Abstract
Infant cries are a critical survival mechanism that draw the attention of adult caregivers, who can then satisfy the basic needs of otherwise helpless infants. Here, we used functional neuroimaging to investigate the effects of infant hunger cries on brain activity of adults who were in a cognitively non-demanding mental state of awake rest. We found that the brains of males and females, independent of parental status (parent or non parent), reacted differently to infant cries. Specifically, dorsal medial prefrontal and posterior cingulate areas, known to be involved in mind-wandering (the stream of thought typical of awake rest), remained active in men during exposure to infant cries, whereas in women activity in these regions decreased. These results reveal gender-dependent modulation of brain responses to infant requests to be fed, and specifically they indicate that women interrupt mind-wandering when exposed to the sounds of infant hunger cries, whereas men carry on without interruption.
Keywords: Parenting, infant cry, mind-wandering, brain, medial prefrontal cortex, posterior cingulate cortex, default-mode network
Introduction
Lacking agency, new-born babies and young infants communicate their needs and physiological states mainly through vocalizing and facial expression. Infant cries communicate hunger, distress, or the desire for physical closeness [1], and they effectively induce a broad range of caregiver responses, such as proximity [2] or endocrine reactions [3].
In the present study, we analysed gender differences in brain activations in response to infant hunger cries. Women and men, mothers and fathers have different relationships with their children. By many accounts, several cross-cultural in nature, human mothers provide more direct care to children than do fathers [4,5]. Maternal responses to infant cries are critical for infant survival and the development of wholesome mother–infant emotional bonding [6]. Women rate infant cries as more likely to evoke sympathy and caregiving than men, and men rate cries as more aversive, as eliciting more irritation and anger, and evaluate infants who cry as more spoiled [7,8].
Several neuroimaging studies have investigated adult brain activity to infant cries, but so far have produced conflicting findings [9]. Few neurobiological studies have included males. In one exception, Seifritz et al. [10] assessed female and male (parents’ and nonparents’) responses to crying and laughter of unfamiliar infants; females showed greater prefrontal activation than males to infant vocalizations regardless of parental status.
The main goal of our study was to investigate neural responses associated with listening to infant cries during rest and mind-wandering. We asked participants to lie in the functional Magnetic Resonance Imaging (fMRI) scanner, and to think of nothing in particular, and so let their minds wander freely. They were told that they would hear various sounds intermixed with phases of rest, and that they were required to do nothing with these sounds. In the key condition, participants heard cries of hungry babies.
We chose to study the brain’s spontaneous reactions to infant cry, because immersion in “internal” thoughts, without being focused on external stimuli, is acknowledged to be the most common mode of thinking, occupying almost half of our daily awake mental activity [11]. This situation has the additional advantage that it emulates ecological validity: when a baby begins to communicate a physiological or psychological need through crying, this signal likely reaches a caregiver who is not already attending to the baby, but who is instead mind-wandering or may be mentally focused elsewhere. The developing neuroimaging literature on mind-wandering has revealed that this mental state usually involves coherent and spontaneous low-frequency fluctuations in a specific brain network that includes medial prefrontal, posterior cingulate, hippocampus, superior temporal, and inferior parietal regions, collectively called the default-mode network [12]. In particular, medial regions of the default-mode network (the medial prefrontal and posterior cingulate cortex) are involved in self-reflection and self-centred thinking [13]. By contrast, when a person focuses on external stimuli, the default-mode network is deactivated [14]. Thus, the deactivation of the default-mode network is a reliable marker of the extent and efficiency of the observer’s engagement with the external world [15]; for example, it is interpreted to indicate awareness in patients with disorders of consciousness (minimally conscious or vegetative state) during passive sentence listening [16]. The default-mode network encompasses brain regions that show task-induced deactivation, and while its activity is thought to represent on-going and organized cognitive processing that occurs when the brain is not actively involved in another task, its deactivation reflects an interruption of on-going mind-wandering [17].
Materials and Methods
Participants
Eighteen healthy adults (9 females: M age = 31.50 years, SD = 4.27; and 9 males: M age = 35.38 years, SD = 4.63) were recruited through the University of Trento webpage and local advertisements. Nine were parents of children older than 4 years (4F/5M), and nine were non-parents (5F/4M). All participants were right-handed, and none reported neurological or psychiatric disorders. Participants were ethnically homogeneous of European heritage. All gave written informed consent for participation. The procedures were approved by the ethical committee for experiments involving humans at the University of Trento.
Acoustic Stimuli
In our main condition, we analysed brain responses to infant hunger cries vis-á-vis the adult at rest. We used 14 sec for the rest period between acoustic stimuli. We also compared responses during hunger cries to two types of auditory control stimuli: white noise and atypical cries. All acoustic files were edited using the computer software Praat (www.praat.com) to normalize volume, equate for duration (10 sec), and remove all background noise. A total of 10 acoustic excerpts of natural infant hunger cries from 10 1-year-old infants (5 girls/5boys) were selected from a pool of home videos of typically developing infants who did not present any significant medical or developmental concerns (confirmed by their normal scores on the Child Behavior Checklist; [18]). Atypical cries were extracted from home videos of 10 (5 girls/5boys) approximately 1-year-old infants who later, as children of ~3 years, were diagnosed with Autism Spectrum Disorder (clinical diagnosis was made by a child psychiatrist according to DSM-IV-R criteria and confirmed by ADI-R, ADOS-G). Atypical cries differ from hunger cries in morphological characteristics, especially their fundamental frequency (atypical cries > hunger cries). The fundamental frequency (f0) is generated by vibration of the vocal folds and is heard as the pitch of the cry. f0 influences adult responses and caregiver perceptions: for example, higher-frequency cries are regularly perceived as more aversive and distressed than low-frequency cries [19]. To ensure that hunger cries and atypical cries were representative of the typical expected range of cry sounds for typical and atypical children, respectively, we analysed their f0. A long-term average spectrum (LTAS) was employed to provide spectral information for each cry. For all cry stimuli the First Spectral Peak (FSP) of the LTAS was obtained. FSP is the frequency value (in Hz) of the first amplitude peak across the LTAS. It is an estimate of the average f0 of the cry episodes. The FSPs of hunger cries were M = 526.87 (SD = 60.33) and of atypical cries were M = 637.87 (SD = 66.56), F(1,16)=18.80, p<.001.
fMRI Protocol
During functional scanning, participants were asked to listen passively to the acoustic stimuli presented binaurally at ~75 dB SPL using Serene Sound (Resonance Technologies, Northridge, CA) headphones, with stereo quality sound (40 Hz to 40 KHz frequency response) and passive scanner noise attenuation (30 dB). Participants underwent a single fMRI run in which stimuli were presented in a blocked design. Acoustic stimuli of each category (hunger cries, atypical cries, white noise) lasted 10 sec, with an inter-stimulus interval of 14 sec during which no stimuli was presented (rest). These stimulus categories were presented in a fixed order a total of 10 times. The 10 cry stimuli in each category were different, and they were pseudo-randomized between participants. Each cry was presented once to minimize habituation effects.
fMRI Data Acquisition
Participants underwent MRI scanning at 4 Tesla in a MedSpec Biospin MR scanner (Bruker Ettlingen, Germany) using an 8-channel birdcage head coil. Mild external head restraint was used to minimize head movement. Before collecting functional images, a high-resolution T1 weighted image of the whole brain (MPRAGE: 176 slices, GRAPPA acquisition with an acceleration factor of 2, FOV=256×256 mm2, voxel size = 1×1×1 mm, TI = 1020 ms TE = 4.18 ms, TR = 2700 ms) was acquired for the purpose of spatial coregistration. Whole-brain functional data were acquired using echoplanar imaging, sensitive to Blood Oxygenation Level Dependent (BOLD) contrast (34 slices, tilted 18° from intracommisural plane, FOV=192×192 mm2, voxel size = 3×3×3 mm3, slice gap = 15%, flip angle (FA), 73°, TE = 33 ms, TR = 2 s per volume). We performed an additional scan to measure the point-spread function (PSF) of the acquired sequence, which served for distortion correction as is expected with high-field imaging. The experimental session consisted of 489 whole brain images per participant, including four dummy scans at the start of each time series to allow for T1 equilibration. The experiment lasted 16 min and 10 sec.
fMRI Data Analysis
Imaging data analyses were performed with BrainVoyager QX 2.1 (BrainInnovation). For preprocessing, we corrected for distortions in geometry and intensity in the EPI images, and we applied distortion correction on the basis of the PSF data acquired before the EPI scans. Then, we performed 3D motion correction with trilinear interpolation and slice timing correction with ascending interleaved order, using the first slice as reference. Functional data were temporally high-pass filtered at 3 cycles/run length. A Gaussian kernel of 8 mm was applied to spatially smooth the images. Next, we aligned the first volume of each functional run to the high-resolution anatomy. Both functional and anatomical data were transformed into Talairach space [20] using trilinear interpolation. Predictor time courses were convolved with a canonical hemodynamic impulse response function starting at the beginning of each trial and included 3D-motion correction parameters.
Using general linear models, we explored the sensitivity of brain regions to cries of hungry healthy infants. We identified brain regions that were more or less active during white noise compared to rest for all participants. This contrast allowed us to localize brain regions that – independent of gender or parent status – were responsive to sudden neutral sounds while individuals were resting and free to let their minds wander. Subsequently, we conducted orthogonal region of interest (ROI) analyses within the individual regions identified in the previous contrast. For all reported contrasts, we used random effects and False Discovery Rate (FDR) to correct for multiple comparisons.
RESULTS
Consistent with extant literature [21], the first analysis showed that, during white noise auditory stimulation, left and right superior temporal lobe regions were more active (in particular, centred on the Heschl’s gyri, otherwise known as transverse temporal gyri; Brodmann area 41), whereas the dorsal medial prefrontal and posterior cingulate cortex (BA 23) regions – two key medial components of the default-mode network - were less active (see Table 1). A follow-up comparison of group differences (male versus female; parent versus non parent) in activity during white noise compared to rest revealed no differences (minimum p = 0.12), indicating that neither gender nor parenthood status affects these regions differently when listening to white noise compared to rest (see Fig.1, panel B for the male versus female comparison).
Table 1. Passive-listening brain regions.
Brain regions resulting from the contrast white noise against rest in the whole group of participants. These regions serve as a mask for auditory sensitive regions (both increase and decrease of activation) during passive exposure to sudden sounds.
| Auditory Brain Regions (White Noise<>Rest) |
BA | Peak X | Peak Y | Peak Z | Number of Voxels at q(FDR)<0.01 |
|---|---|---|---|---|---|
|
Decreased Activation (White
Noise<Rest) |
|||||
| Dorsal Medial Prefrontal Cortex | 9/10 | 3 | 47 | 28 | 3926 |
| Posterior Cingulate Cortex | 23 | 0 | −46 | 25 | 1827 |
|
Increased Activation (White
Noise>Rest) |
|||||
| Left Heschl’s (Transverse Temporal) Gyrus |
41 | −45 | −19 | 7 | 6368 |
| Right Heschl’s (Transverse Temporal) Gyrus |
41 | 48 | −13 | 4 | 7379 |
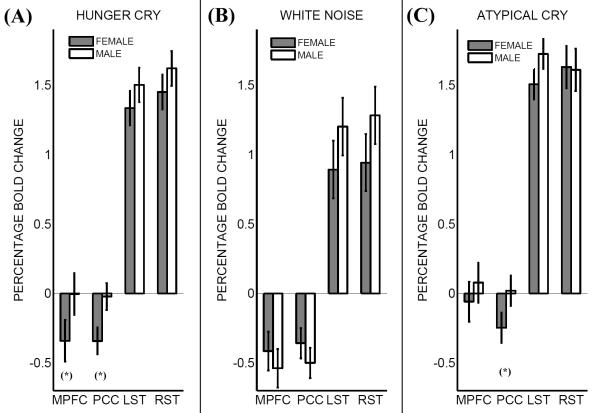
Figure 1.
Percentage BOLD (Blood Oxygenation Level Dependent) signal change versus rest in males and females in three conditions: (panel A) Hunger Cry, (panel B) White Noise, and (panel C) Atypical Cry. Asterisks denote significant differences between males and females’ percentage bold change (p<0.05). MPFC stands for dorsal medial prefrontal cortex, PCC for posterior cingulate cortex, LST for left superior temporal gyrus (Heschl’s gyrus), and RST for right superior temporal gyrus (Heschl’s gyrus). For precise coordinates of regions, see Table 1. Error bars are standard errors. Deactivation in dorsal medial prefrontal cortex and posterior cingulate cortex during hunger cry – distinctive of the mind-wandering interruption – took place only for the female group, but not for the male group. During atypical cry, this gender difference was significant only in posterior cingulate cortex, but not in dorsal medial prefrontal cortex.
Our main analysis looked for group differences during passive listening to hunger cries versus rest (male versus female, and parent versus non parent) within each of the localized regions identified as more sensitive to sounds. The parenthood group contrast showed no difference (minimum p=0.16). However, the gender group contrast revealed significant differences in the two medial regions, namely the dorsal medial prefrontal cortex (df=16; M female group BOLD= −0.341; M male group BOLD= − 0.004; standard error= 0.15; t=2.22; p=0.041) and the posterior cingulate cortex (df=16; M female group BOLD= −0.342; M male group BOLD= −0.022; standard error= 0.097; t=3.76; p=0.001). No gender group difference emerged in the left superior (p=0.10) or right superior temporal regions (p=0.57). Thus, the gender difference consisted in males not deactivating in dorsal medial prefrontal cortex and posterior cingulate cortex during hunger cries relative to rest (Fig. 1, panel A). Gender differences again emerged as significant when we directly compared hunger cries to white noise, in both dorsal medial prefrontal cortex (df=16; M female group BOLD =−0.268; M male group BOLD = 0.531; standard error=0.354; t=2.26; p=0.03) and posterior cingulate cortex (df=16; M female group BOLD = −0.327; M male group BOLD = 0.544; standard error=0.193; t=4.50; p=0.0003), but not significant in either the left (p=0.75) or right (p=0.69) superior temporal regions.
When looking at gender group differences within the same regions for atypical cries (another emotionally charged stimulus produced by infants, but with a difference in frequency; see methods) versus rest (Fig. 1, panel C), we observed a significant difference only in posterior cingulate cortex (df=16; M female group BOLD =−0.247; M male group BOLD = 0.019; standard error=0.109; t=2.44; p=0.02). There was no difference in the dorsal medial prefrontal cortex (p=0.35), in right superior temporal lobe (p=0.89), or left superior temporal lobe (p=0.09). Thus, the gender difference in the dorsal medial prefrontal cortex (i.e., only the female group deactivated) was specific of hunger cries; it was not found to closely matched atypical cries.
DISCUSSION
In this experiment, adult participants passively listened to hunger cries of infants, cries of atypically developing infants, and white noise. Participants were simply resting in the scanner and not required to perform any task or even to pay attention to the auditory stimuli. During exposure to hunger cries compared to rest and white noise, brain activity of women and men differed considerably in two key medial nodes of the default-mode network, namely the dorsal medial prefrontal cortex and the posterior cingulate cortex (BA 23). Specifically, activity in these regions decreased in women, but not in men. During exposure to atypical cries (sounds closely matching hunger cries, but with higher fundamental frequency), the gender difference emerged only in posterior cingulate cortex, but not in dorsal medial prefrontal cortex.
Deactivation in default-mode network is a common response to sounds that recruit attention during passive sensory tasks [21]. Researchers studying brain function generally assume that presentation of a stimulus or task will increase neural activity in the brain relative to a resting state. However, many tasks result in decreases in blood flow or blood-oxygenation indexes of neural activity relative to a “resting” baseline, a phenomenon referred to as task-induced deactivation. This change may occur when resources shift from on-going, internally generated processing typical of “resting” states to processing required by an exogenous task. Research demonstrates that task-induced deactivation occurs consistently in default-mode network regions, and researchers speculate that “rest” is actually a state of organized cognitive activity involving many processes, including monitoring the external environment, monitoring body image and state, and processing emotions [15,17,22,23]. Included among these possible resting activities is on-going internal “thought” processes that humans experience during resting consciousness, sometimes referred to as “stream of consciousness”. Because these “thought” processes are generally self-initiated and self-referential, and not related to specific exogenous task demands, they have been reported as “task-unrelated thoughts” [24]. As task-induced deactivation magnitude increases across task conditions, the frequency of task-unrelated thoughts declines [17]. Internally generated cognitive activities (such as task-unrelated thoughts) are suspended due to reduced availability of resources when attention shifts from on-going, internal processes to performance of an exogenous task. As an auditory target detection task increases in difficulty (i.e., increased processing demands), multiple brain regions show correlated decreases in the BOLD signal (i.e., more negative task-induced deactivations values) relative to the resting state [17].
In this experiment we observed a medial default-mode network deactivation in both gender groups during passive exposure to white noise; by contrast, dorsal medial prefrontal and posterior cingulate cortical activity did not deactivate in men during exposure to hunger cries, but instead remained at the same level as during rest. These medial cortical regions are key nodes of the default-mode network associated with self-reflection activities [13], and thus these results suggest that women interrupt their mind-wandering when they are exposed to infant hunger cries, whereas men carry on without interruption.
We found no difference in response to hunger cries depending on parental status. The size of sample did not allow us to make further gender comparisons within each parental group (parents and parents), Nonetheless, our results are indicative of an “alloparental care” predisposition among females, a result common in several mammalian species where adult females act as cooperative breeders and share responsibility for infant care [25].
Conclusion
We found a gender difference in brain responses to hunger cries in medial default-mode network regions: specifically, women decrease activity in these regions when they suddenly and passively hear infant hunger cries, but men do not. In functional terms, this finding suggests that, whereas the female brain during hunger cries interrupts on-going mind-wandering, the male brain continues in self-reflection typical of awake resting states.
Acknowledgments
source of funding This research was supported by the Intramural Research Program of the NIH, NICHD.
Footnotes
Statement of Conflicts of Interest We declare no conflict of interest relatively to this study.
References
- [1].Zeifman DM. An ethological analysis of human infant crying: answering Tinbergen’s four questions. Developmental psychobiology. 2001;39:265–285. doi: 10.1002/dev.1005. [DOI] [PubMed] [Google Scholar]
- [2].Bowlby J. Attachment and loss. Basic Books; New York: 1969. [Google Scholar]
- [3].Swain JE, Kim P, Ho SS. Neuroendocrinology of parental response to baby-cry. J Neuroendocrinol. 2011;23:1036–1041. doi: 10.1111/j.1365-2826.2011.02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whiting BB, Edwards CP. Children of Different Worlds. Harvard University Press; Cambridge, MA: 1988. [Google Scholar]
- [5].Clutton-Brock TH. Mammalian mating systems. Proc R Soc Lond B Biol Sci. 1989;236:339–372. doi: 10.1098/rspb.1989.0027. [DOI] [PubMed] [Google Scholar]
- [6].Feldman R. Maternal versus child risk and the development of parent-child and family relationships in five high-risk populations. Development and psychopathology. 2007;19:293–312. doi: 10.1017/S0954579407070150. [DOI] [PubMed] [Google Scholar]
- [7].Boukydis CF, Burgess RL. Adult physiological response to infant cries: effects of temperament of infant, parental status, and gender. Child development. 1982;53:1291–1298. [PubMed] [Google Scholar]
- [8].M. ZD. Predicting adult responses to infant distress: Adult characteristics associated with perceptions, emotional reactions, and timing of intervention. Infant Mental Health Journal. 2003;24:597–612. [Google Scholar]
- [9].Swain JE. Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry. 2008;5:28–36. [PMC free article] [PubMed] [Google Scholar]
- [10].Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiat. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- [11].Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- [12].Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann Ny Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- [13].Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in cognitive sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [14].Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- [15].De Pisapia N, Turatto M, Lin P, Jovicich J, Caramazza A. Unconscious priming instructions modulate activity in default and executive networks of the human brain. Cereb Cortex. 2012;22:639–649. doi: 10.1093/cercor/bhr146. [DOI] [PubMed] [Google Scholar]
- [16].Crone JS, Ladurner G, Holler Y, Golaszewski S, Trinka E, Kronbichler M. Deactivation of the default mode network as a marker of impaired consciousness: an fMRI study. PLoS One. 2011;6:e26373. doi: 10.1371/journal.pone.0026373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. [Google Scholar]
- [19].Venuti P, Caria A, Esposito G, De Pisapia N, Bornstein MH, de Falco S. Differential brain responses to cries of infants with autistic disorder and typical development: an fMRI study. Res Dev Disabil. 2012;33:2255–2264. doi: 10.1016/j.ridd.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- [21].Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cognitive Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- [22].Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- [23].Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain research bulletin. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- [24].Giambra LM. Task-unrelated-thought frequency as a function of age: a laboratory study. Psychology and aging. 1989;4:136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- [25].Briga M, Pen I, Wright J. Care for kin: within-group relatedness and allomaternal care are positively correlated and conserved throughout the mammalian phylogeny. Biology letters. 2012;8:533–536. doi: 10.1098/rsbl.2012.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]