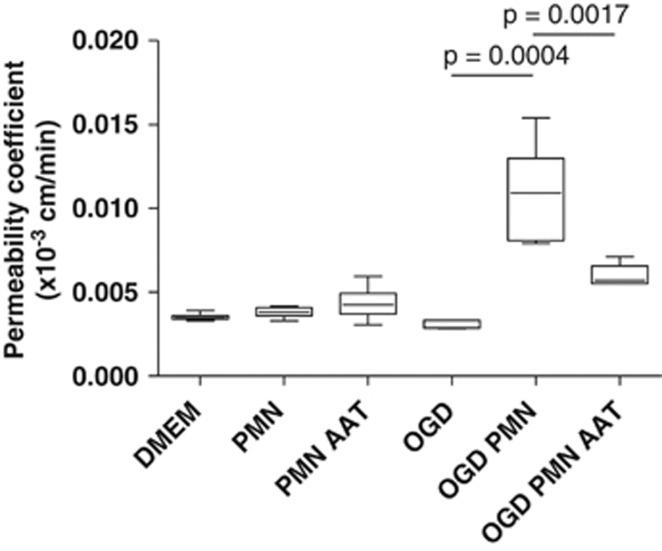
Elastase Inhibition Reduces Polymorphonuclear Neutrophil-Induced Blood–Brain Barrier Destabilization in Ischemic Conditions
To address the role of elastase in our in vitro model of BBB aggression by PMNs in ischemic conditions, we assessed the effect of AAT, the natural inhibitor of elastase, on this increase of BBB permeability. After 4 hours of treatment, the integrity of the BBB was assessed in each condition. As shown in Figure 6, coincubation with AAT significantly inhibited the increase in permeability induced by PMNs under OGD conditions (Figure 6). Alpha-1 antitrypsin totally inhibited elastase activity in both OGD and non-OGD conditions (Supplementary Figure 5). Immunodetection of VE-cadherin and western-blot analysis of fibronectin showed an important protective effect of AAT both on stabilization of intercellular junctions and on proteolysis (Supplementary Figures 6 and 4B). These results suggest that elastase is the major determinant of pericellular proteolysis and subsequent BBB disorganization leading to increased permeability induced by PMNs under OGD conditions.
Figure 6.
Effects of alpha-1-antitrypsin (AAT) on the permeability induced by polymorphonuclear neutrophils (PMNs) under oxygen-glucose deprivation (OGD) conditions. Cells were incubated with PMNs (1 × 106 cells/mL)±AAT (1 μM)±high-density lipoproteins (HDLs) (0.4 g/L) for 4 hours under OGD or non-OGD conditions (n⩾6 for each condition).
Discussion
In our study, we have shown in vitro that PMNs were able to induce BBB disruption when coincubated with cerebral endothelial cells and submitted to OGD conditions for 4 hours. In particular, elastase was shown to be the major determinant of BBB breakdown. We also propose that HDLs may represent a promising therapy in stroke by preventing PMN-induced BBB permeability under ischemic conditions, in particular by inhibiting elastase.
In contrast to other studies, we show that ischemic conditions were not sufficient to induce an increased permeability.22, 23 Wachtel et al23 used human umbilical veni endothelial cells submitted to 5 hours of ischemia and permeability was assessed using 4.4 kDa FITC-Dextran versus 70 kDa FITC-Dextran in our study. In addition to the increased ability of lower molecular mass dextran to cross the endothelial layer, human umbilical veni endothelial cells establish less impermeable junctions relative to the hCMEC/D3 cells used in our study (as shown in Supplementary Figure 2). More recently, Cowan et al24 reported an increased, but reversible, permeability in hCMEC/D3 cells subjected to OGD for 1 hour and then reoxygenated for 30 minutes or 1 hour. A permanent increase in permeability was only achieved after 12 hours of OGD and was associated with cytotoxicity. Intermediate incubation times, i.e., 4 hours, were not tested in this study. We have also observed an increased BBB permeability in ischemic conditions (4 hours) using hCMEC/D3 cells grown 10 days versus 14 days post confluence in the present study (not shown).
Interestingly, transendothelial migration of PMNs after stimulation of the BBB by tumor necrosis factor (TNF)α was accompanied by an increased permeability, but after the migration period, the endothelial layer resumed its continuity.25 This suggests that activation of endothelial cells is sufficient for expression of adhesion molecules and recruitment of PMNs, but a sustained BBB disruption may require additional factors such as PMN activation.
Conversely, Joice et al26 tested the effect of PMN activation by TNFα or LTB4 on the same BBB model that we used, consisting of the hCMEC/D3 endothelial cell line cultured on collagen. Interestingly, they showed that nonstimulated PMNs reduced endothelial permeability whereas activated PMNs returned permeability to baseline. The same group showed that PMNs blocked the OGD-induced increase in permeability when added 1 hour after OGD or at the onset of OGD (for 1 hour). Whereas longer exposure of hCMEC/D3 to OGD (12 and 24 hours) led to increased permeability associated with cytotoxicity, PMNs did not show any effect on the permeability.24
In our study, we hypothesized that a sustained activation of both PMNs and endothelial cells may be required to mimic the conditions leading to BBB disruption in stroke. For this reason, we tested in vitro the permeability of the BBB20 in the presence of PMNs under OGD conditions for 4 hours. These conditions were chosen to reproduce in vitro ischemic conditions that could be found in a clinical situation of thrombo-embolic stroke. In humans, this condition (4 hours of ischemia) may be similar when patients with acute cerebral artery occlusion are recanalized by thrombectomy.27
We have shown that elastase was sufficient to induce an increased permeability of the BBB and participated in PMN-induced BBB disruption under ischemic conditions. In contrast to the study by Cowan et al,24 we have shown that PMN activation under OGD conditions was able to destabilize the BBB leading to increased permeability. This may be because of the 4-hour incubation time that might be necessary for the proteolytic action of elastase on pericellular matrix and junction proteins. In this respect, it was shown that endothelial cells exposed to anoxia-reoxygenation induced elastase release by PMNs.28 Elastase was shown to increase the permeability of an endothelial monolayer via its proteolytic effects.29 This protease was showed to be, at least in part, responsible for microvascular endothelial cell injury after activation of PMNs by lipopolysaccharide and N-formyl-methionyl-leucyl-phenylalanine.30 In this latter study, either purified elastase or stimulated PMNs produced significant endothelial injury after 4 hours of treatment. In vivo, stimuli other than ischemia may participate in PMN activation, such as TNFα or interleukin 1β. For example, interleukin 1β was reported to induce PMN adhesion and migration associated with an increased permeability of the BBB and a disorganization of junction proteins (occludin, ZO-1, etc.).31
Other studies have suggested that elastase could be an important mediator of endothelial layer permeability.18, 32, 33 For this reason, the recently reported anti-elastase function of HDLs14 may represent a novel protective effect of these particles in pathologic conditions that involve PMN activation and subsequent elastase release. In particular, HDLs may be able to transport AAT into the cells where it could thwart the deleterious effects of intracellular elastase.34 Activation of PMNs was recently reported to be modulated by HDLs.35 In this study, PMNs were stimulated by PMA or lipopolysaccharide for 15 to 60 minutes and CD11b expression was monitored. Although reconstituted HDLs and apoA1 limited membrane expression of CD11b after these inflammatory stimuli, the effect of rHDLs alone without stimulation was not reported. We show that HDLs induced release of MMP-9 and cell-free DNA by PMNs when incubated without endothelial cells (Supplementary Figure 3 and data not shown). This point should be explored in further experiments.
In addition to their anti-elastase activity it cannot be totally excluded that HDLs may limit PMN activation in coincubation with endothelial cells. Indeed, a trend toward reduced MMP-9 activity and a significant decrease in MPO release were observed in the supernatant of the endothelial cells incubated with PMNs+HDLs (respectively, P=0.057 and P=0.02 in Figures 2A and 2C). We can speculate that HDLs may limit the exposure of adhesion molecules by endothelial cells and thus decrease PMN adhesion and subsequent activation, as previously reported after stimulation by TNFα and lipopolysaccharide.36 It was recently reported that hypoxia caused an increased expression of E-selectin and VCAM-1 by endothelial cells,37 which could mediate PMN adhesion. Further experiments would be required to test this hypothesis in our in vitro model. Finally, we tested the effect of LDLs, which do not display anti-elastase activity, on the permeability of the BBB in vitro.14 These lipoproteins neither prevented OGD/PMN-induced increased permeability of the BBB nor VE-cadherin redistribution (Supplementary Figures 8A to 8C).
Clinical Perspectives
In spite of promising results obtained in rat models,38, 39 different clinical trials that aimed at limiting PMN infiltration (anti-ICAM-140 and anti-CD11b41) have failed to show an improved outcome in patients with acute stroke. Other drugs targeting PMN elastase inhibition have been used successfully in experimental models,42, 43, 44 but were not tested in humans. In these two latter studies, synthetic elastase inhibitors43, 44 and genetic deletion of elastase44 were shown to attenuate BBB disruption and to limit the infarct volume. Our previous study in a rat model of thromboembolic stroke showed that intravenous injection of HDLs at the acute phase of stroke limited both BBB breakdown and infarct volume, and eventually reduced the mortality.16 Our present results indicate that HDLs may provide a protective effect, at least in part, via their anti-elastase action that limited PMN-induced BBB disruption under ischemic conditions. All other documented beneficial effects of HDLs on endothelial cells, including junction stabilization via sphingosine 1-phosphate, antioxidant, and anti-thrombotic properties,21 represent additional rationale for the use of HDL-based therapy to limit BBB permeability in acute stroke.
Acknowledgments
The authors would like to thank Dr Mary Osborne-Pellegrin for help in editing this manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported by ANR JCJC-1105 HDLomics, the Fondation de France and the Fondation Coeur et Artère.
Supplementary Material
References
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Ho-Tin-Noe B, Enslen H, Doeuvre L, Corsi JM, Lijnen HR, Angles-Cano E. Role of plasminogen activation in neuronal organization and survival. Mol Cell Neurosci. 2009;42:288–295. doi: 10.1016/j.mcn.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P-selectin and intercellular adhesion molecule-1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25:202–211. doi: 10.1161/01.str.25.1.202. [DOI] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Onodera H, Shiga Y, Nakamura M, Ninomiya M, Kihara T, et al. Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effects of neutrophil depletion. Stroke. 1994;25:1469–1475. doi: 10.1161/01.str.25.7.1469. [DOI] [PubMed] [Google Scholar]
- Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropath Exp Neurol. 2011;70:218–235. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Florentin M, Liberopoulos EN, Wierzbicki AS, Mikhailidis DP. Multiple actions of high-density lipoprotein. Curr Opin Cardiol. 2008;23:370–378. doi: 10.1097/HCO.0b013e3283043806. [DOI] [PubMed] [Google Scholar]
- Ortiz-Munoz G, Houard X, Martin-Ventura JL, Ishida BY, Loyau S, Rossignol P, et al. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 2009;23:3129–3139. doi: 10.1096/fj.08-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill GW, McDonald MC, Mota-Filipe H, Cuzzocrea S, Miller NE, Thiemermann C. High density lipoproteins reduce organ injury and organ dysfunction in a rat model of hemorrhagic shock. FASEB J. 2001;15:1941–1952. doi: 10.1096/fj.01-0075com. [DOI] [PubMed] [Google Scholar]
- Lapergue B, Moreno JA, Dang BQ, Coutard M, Delbosc S, Raphaeli G, et al. Protective effect of high-density lipoprotein-based therapy in a model of embolic stroke. Stroke. 2010;41:1536–1542. doi: 10.1161/STROKEAHA.110.581512. [DOI] [PubMed] [Google Scholar]
- Inglis VI, Jones MP, Tse AD, Easton AS. Neutrophils both reduce and increase permeability in a cell culture model of the blood-brain barrier. Brain Res. 2004;998:218–229. doi: 10.1016/j.brainres.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Nolte A, Wilke A, Drenckhahn D. Human neutrophil elastase increases permeability of cultured pulmonary endothelial cell monolayers. Int J Microcirc Clin Exp. 1993;13:187–203. [PubMed] [Google Scholar]
- Jin X, Liu J, Yang Y, Liu KJ, Yang Y, Liu W. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3 hours after ischemia onset. Neurobiol Dis. 2012;48:309–316. doi: 10.1016/j.nbd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- Muruganandam A, Smith C, Ball R, Herring T, Stanimirovic D. Glutathione homeostasis and leukotriene-induced permeability in human blood-brain barrier endothelial cells subjected to in vitro ischemia. Acta Neurochir Suppl. 2000;76:29–34. doi: 10.1007/978-3-7091-6346-7_6. [DOI] [PubMed] [Google Scholar]
- Wachtel M, Frei K, Ehler E, Bauer C, Gassmann M, Gloor SM. Extracellular signal-regulated protein kinase activation during reoxygenation is required to restore ischaemia-induced endothelial barrier failure. Biochemical J. 2002;367 (Pt 3:873–879. doi: 10.1042/BJ20020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan KM, Easton AS. Neutrophils block permeability increases induced by oxygen glucose deprivation in a culture model of the human blood-brain barrier. Brain Res. 2010;1332:20–31. doi: 10.1016/j.brainres.2010.03.066. [DOI] [PubMed] [Google Scholar]
- Wong D, Prameya R, Dorovini-Zis K. Adhesion and migration of polymorphonuclear leukocytes across human brain microvessel endothelial cells are differentially regulated by endothelial cell adhesion molecules and modulate monolayer permeability. J Neuroimmunol. 2007;184:136–148. doi: 10.1016/j.jneuroim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Joice SL, Mydeen F, Couraud PO, Weksler BB, Romero IA, Fraser PA, et al. Modulation of blood-brain barrier permeability by neutrophils: in vitro and in vivo studies. Brain Res. 2009;1298:13–23. doi: 10.1016/j.brainres.2009.08.076. [DOI] [PubMed] [Google Scholar]
- Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- Inauen W, Granger DN, Meininger CJ, Schelling ME, Granger HJ, Kvietys PR. An in vitro model of ischemia/reperfusion-induced microvascular injury. Am J Physiol. 1990;259 (1 Pt 1:G134–G139. doi: 10.1152/ajpgi.1990.259.1.G134. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Ishii Y, Kitamura S. Mechanism for the increased permeability in endothelial monolayers induced by elastase. Mediators Inflamm. 1994;3:11–16. doi: 10.1155/S0962935194000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedly LA, Tonnesen MG, Sandhaus RA, Haslett C, Guthrie LA, Johnston RB, et al. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986;77:1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Killackey JJ, Killackey BA. Neutrophil-mediated increased permeability of microcarrier-cultured endothelial monolayers: a model for the in vitro study of neutrophil-dependent mediators of vasopermeability. Can J Physiol Pharmacol. 1990;68:836–844. doi: 10.1139/y90-127. [DOI] [PubMed] [Google Scholar]
- Carl VS, Moore EE, Moore FA, Whalley ET. Involvement of bradykinin B1 and B2 receptors in human PMN elastase release and increase in endothelial cell monolayer permeability. Immunopharmacology. 1996;33:325–329. doi: 10.1016/0162-3109(96)00055-0. [DOI] [PubMed] [Google Scholar]
- Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- Moudry R, Spycher MO, Doran JE. Reconstituted high density lipoprotein modulates adherence of polymorphonuclear leukocytes to human endothelial cells. Shock. 1997;7:175–181. doi: 10.1097/00024382-199703000-00004. [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Cromer W, Wells S, Jennings M, Mathis JM, Minagar A, et al. Metabolic modulation of cytokine-induced brain endothelial adhesion molecule expression. Microcirculation. 2012;19:155–165. doi: 10.1111/j.1549-8719.2011.00141.x. [DOI] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Chahwala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res. 1998;788:25–34. doi: 10.1016/s0006-8993(97)01503-5. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Li Y, Zaloga C, Jiang N, Jones ML, et al. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- Investigators EAST Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo JM, Ford GA. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umemura K. Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res. 2000;858:55–60. doi: 10.1016/s0006-8993(99)02431-2. [DOI] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi S, Yoshimura S, Nakashima S, Iwama T. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertens Res. 2010;33:703–707. doi: 10.1038/hr.2010.58. [DOI] [PubMed] [Google Scholar]
- Stowe AM, Adair-Kirk TL, Gonzales ER, Perez RS, Shah AR, Park TS, et al. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35:82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.