Abstract
Beneficial effects of the ovarian steroid, 17β-estradiol (E2), for affective behavior have been reported in young individuals, but less is known about the effects of E2 among older individuals, and the capacity of older individuals to respond to E2 following its decline. In the present study, the effects of acute E2 administration to aged mice for anxiety-like and depression-like behaviors were investigated. Intact female C57BL/6 mice (N=18) that were approximately 24 months old were administered vehicle (sesame oil, n=9) or E2 (10 μg, n=9) subcutaneously 1h prior to behavioral testing. Mice were tested for anxiety-like behavior (open field, elevated plus maze, mirror chamber, light–dark transition task, Vogel conflict task) and depression-like behavior (forced swim task). To assess the role of general motor behavior and coordination in these aged mice, performance in an activity monitor and rotarod task, and total entries made in tasks (open field, elevated plus maze, light–dark transition task) were determined. Mice administered E2, compared to vehicle, demonstrated anti-anxiety behavior in the open field, mirror chamber, and light–dark transition task, and anti-depressive-like behavior in the forced swim task. E2 also tended to have anti-anxiety effects in the elevated plus maze and Vogel task compared to vehicle administration, but these effects did not reach statistical significance. E2 did not alter motor behavior and/or coordination in the activity monitor, open field, or rotarod tasks. Thus, an acute E2 regimen produced specific anti-anxiety and anti-depressant effects, independent of effects on motor behavior, when administered to aged female C57BL/6 mice.
Keywords: 17β-estradiol (E2), Aging, Anxiety, Depression, Senescent, Motor activity
1. Introduction
The ovarian hormone, 17β-estradiol (E2), has pleiotropic effects in the brain and body. A situation in which these effects may be most evident is when individuals experience robust changes in ovarian function with aging and the onset of reproductive senescence, which can produce changes in quality of life (i.e. mood, anxiety, forgetfulness, sleepiness, etc.), as well as physical changes (i.e. changes in bone density, hot flashes, drying of mucosal membranes, etc.). Given that in many cases the source and target of E2 are the same (e.g. brain, ovaries), it can be difficult to parse out these effects and determine E2’s mechanisms. A typical approach that has been taken to address this in the laboratory is to ovariectomize (OVX) rodents and replace them back with E2 or E2-mimetics, such as selective estrogen receptor modulators (SERMs), which have discrepant effects at different receptor targets. Although this has been a fruitful approach, differences in the timing of OVX and replacement can obscure some of the effects. For instance, there may be differences on anxiety measures in rats depending upon how long they are OVX, with or without E2 replacement [1–3]. As well, the clinical relevance of using OVX as a model of reproductive senescence may be less than ideal. In support, differences in cognitive performance of women who have undergone natural, compared to surgical, menopause have been noted [4]. However, the functional effects of E2 replacement to aged mice have not been well-characterized and are of interest.
Given that life expectancy of women has risen, the population is aging, and more women will live longer in a post-menopausal, E2-deficient state [10], it is important to understand further the effects of E2 in anxiety/depression among older individuals. Although most women do not become depressed during perimenopause, reproductive events may play a role in the onset of depression among some perimenopausal women [6]. Further, E2 may be efficacious as a primary and/or adjunctive therapy for some perimenopausal women [5–7]. Indeed, some post-menopausal women have significantly higher depression scores, when evaluated, than do pre- and perimenopause women [8]. Some prospective, controlled studies show that E2 can dose-dependently alleviate depressive symptoms [9]. As in the clinical literature, few studies reported in the animal literature have directly investigated the responses of older individuals to E2 on affective measures.
In animal models, E2 to young individuals can have anxiety- and depression-reducing effects [2,11]. Although the majority of these studies have been done in rats, there is accumulating evidence that similar effects are observed in mice. For example, when tested in the high (behavioral estrous), versus the low (diestrous), steroid hormone phase of the estrous cycle, mice show less anxiety behavior in the plus maze [9,12,13]. Physiological dosing with E2 reverses OVX-induced increases in depressive behavior in the forced swim test and in anxiety behavior in the elevated plus maze of young adult mice [2,14–18]. How alterations in ovarian function with aging of mice alter affective behavior is of interest. To this end, we investigated the effects of E2 administration to aged female mice in a variety of behavioral tasks to assess its functional effects to test the hypothesis that aged mice would respond favorably to E2 administration.
2. Methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at the University at Albany-SUNY.
2.1. Subjects and housing
Intact, female mice (N=18, mean age 24 months, range 20–28 months), were bred in the Social Sciences Laboratory Animal Care Facility at SUNY-Albany, on a congenic C57BL/6 background. Mice were group-housed, on a 12/12 hour light/dark cycle (lights on 0800h) with free access to Purina Rodent Chow and water in their home cages.
2.2. Hormonal milieu
The pattern of female reproductive aging in mice is similar, but not analogous to that of women [19,20]. Although there is considerable variation at midlife, ovarian follicles become depleted and steroid production decreases to levels akin to that of OVX mice during reproductive aging. By 20–26 months of age, mice without pituitary tumors have gonadatropin levels which are similar to that of OVX mice. As such, mice without pituitary tumors (as determined at autopsy) were utilized intact to prevent attrition from surgery. Mice were randomly assigned to receive E2 (10 μg/0.2cm3; n =9) or vehicle (sesame oil; n=9) by SC injection 1h prior to behavioral testing. This E2 regimen produces concentrations of E2 in plasma of young female mice [21] and hippocampus of senescent female mice [22], akin to that of young, intact mice in behavioral estrus.
2.3. Behavioral testing
Mice were tested approximately once weekly until all tasks, described below, were completed. It was important to test mice across several tasks to account for potential age-related differences in locomotion and/or coordination in these affective tasks and control tasks. As such, in addition to reporting on the effects on anxiety and depression tasks, performance of mice for locomotor behavior in the activity monitor and open field and motor coordination in the rotarod were determined. A description of the tasks that mice were tested in is as follows. Mice were tested in one task per day, in the order that tasks are described below. Although the authors acknowledge that this order of testing may have confounded results, it was deemed necessary in a study such as this using a small number of aged individuals that may be particularly sensitive to the physical demands, or stress of being exposed to a shock stimulus, in the forced swim test and Vogel conflict task, respectively. Experience in these tasks may have altered typical performance in the other behavioral tasks utilized. As such, mice were tested in the following tasks in the order indicated.
2.3.1. Activity monitor
A Digiscan Optical Animal Activity Monitor (39×39×30 cm; Accuscan Instruments Inc) recorded the number of horizontal beam breaks in 5 min [23]. The number of beam breaks made is utilized as an index of general motor behavior.
2.3.2. Open field
Entries into central and peripheral squares of an open field (39 cm×39 cm×30 cm), with 16 squares on the grid floor, was recorded for 5 min [23]. The total and central square entries made in the open field are utilized as indices of general motor and anti-anxiety-like behavior, respectively.
2.3.3. Rotarod
The latency of mice to fall (3 min max, a 48 cm fall height) from The Accurotor Rotorod Apparatus (Accuscan Instruments Inc), with a 70 mm drum, set to rotate at accelerating speeds (0–60 rpm/min), was recorded [23]. The latency to fall from the rotarod is utilized as a measure of motor coordination.
2.3.4. Elevated plus maze
The time spent by mice in the 2 open (5×40 cm) or closed (5×40×20 cm) arms was recorded for 5 min [24]. The time spent in the open arms of the plus maze is utilized as a measure of anti-anxiety-like behavior in this task. Measures of motor activity in this task are the number of entries to the open arms and the total number of arm entries made.
2.3.5. Mirror chamber
The time spent by mice in the mirrored chamber (30 cm×30 cm× 30 cm) or the adjoining alleyway (30 cm×5 cm×30 cm) without mirrors was recorded for 5 min [23]. The time spent in the mirrored chamber is considered an index of anti-anxiety-like behavior in this task. General motor activity in this task was assessed by determining the number of entries mice made to the mirrored chamber.
2.3.6. Light–dark transition task
The time spent by mice in the light or the dark compartments of the testing chamber (24.5 cm×23.5 cm×35 cm) were recorded for 5 min [23]. The time spent in the light compartment is considered an index of anti-anxiety-like behavior in this task. The number of entries made to the light side and the total number of entries made in this task were utilized as indices of general motor activity in this task.
2.3.7. Vogel conflict task
The number of licks made by water-deprived (24h) mice of an electrified water bottle that delivered a shock every 20 licks was recorded for 15 min [23]. The number of licks that are made in this task are utilized as an index of anti-conflict/anti-anxiety-like behavior in this task. The number of times that mice approached and began licking from the spout (“bouts”) was utilized as an index of general motor activity.
2.3.8. Porsolt forced swim task
The duration of immobility when mice were placed in a glass cylinder (20.5 cm diameter, 21.5 cm depth), which contained 18 cm of 25 °C water was recorded for 5 min [25]. The time spent immobile is considered an index of anti-depressive-like behavior of mice in this task. Other measures that were assessed in this task was the amount of time spent swimming (paddling in the chamber) and struggling (making movements in which paws break the surface of the water, generally on the sides of the chamber, as an attempt to climb out of the chamber).
2.4. Data analyses
One-way analyses of variance (ANOVAs), with Fisher’s post hoc tests, were used to evaluate effects of hormone condition (E2 or vehicle) on behavioral indices. The α level for statistical significance was p<0.05 and a statistical tendency was considered when p<0.10.
3. Results
3.1. Activity monitor
There was no significant difference between vehicle and E2 to mice for general motor activity in the Activity monitor. Mice administered E2 or vehicle had a similar number of beam breaks in this task (Table 1).
Table 1.
Indices (mean±S.E.M.) of general motor activity (Activity monitor), coordination (Rotarod), and motor behavior in the tasks utilized (open field, elevated plus maze, mirror maze, light–dark transition, Vogel conflict task, forced swim test) of aged mice administered vehicle or estradiol (E2) 1h before testing.
| Measures | Condition
|
|
|---|---|---|
| Vehicle | E2 | |
| Activity monitor — # of beam breaks | 1276±128 | 1156±110 |
| Rotarod — fall latency (s) | 9±5 | 8±2 |
| Elevated plus maze — open arm entries | 6.3±0.5 | 6.9±0.6 |
| Elevated plus maze — total arm entries | 13.1±1.1 | 13.9±1.3 |
| Mirror maze-total entries to mirrored chamber | 7.0±0.9 | 7.1±0.8 |
| Light–dark transition — light side entries | 5.6±0.4 | 6.0±0.4 |
| Light–dark transition — total entries | 21.7±4.0 | 25.7±5.0 |
| Vogel conflict task — total drinking bouts | 6.2±1.0 | 6.6±0.4 |
| Forced swim test — time spent swimming (s) | 93.3±10.7 | 112.4±3.5 |
| Forced swim test — time spent struggling (s) | 18.0±4.8 | 31.9±4.9a |
Indicates a tendency for a difference from vehicle (p=0.06).
3.2. Open field
There was a statistically significant main effect of condition for the number of central squares entered by mice F (1, 16)=65.35, p<0.01. Mice administered E2 entered more central squares than did mice administered vehicle (Fig. 1). However, the total number of square entries was not significantly different for E2- or vehicle-administered mice (Table 1).
Fig. 1.
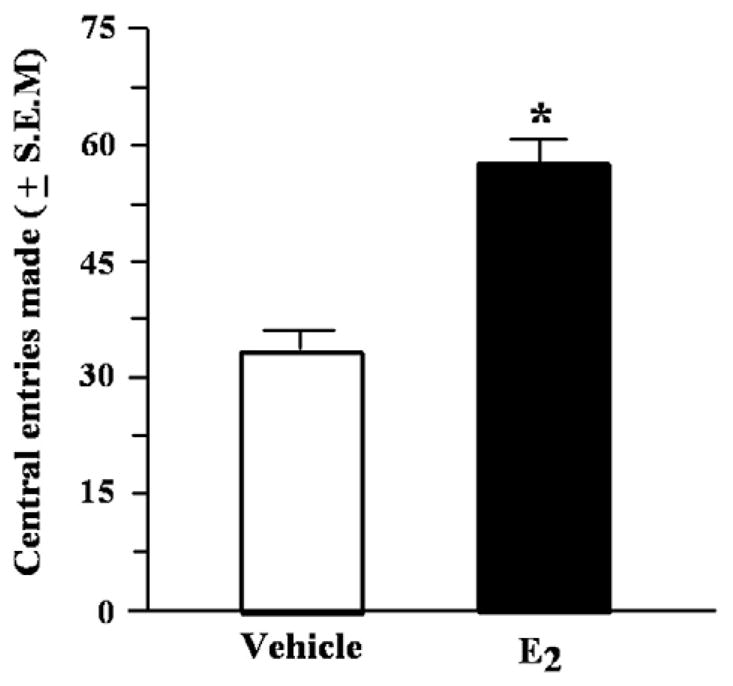
Central entries (mean±S.E.M.) made in the open field of aged mice administered vehicle or estradiol (E2) 1h before testing. *Indicates a significant difference from vehicle (p<0.05).
3.3. Rotarod
There was no significant difference between conditions for fall latencies in the rotarod. Mice administered E2 or vehicle had similar short fall latencies in this task (Table 1).
3.4. Elevated plus maze
Although the data were not significant, the time spent on the open arms of mice tended to be greater for E2 (170±24s) than for vehicle (118±16s) administered mice F (1, 16=3.22, p<0.09. There were no differences between mice administered vehicle or E2 for the open arm entries made or the total arm entries made in this task (Table 1).
3.5. Mirror chamber
There was a significant main effect of condition for the time spent in the mirrored chamber F (1, 16)=9.97, p<0.01. Mice administered E2 spent significantly more time in the mirrored chamber than did vehicle controls (Fig. 2).
Fig. 2.
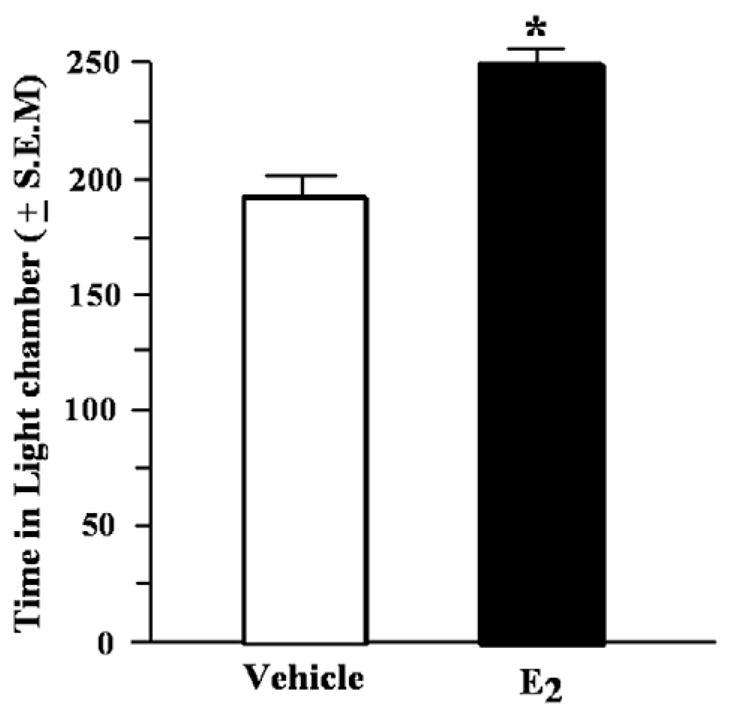
Time spent in the mirrored chamber (mean in s ±S.E.M.) of aged mice administered vehicle or estradiol (E2) 1h before testing. *Indicates a significant difference from vehicle (p<0.05).
3.6. Light–dark transition
There was a significant main effect of condition for the time spent in the light chamber in the light–dark transition task F (1, 16)=33.62, p<0.01. E2-administered mice spent significantly more time in the light compartment than did vehicle mice (Fig. 3). There were no differences in the total entries made in the task to both chambers, or to the light chamber (Table 1).
Fig. 3.
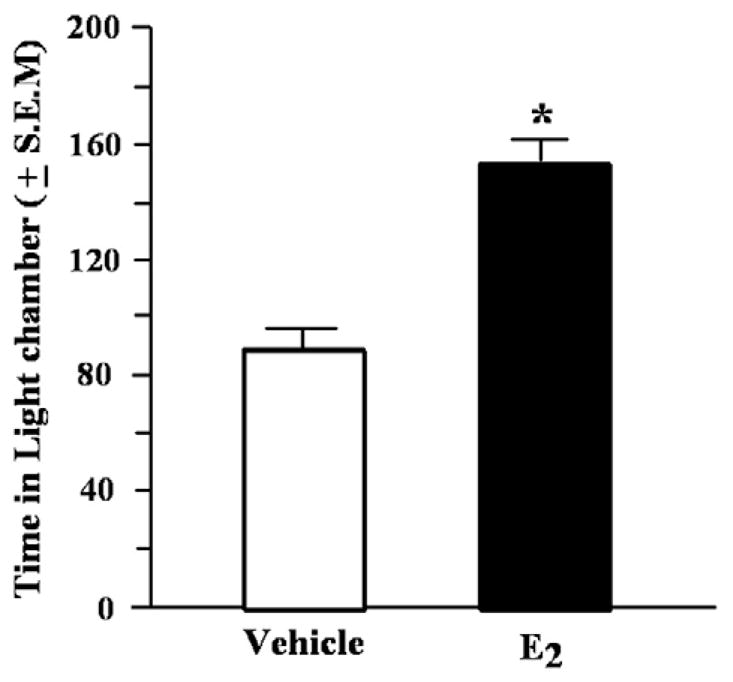
Time spent in the light side of the light-dark transition task (mean in s ±S.E.M.) of aged mice administered vehicle or estradiol (E2) 1h before testing. *Indicates a significant difference from vehicle (p<0.05).
3.7. Vogel conflict task
Although the main effect was not statistically significant, the number of punished licks tended to be greater for E2- (222±15) than for vehicle- (158±32) administered mice F (1, 16)=3.31, p<0.08. There were no differences between these groups for the number of drinking bouts in this task (Table 1).
3.8. Forced swim task
E2-administered mice had significantly F (1,16=12.3; p < 0.01) shorter duration of immobility than did vehicle-administered mice (Fig. 4). There were no differences between groups in the time spent swimming in this task (Table 1). There was no statistically significant difference for time spent struggling, but mice administered E2 tended to spend more time struggling than mice administered vehicle F (1, 16)=4.03, p<0.06 (Table 1).
Fig. 4.
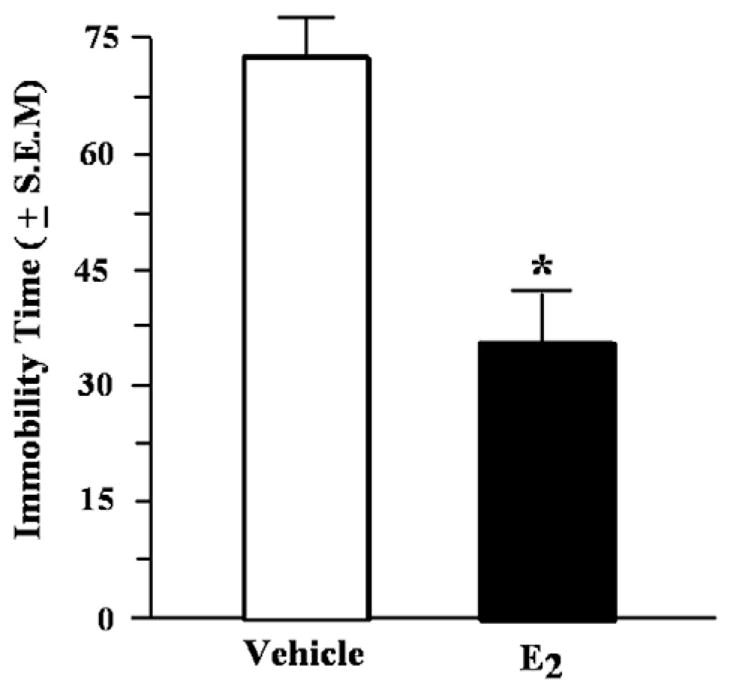
Time spent immobile in the forced swim test (mean in s±S.E.M.) of aged mice administered vehicle or estradiol (E2) 1h before testing. *Indicates a significant difference from vehicle (p<0.05).
4. Discussion
Our hypothesis that E2 administration to aged female mice would decrease anxiety and depressive behaviors was generally supported. Administration of E2, in an acute regimen that produces physiological concentrations of E2 (10 μg SC, 1h prior to testing), significantly decreased anxiety and depressive behavior in the open field, mirror chamber, light–dark transition and forced swim test, compared to that produced by vehicle. A similar pattern of effects was observed in the elevated plus maze and Vogel conflict task, but differences between conditions in these tasks did not reach statistical significance. Interestingly, these effects of E2 on anxiety and depressive behavior occurred independent of changes in motor activity and/or coordination. With the exception of a tendency for differences in the duration spent struggling by mice in the forced swim test, there were no differences in the number of beam breaks in an activity monitor, latencies to fall from the rotarod, or any differences in the motor indices in the anxiety tasks or forced swim test. Thus, E2 administration to aged female mice produces specific effects to reduce anxiety and/or depression behavior.
The present results confirm that E2 administration to young, OVX mice can produce anti-depressant effects in the forced swim task and extend them to senescent intact mice. Young adult, OVX, Swiss-albino mice spent approximately 113s immobile in the forced swim task when administered vehicle compared to 50s when administered 100μg/kg E2 [14]. These results are similar to that observed for young OVX, CD1 and 129S6 mice that spent ~100 and 75s immobile, respectively, when administered vehicle and 75 and 40s immobile when administered 100 μg/kg E2 [16]. Given that there are well known differences between mouse strains in response to steroid administration and for their behavioral effects [26], the effects of E2 on depressive behavior seem very consistent and robust across studies. Our aged intact C57BL/6 congenic mice were immobile for ~70 and ~35s when administered vehicle and E2 (10 μg, SC) 1h before testing, respectively. Although the pattern and magnitude of E2’s effects are clearly consistent across these studies, whether the overall lower levels of immobility observed among our mice is attributable to E2 dosing, aging and/or strain differences is unclear. Indeed, we have recently reported that young OVX mice on a C57BL/6 background have about a 33% reduction in immobility when administered 10 μg E2 44–48h before forced swim testing [18]; whereas the older mice in the present study, and mice of a different strain in the aforementioned studies, had a greater (~100% reduction) response. Current studies are investigating the extent to which aging, strain differences, and/or exposure to other stressors are contributing factors to responsiveness of mice to physiological E2 dosing.
Another factor to consider is the extent to which the duration of E2 deprivation may have influenced the results. Indeed, given the results of recent basic research and clinical trials which suggest that beneficial effects of E2 may be limited after prolonged periods of E2 deprivation and/or neuronal compromise [3,27–29]. It is important to note that we do not know precisely for how long mice in the present study were steroid-deprived. It has been reported that about 80% of 17 month old C57BL/6 mice have irregular or absent cycles, whereas 100% of their 25 month old counterparts are acyclic [30]. These findings suggest that our mice may have experienced E2 (progestin and androgen) deprivation for up to 25% of their lifespan before administration of E2 or vehicle and behavioral testing. As such, the favorable response to E2 observed may suggest that anxiety and/or depressive behavior can be improved following E2 deprivation among some aged individuals. This is in direct contrast to the effects that we observed on motor measures in these tasks. Although these effects support the notion that E2’s functional effects were specific to the anxiety and depression-like behaviors, and not secondary to activity, among aged mice, E2 has a well-known clear role in behavioral arousal and motor activity among young mice [31]. In support, over the estrous cycle, and with OVX and E2 replacement, greater motor activity and exploration by mice of their homecage or novel environment is noted [32–36], and may be related to reproduction [37]. In the present study, aged mice did not have an increase in motor activity in any task following administration of E2. Mice of this age were likely reproductively-senescent so these data lend some support to the notion that E2’s enhancement of motor activity may subserve reproduction-related function.
This study confirms results of prior investigations which demonstrate that E2 has anti-anxiety effects in young rodents and extends them to show effects in aged mice across multiple behavioral assays, but not all tasks utilized. Effects of E2 on anxiety behavior of young mice have been investigated primarily in the plus maze. Mice in behavioral estrous, which have elevated levels of E2 and progestins, spend typically twice as long on the open arms of the elevated plus maze as do diestrous mice, which have low levels of E2 and progestins [12]. However, when young adult, cycling mice were subsequently OVX and administered E2, only modest effects of E2 on plus maze behavior were observed [12] as was observed in the present study in aged mice. This was not a test decay effect, as controls were not different across experiments. Similar to the present study, other studies have demonstrated a robust effect of endogenous and exogenous E2 in young to reduce anxiety-like responses in additional tasks (open field, elevated zero maze, light–dark transition, mirror maze, social interaction) [13,17,38]. Furthermore, the role of progesterone (P) should be considered in this context. P administration to young or aged wild type or progestin receptor knockout mice decreases anxiety behaviors in all tasks, except the plus maze, in part through increasing formation of the P metabolite 5α-pregnan-3α-ol-20-one (3α,5α-THP) and its subsequent enhancement of GABAA receptor function [23]. Consistent with this, P administration did not alter open arm time in the plus maze when administered to 5α-reductase or wild type mice although effects on other anxiety measures were observed [25]. Thus, these data suggest that there may be differential sensitivity of mice to hormonal modulation of anxiety-like behavior across tasks.
An important question is whether some of the effects of E2 for anxiety-related behavior may involve actions of 3α,5α-THP. E2 can increase activity of the 5α-reductase enzymes [39] and enhance de novo formation of 3α,5α-THP [40,41]. Indeed, we have found that E2 to OVX mice that do not express a molecular target of E2, the estrogen receptor β (ERβ), do not have increased 3α,5α-THP in the hippocampus and enhanced cognitive performance as do their wild type counterparts [42]. In addition to other stress systems (e.g. cholinergic; [43]), the hippocampus is a likely central target for E2’s anti-anxiety and anti-depressive effects. In support, administration of this same E2 regimen to aged, intact, female C57BL/6 mice produces physiological concentrations of E2 in the hippocampus compared to levels observed in aged intact female mice administered vehicle, which were at nadir [22]. Administration of E2, compared to vehicle, subcutaneously or to the hippocampus of OVX rats similarly increases anti-anxiety and anti-depressive behavior [2,44]. As well, whether actions of E2 at the β isoform of the E2 receptor (ERβ) in the hippocampus may underlie the anti-anxiety and anti-depressant effects observed herein was not established. Among young OVX rats and young, intact of OVX and E2- or ERβ-SERM-replaced mice, actions at ERβ are sufficient to produce anti-anxiety and anti-depressant effects; but this effect is abrogated in ERβ knockout mice [2,11,13,15–18,38,45]. We plan to address this critical question further by examining effects of E2 administration to aged ERβ knockout mice and their wild type counterparts. Thus, further investigation of how some of E2’s functional effects may involve interactions between ERβ and its metabolism to 3α,5α-THP in the hippocampus in young and aged individuals is of interest.
In summary, our results demonstrate that aged female mice respond favorably to E2 administration and exhibit greater anti-anxiety and anti-depressant behavior than is observed following vehicle administration. These effects on affective measures can be parsed out from general motor activity. Given the increases in anxiety and depression among post-menopausal women, these findings have clinical relevance. Further studies are needed to elucidate the mechanisms by which E2 influences anxiety and depressive behavior in aged individuals.
Acknowledgments
Support for this research was provided by extramural funding to CAF from The National Institute of Mental Health (MH06769801) and The National Science Foundation (IBN 98-96263, IBN 03-16083) and an intramural faculty research award program grant to CAF. Technical assistance provided by Kanako Sumida is greatly appreciated.
References
- 1.Picazo O, Estrada-Camarena E, Hernandez-Aragon A. Influence of the post-ovariectomy time frame on the experimental anxiety and the behavioural actions of some anxiolytic agents. Eur J Pharmacol. 2006;530:88–94. doi: 10.1016/j.ejphar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–16. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson VW, Sherwin BB. Surgical versus natural menopause: cognitive issues. Menopause. 2007;14:572–9. doi: 10.1097/gme.0b013e31803df49c. [DOI] [PubMed] [Google Scholar]
- 5.Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt PJ. Mood, depression, and reproductive hormones in the menopausal transition. Am J Med. 2005;118:54–8. doi: 10.1016/j.amjmed.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt PJ. Depression, the perimenopause, and estrogen therapy. Ann NY Acad Sci. 2005;1052:27–40. doi: 10.1196/annals.1347.003. [DOI] [PubMed] [Google Scholar]
- 8.Maartens LW, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas. 2002;25:195–200. doi: 10.1016/s0378-5122(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 9.Sherwin BB. Sex hormones and psychological functioning in postmenopausal women. Exp Gerontol. 1994;29:423–30. doi: 10.1016/0531-5565(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 10.Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–5. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 11.Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–7. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- 13.Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–60. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–8. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- 15.Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 β-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-β knockout (βERKO) mice. Psychopharmacology. 2005;179:637–43. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- 17.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor β knockout, mice. Behav Neurosci. 2008;122:974–81. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walf AA, Koonce CJ, Frye CA. Adult female wildtype, but not oestrogen receptor β knockout, mice have decreased depression-like behaviour during pro-oestrus and following administration of oestradiol or diarylpropionitrile. J Psychopharmacol. 2009;23:442–50. doi: 10.1177/0269881108089598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finch CE, Felicio LS, Mobbs CV, Nelson JF. Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev. 1984;5:467–97. doi: 10.1210/edrv-5-4-467. [DOI] [PubMed] [Google Scholar]
- 20.vom Saal FS, Finch CE, Nelson JF. In: Physiology of reproduction. Knobi E, editor. Vol. 2. New York: Raven Press; 1994. p. 1240. [Google Scholar]
- 21.Edwards DA. Induction of estrus in female mice: estrogen-progesterone interactions. Horm Behav. 1970;1:299–304. [Google Scholar]
- 22.Frye CA, Rhodes ME, Dudek BC. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–8. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, Pfaff DW, Rhodes ME. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology. 2006;186:312–22. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- 24.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc Dir. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–24. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Svare B. Genotype modulates the aggression-promoting quality of progesterone in pregnant mice. Horm Behav. 1988;22:90–9. doi: 10.1016/0018-506x(88)90033-5. [DOI] [PubMed] [Google Scholar]
- 27.Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 28.Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–7. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 29.Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- 30.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 31.Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- 32.Fahrbach SE, Meisel RL, Pfaff DW. Preoptic implants of estradiol increase wheel running but not the open field activity of female rats. Physiol Behav. 1985;35:985–92. doi: 10.1016/0031-9384(85)90270-7. [DOI] [PubMed] [Google Scholar]
- 33.Frohlich J, Morgan M, Ogawa S, Burton L, Pfaff D. Statistical analysis of hormonal influences on arousal measures in ovariectomized female mice. Horm Behav. 2002;42:414–23. doi: 10.1006/hbeh.2002.1832. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro AC, Pfaff DW, Devidze N. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci. 2009;29:795–801. doi: 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- 35.Gray P, Cooney J. Stress-induced responses and open-field behavior in estrous and nonestrous mice. Physiol Behav. 1982;29:287–92. doi: 10.1016/0031-9384(82)90017-8. [DOI] [PubMed] [Google Scholar]
- 36.Wade GN, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J Comp Physiol Psychol. 1970;72:328–36. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- 37.Pfaff DW. Brain arousal and information theory: neural and genetic mechanisms. Cambridge, MA: Harvard University Press; 2006. [Google Scholar]
- 38.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–6. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3, 20-dione and 3α-hydroxy-5α-pregnan-20-one by rat medical basal hypothalami and the effects of estradiol and stage of estrous cycle on the conversion. Endocrinology. 1973;93:1157–62. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- 40.Frye CA, Rhodes ME. Estrogen-priming can enhance progesterone’s anti-seizure effects in part by increasing hippocampal levels of allopregnanolone. Pharmacol Biochem Behav. 2005;81:907–16. doi: 10.1016/j.pbb.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 41.Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacol Biochem Behav. 1999;64:777–85. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 42.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–21. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serova LI, Maharjan S, Sabban EL. Estrogen modifies stress response of catecholamine biosynthetic enzyme genes and cardiovascular system in ovariectomized female rats. Neuroscience. 2005;132:249–59. doi: 10.1016/j.neuroscience.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 44.Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007;86:407–14. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor β-deficient mice. PNAS. 2001;98:12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]


