Summary
Purpose
Ethosuximide (ESX) is a drug of choice for the symptomatic treatment of absence seizures. Chronic treatment with ESX has been reported to have disease-modifying anti-epileptogenic activity in the WAG/Rij rat model of genetic generalised epilepsy (GGE) with absence seizures. Here we examined whether chronic treatment with ESX (i) possesses anti-epileptogenic effects in the GAERS model of GGE, (ii) is associated with a mitigation of behavioural comorbidities, and (iii) influences gene expression in the somatosensory cortex region where seizures are thought to originate.
Methods
GAERS and Non-Epileptic Control (NEC) rats were chronically treated with ESX (in drinking water) or control (tap water) from 3 to 22 weeks of age. Subsequently, all animals received tap water only for another 12 weeks to assess enduring effects of treatment. Seizure frequency and anxiety-like behaviours were serially assessed throughout the experimental paradigm. Treatment effects on the expression of key components of the epigenetic molecular machinery, the DNA methyltransferase enzymes, were assessed using qPCR.
Key findings
ESX treatment significantly reduced seizures in GAERS during the treatment phase, and this effect was maintained during the 12 weeks post-treatment phase (p<0.05). Further, the anxiety-like behaviours present in GAERS were reduced by ESX treatment (p<0.05). Molecular analysis revealed that ESX treatment was associated with increased expression of DNA methyltransferase enzyme mRNA in cortex.
Significance
Chronic ESX treatment has disease-modifying effects in the GAERS model of GGE, with anti-epileptogenic effects against absence seizures and mitigation of behavioural comorbidities. The cellular mechanism for these effects may involve epigenetic modifications.
Keywords: GAERS, ethosuximide, epileptogenesis, seizures, anxiety, DNA methylation
Introduction
The epilepsies are a group of debilitating and progressive neurological conditions affecting about 1% of the population (Hauser, et al. 1993), and are characterized by spontaneous recurrent seizures. All current medical therapies for epilepsy symptomatically suppress seizure activity, but are not disease-modifying, having no effect on the underlying propensity of the brain to generate seizures. Current treatments also have no significant effects on the psychiatric co-morbidities associated with epilepsy, such as anxiety, depression and psychosis, which are common in patients and significantly add to the disability burden (Hermann, et al. 2008, Tellez-Zenteno, et al. 2007, Vega, et al. 2011). A major goal of translational epilepsy research is to identify treatments which are not merely symptomatic, but truly disease-modifying (Galanopoulou, et al. 2012). There have been several recent reports of experimental successes using therapies which interfere with epileptogenesis (Blumenfeld, et al. 2008, McClelland, et al. 2011, Russo, et al. 2010, Wong 2010), suggesting that this goal is achievable.
Ethosuximide (ESX) is a first-line clinical symptomatic treatment for absence seizures. Recent research has demonstrated that chronic treatment with ESX, when initiated prior to the onset of the epilepsy, has anti-epileptogenic effects in the WAG/Rij rat model of genetic generalized epilepsy (GGE) with absence seizures (Blumenfeld, et al. 2008, Russo, et al. 2010, Russo, et al. 2011, Sarkisova, et al. 2010). This treatment regime also reduces the depression-like behaviours that are present in WAG/Rij rats (Sarkisova, et al. 2010). Without corroboration of the anti-epileptogenic potential of ESX in other models, it is unclear whether this property is limited to an isolated cause of GGE (thereby limiting the translatability of this finding), or whether it is effective in other GGE models, thereby establishing a broad applicability of this drug across animal models, and potentially extending to human cases. In this study, we investigated whether chronic treatment with ESX has disease-modifying effects against epilepsy and behavioural comorbidities in a different model of GGE with absence seizures, Genetic Absence Epilepsy Rats from Strasbourg (GAERS).
GAERS are a well-validated polygenic model of GGE (Danober, et al. 1998), with a genetic causation that is different from that of WAG/Rij rats (Gauguier, et al. 2004, Powell, et al. 2009, Rudolf, et al. 2004). The epilepsy develops in GAERS at around 8–9 weeks of age in our colony (Jones, et al. 2008), manifesting as bilateral spike-and-wave discharges on the EEG which are responsive to drugs used clinically to treated absence seizures, including ESX (Tringham, et al. 2012). In addition, we have shown previously that GAERS from our colonies in Melbourne exhibit an anxiety-like behavioural phenotype using the elevated plus maze and open field tests of anxiety (Jones, et al. 2008). Since anxiety disorders are prevalent in pediatric generalized epilepsy patients (Caplan, et al. 1998, Caplan, et al. 2005, Caplan, et al. 2008, Jones, et al. 2007, Ott, et al. 2001, Ott, et al. 2003, Vega, et al. 2011), this high-anxiety phenotype makes GAERS an appropriate model to study the influence of disease-modifying drugs on behavioural outcomes (Jones and O’Brien 2012).
We also investigated whether ESX treatment alters expression of key components of the epigenetic molecular machinery, which may implicate an epigenetic mechanism in any long-term effects on the epilepsy and behavioral phenotype. Specifically we examined the expression levels of the DNA methyltransferase (DNMT) family of enzymes in the somatosensory cortex - a region shown previously to play a key role in spike-wave seizure expression in rodent models (David, et al. 2008, Meeren, et al. 2002, Polack, et al. 2007, Zheng, et al. 2012) following both chronic and acute ESX treatment. DNMTs catalyse the covalent attachment of methyl (CH3) groups to specific cytosine residues in the DNA sequence – an epigenetic modification termed DNA methylation which is strongly associated with transcriptional repression of genes (Attwood, et al. 2002). Any change in DNMT enzyme expression would be expected to induce enduring alterations of the expression patterns of key genes, an effect which may influence epileptogenesis.
Methods
Animals
This study used male Genetic Absence Epilepsy Rats from Strasbourg (GAERS; n=16) and Non-Epileptic Control rats (NEC; n=16) bred in the Department of Zoology, University of Melbourne Biological Research Facility (BRF). These animals were derived from the original GAERS/NEC colony from Strasbourg in 2007, and inbred at the University of Melbourne for 7 generations prior to use in this study. Rats were weaned and transferred at 3 weeks of age to the Department of Medicine (Royal Melbourne Hospital), University of Melbourne BRF, where they were housed until the completion of the study. Both facilities were kept on a 12h light/dark cycle, with lights on at 7am at a constant temperature of 22°C. For chronic treatment experiments, animals were group-housed up until the surgical procedure, and from then on, they were housed individually. For acute treatment studies, animals were group-housed until 14 weeks of age, when the treatments and post-mortem experiments took place. All procedures were approved by the University of Melbourne Animal Ethics Committee.
Drug regimes
For the chronic treatment studies, wherever possible, animals from a single litter were divided and allocated as either ESX-treated or control (tap water)-treated animals in a paired fashion. Ethosuximide syrup was purchased from the Royal Melbourne Hospital pharmacy (Zarontin, Pfizer Pharmaceuticals Group). At 3 weeks of age, ESX treatment was initiated in GAERS (n=5) and NEC (n=7) rats, attempting to achieve a palatable dose of 300mg/kg/day in the drinking water. Volumes consumed and animal weights were measured daily for the first week, and then weekly thereafter, and the drug dosage received (mg/kg/day) was calculated. The concentration of drug in the water bottles was updated weekly to maintain the appropriate dosage in an iterative fashion. All animals were able to drink independently when the study began. Control-treated rats received tap water ad libitum for the duration of the study. Due to the light sensitive nature of ESX, drinking bottles were wrapped with black tape. At 22 weeks, the drug treatment ceased, and all animals reverted to tap water (see Figure 1A for study timeline) until 34 weeks of age.
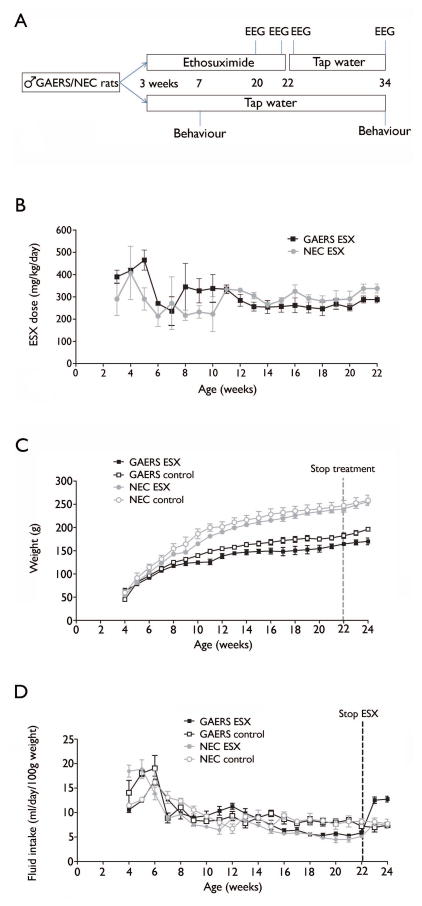
Figure 1. Study timeline and ethosuximide dosing.
(A) Littermates were separated to receive ESX or control treatment at 3 weeks of age, which persisted until 22 weeks. EEG recordings were made at 20 and 34 weeks of age, as well as 1 day prior to, and following, treatment cessation in GAERS. Behavioural assessments were conducted at 7 and 34 weeks in all rats. (B) Over the course of the study, the concentration of ESX in the drinking water presented to GAERS and NEC rats was altered depending on their drinking habits. This stabilized for each rat at around 10 weeks of age, and thereafter, all animals consistently received 300mg/kg/day. (C) GAERS weigh significantly less than NEC rats across the study, and ESX treatment also results in lower weights. (D) Fluid intake was equal between GAERS and NEC, but reduced in ESX-treated rats. Once treatment was removed (ie: 22 weeks), drinking volumes returned to control levels, and actually rebounded above control levels in GAERS.
For the acute treatment studies, GAERS were administered ESX (Sigma: 200mg/kg ip, n=5) or vehicle (0.9% saline, n=5), and culled 90 minutes later, a time when the acute seizure-suppressing effects of ESX are present (Tringham, et al. 2012). By comparing acute and chronic effects of drug treatment, we could discern whether any observed genomic effects were related to acute anti-seizure effects of ESX, or whether they were specifically associated with chronic treatment, and therefore more closely linked to any disease-modifying effects.
Surgery and EEG
At 11 weeks, animals underwent surgical implantation of EEG recording electrodes, as previously described (Hakami, et al. 2009), with minor modifications. Briefly, under isoflurane anaesthesia, a midline incision was made on the scalp and the connective tissue removed. 6 small burr holes were made in the skull, and electrodes implanted (Plastics One, Bioscientific) to facilitate recording of the EEG. The electrodes were held in place with dental cement (Henry Schein Halas, Australia) applied to the skull, and animals left to recover for one week in individual cages.
To assess the effects of ESX treatment on seizures, electrical cables (Plastics One, Bioscientific) were connected to the implanted electrodes, and the EEG recorded continuously for 24 hours, beginning at midday, using Compumedics software. These recordings occurred four times: at week 20, one day prior to drug cessation, one day following drug cessation, and then again at week 34. Recordings were performed in the home cages in a quiet room under standard lighting conditions of the BRF. Analysis of the seizure frequency was performed by a reviewer blinded to treatment using Spike-and-Wave seizure detection program (Netherlands) over the entire 24 hour recording period. This program automatically identifies spike-wave episodes, with each episode then visually verified. Inclusion criteria for seizures was a Spike-Wave discharge of amplitude more than two times baseline, a frequency of 7 to 12 Hz, and a duration of longer than 1 sec (see Figure 2A).
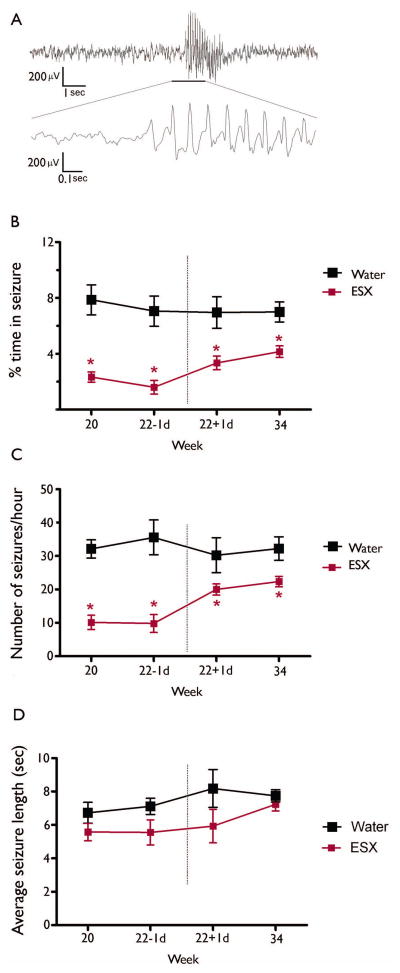
Figure 2. Ethosuximide reduces seizures during the treatment period, and this effect endures following treatment cessation.
(A) An example of a recorded SWD from the EEG. GAERS exposed to ESX (n=5) show significantly reduced % time in seizure (B) and the number of seizures recorded (C) during the 24 hour recording period both during and after the treatment period, compared to control-treated GAERS (n=6). No differences were observed in seizure duration throughout the study period (D). * indicates significant (p<0.05) post-hoc comparison with control-treated GAERS. Data represent group mean + SEM.
Anxiety-like behaviour
To determine the effects of ESX treatment on behavioural comorbidity, at 7 and 34 weeks of age, all rats underwent assessment of anxiety-like behaviour in an open field. This was performed in a closed, quiet, light-controlled room in the Behavioural Testing Facility at the Department of Medicine, Royal Melbourne Hospital, University of Melbourne. The testing protocol was as previously described (Jones, et al. 2008). Briefly, animals were individually placed in the centre of a 1m diameter circular arena with an inner circle (diameter 66 cm) separating the inner area from the outer, and allowed to explore the arena for 10 min. The lighting level within the maze was set at ~90 lux. Quantification of the total distance travelled, and the time spent and number of entries made into the inner area of the maze was objectively assessed using Ethovision Tracking Software (Noldus, Netherlands). Our previous data have demonstrated robust differences in open field behaviour between GAERS and NEC, particularly the extent of exploration of the environment (Bouilleret, et al. 2009, Jones, et al. 2010, Jones, et al. 2008).
Post-mortem analysis
At the conclusion of the chronic study, animals were cardiac-perfused with 4% paraformaldehyde, the brains extracted and sectioned on a cryostat at 40μm thickness. The somatosensory cortex region was microdissected from 5–6 sections, and RNA was extracted using the RNeasy FFPE kit (QIAGEN). 1μg of RNA was reverse transcribed to cDNA using the Omniscript Reverse Transcription kit (QIAGEN) in the presence of an RNase inhibitor. Taqman quantitative PCR was performed on 50ng cDNA (Powell, et al. 2008) using custom designed taqman gene expression assays (Applied Biosystems) for Dnmt1 (Assay ID Rn00709664_m1), Dnmt3a (Assay ID Rn01469994_g1), Dnmt3b (Assay ID Rn01536414_g1). Relative quantification of mRNA levels were normalized to the reference genes, GAPDH (Assay ID Rn99999916_s1) using the ΔΔCT method (Schmittgen and Livak 2008).
For the acute study, rats were culled 90 minutes after ESX or vehicle treatment, and the somatosensory cortex region dissected and frozen on dry ice. RNA was extracted from these samples using RNeasy Mini Kit (QIAGEN), and gene expression analysis performed as above.
Statistical comparisons
All EEG parameters were compared using two-way ANOVA with repeated measures, separating the periods during and following the treatment period into different analyses. The between subject factor was treatment (either ESX or control), and the within subject factor was age of recording. In addition, we performed ANOVA to assess whether EEG parameters were altered following removal of treatment, using the same parameters as above. Bonferroni’s post-hoc analysis was used when appropriate. Behaviour was assessed at 7 and 34 weeks separately, and used two-way ANOVA. Molecular data was analysed using two-way ANOVA, or Student’s t-tests. All comparisons were conducted using GraphPad Prism v5.04.
Results
Drug dosage and animal weights
Through management of the concentration of ESX in the drinking bottles, we were able to achieve stable doses of 300mg/kg/day for chronically treated animals (Figure 1B). Over the course of the study, GAERS weighed significantly less than NEC rats (F(3,22)=80.04; p<0.0001: Figure 1C), and weights were also significantly reduced by ESX treatment (F(3,22)=4.94; p=0.039). This drug effect may be partially explained by the slight, but significant, reduction in fluid intake of ESX-treated rats (F(3,22)=14.79; p=0.001: Figure 1D). After cessation of drug treatment, fluid consumption returned to control levels, and actually increased in treated GAERS to well above control. Over the treatment period, fluid intake did not differ between GAERS and NEC rats (F(3,22)=0.002; p=0.96).
Ethosuximide limits disease severity in GAERS
As expected, the percentage of time spent in seizure was significantly reduced during ESX treatment, compared to water-treated GAERS (F(1,11)=21.70; p=0.0012). Following cessation of ESX treatment, this seizure-suppressing effect was maintained for the three month follow-up period (F(1,11)=11.92; p=0.0072: Figure 2B), indicative of a disease-modifying effect of the ESX treatment. Likewise, the number of seizures experienced was significantly reduced in treated animals both during treatment (F(1,11)=31.13; p=0.0003) and this persisted after treatment had stopped (F(1,11)=5.36; p=0.046: Figure 2C). When examining treated GAERS, it should also be noted that, following cessation of treatment, both the percentage time in seizure (F(1,11)=9.92; p=0.013) and seizure frequency (F(1,11)=32.20; p<0.001) increased compared to during the treatment period. However, these outcomes still remained significantly lower than control-treated rats. No significant differences were observed in seizure duration either during (F(1, 11)=2.99; p=0.117) or after (F(1,11)=2.95; p=0.120) ESX treatment (Figure 2D).
Chronic ethosuximide treatment alleviates behavioural disturbance in GAERS
At 7 weeks, the distance travelled in the open field was significantly reduced in GAERS compared to NEC rats (F(3, 22)=25.61; p<0.001; Figure 3), but this measure was not significantly influenced by ESX treatment (F(3, 22)=1.96; p=0.178). At 34 weeks, when all GAERS are exhibiting seizures, a different profile was observed: the significant effect of strain on behaviour was maintained (F(3, 22)=4.57; p=0.046), but we now also observed a significant effect of treatment (F(3, 22)=12.08; p=0.003), and a significant strain vs. treatment interaction (F(3, 22)=5.125; p=0.036), such that ESX treatment increased the extent of exploration of the open field but only in GAERS (Figure 3C).
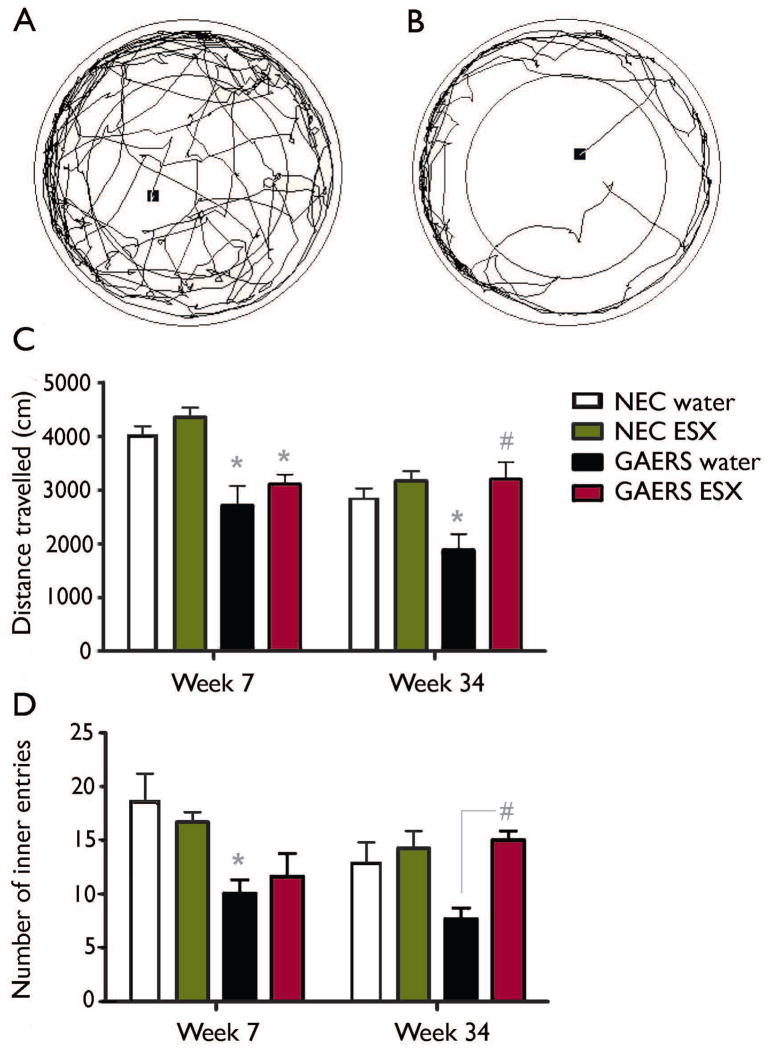
Figure 3. Suppression of seizures is accompanied by improved behavioural outcome in GAERS.
Representative traces of the path travelled by an NEC rat (A) and a GAERS (B) in the open field – the black square represents the trial start position. Note the reduced total distance and entry into the central area of the maze of the highly anxious GAERS. At 7 weeks, prior to the onset of seizures, GAERS displayed an anxiogenic phenotype compared with NEC rats, as evidenced by (C) significantly reduced distance travelled in the open field and (D) significantly reduced entries into the central area, and this phenotype was not influenced by ESX treatment. At 34 weeks however, this anxiogenic phenotype was ameliorated in ESX-treated GAERS, compared to control treated GAERS. * indicates significant (p<0.05) post-hoc comparison with relevant NEC group. # indicates significant (p<0.05) post-hoc comparison with control-treated GAERS. Data represent group mean + SEM. Sample sizes: GAERS treated with ESX - n=5, with water - n=6; NEC treated with ESX - n=7, with water - n=4.
We observed the same trends when assessing the number of times the animals ventured into the inner area of the open field. At 7 weeks, number of entries were significantly reduced in GAERS compared to NEC rats (F(3, 22)=17.7; p<0.001; Figure 3D), but not affected by ESX (F(3, 22)=0.02; p=0.896). At 34 weeks however, ESX treatment significantly influenced the number of entries into the central area (F(3, 22)=8.95; p=0.0078); but a non-significant effect of strain for this measure (F(3, 22)=2.57; p=0.126). Importantly, we also observed a significant interaction between these variables at this age (F(3, 22)=4.41; p=0.05).
Ethosuximide differentially alters DNA methyltransferase gene expression
To investigate potential molecular mediators of the enduring effects of ESX, we examined the gene expression profiles of the DNA methyltransferase family of enzymes – DNMT1, DNMT3A and DNMT3B – in the somatosensory cortex of treated and untreated rats (Figure 4). Expression of these enzymes is strongly associated with gene transcriptional repression (Attwood, et al. 2002), which we hypothesized as being related to the enduring effects of ESX. Using quantitative PCR, we found that DNMT1 and DMNT3A mRNA expression were significantly elevated in GAERS chronically treated with ESX (Figure 4A, B). Altered expression of these genes, specifically in treated epileptic animals, would suggest a possible role in suppressing epileptogenesis. ANOVA demonstrated increased DNMT1 expression in GAERS compared to NEC rats (F(3, 22)=8.131; p=0.011), and in ESX vs. water-treated rats (F(3,22)=17.99; p=0.0006), but without an interaction (F(3, 22)=1.568; p=0.23). In the case of DNMT3A mRNA expression, significant strain (F(3, 22)=6.199; p=0.023) and treatment (F(3, 22)=6.978; p=0.017) effects were also observed, but this time a significant interaction between groups was observed (F(3, 21)=5.271; p=0.035), with ESX-treated GAERS showing significantly higher expression levels than the other three groups (p<0.05).
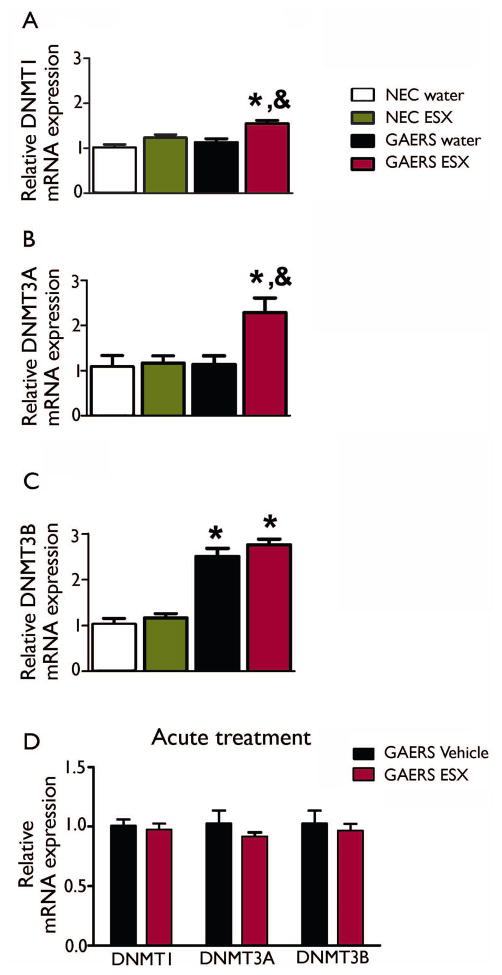
Figure 4. DNA Methyltransferase (DNMT) mRNA expression is differentially altered in GAERS.
Expression of DNMT1 (A) and DNMT3A (B) are elevated in chronically treated ESX-treated GAERS, whereas DNMT3B is elevated in GAERS irrespective of drug treatment (C). No gene expression changes were induced by acute treatment with ESX (D) * indicates significant (p<0.05) post-hoc comparison with relevant NEC group. & indicates significant (p<0.05) post-hoc comparison with relevant control-treated group. Data represent group mean + SEM. Sample sizes as for Figure 3.
Effects of ESX on DNMT1 and DNMT3A expression were not simply a non-specific drug response since ESX did not change expression of these enzymes in NEC animals (Figure 4A, B). In addition, ESX did not affect all methyltransferase genes in GAERS: DNMT3B expression demonstrated a different profile (Figure 4C), with GAERS displaying significantly greater levels of gene expression (F(3, 22)=112.0; p<0.0001). In contrast to the other family members, DNMT3B mRNA expression was not significantly altered by ESX (F (3, 22)=1.852; p=0.191).
To determine whether the effects on DNMT gene expression were related to the anti-epileptogenic effects of ESX, or to a non-specific effect of the drug, we next treated separate adult male GAERS with an acute injection of ESX or vehicle at a dose known to suppress seizures (Tringham, et al. 2012). We found no changes in gene expression of any of the DNMT family of genes between treated and untreated rats (DNMT1: t(8)=0.45; p=0.66, DNMT3A: t(8)=0.96; p=0.36, DNMT3B: t(8)=0.49; p=0.64, Fig 4D), ruling out acute exposure to ESX as a driving factor.
Discussion
Here we demonstrate that chronic treatment with ESX induces a disease-modifying effect in the GAERS rat model of GGE. This extends upon previous research demonstrating an anti-epileptogenic action of chronic ESX treatment in WAG/Rij rats (Blumenfeld, et al. 2008, Russo, et al. 2010, Russo, et al. 2011, Sarkisova, et al. 2010), a model of GGE with different genetic causation (Gauguier, et al. 2004, Powell, et al. 2009, Rudolf, et al. 2004), suggesting that this effect is broadly applicable across absence epilepsy models. Also, we showed that chronic suppression of seizures mitigates the anxiety-like behavioural phenotype in GAERS, an effect which only presented itself after the onset of seizures.
GAERS from our Melbourne colonies exhibit a highly anxious phenotype, which is present prior to the onset of the epilepsy (Jones, et al. 2008). Our previous studies characterizing this phenotype employed GAERS/NEC rats originally obtained from Hull, UK, in 2002 (Bouilleret, et al. 2009, Jones, et al. 2010, Jones, et al. 2008), and contrasted from a much earlier report on the original Strasbourg colony which did not find an hyper-anxious phenotype (Vergnes, et al. 1991). Importantly, we now replicate this phenotype using GAERS/NEC rats from our inbred colonies sourced from the founder colony from Strasbourg in 2007, providing broad validation of the association of this behavioural trait with different GAERS colonies. Exposure to a novel open field is a widely used measure of anxiety-like behaviour which demonstrates predictive validity (Prut and Belzung 2003), and this apparatus gives strong and reproducible data when testing GAERS. However caution must be taken when using only a single test for behavioural outcomes, and future studies should use batteries of assays to comprehensively characterize anxiety-like phenotypes in this, and other rat strains.
An intriguing aspect of the disease-modifying effects of ESX treatment observed here was the mitigation of the anxiety-like behavioural comorbidity. Because the GAERS strain was originally bred for the epilepsy phenotype, the emergence of the behavioural abnormality is likely to be linked in some way to the epilepsy, even though it appears to manifest before seizures begin. Clinically, this ontogeny has also been reported, with pediatric patients with genetic generalised epilepsy frequently experiencing anxiety disorders prior to the first recognised seizure (Jones, et al. 2007). Here we show that the behavioural deficit is significantly attenuated by ESX treatment, but only in chronically epileptic animals, suggesting an indirect effect of the treatment on behaviour which is dependent on the presence of seizures. This finding also agrees with a previous report describing amelioration of depression-like behaviour during chronic ESX treatment in the WAG/Rij model (Sarkisova, et al. 2010), and further enhances the postulate of bi-directionality between epilepsy and psychiatric comorbidities (Kanner 2011). Here, we did not examine any measure of depression-like behaviours, so are unable to comment on whether depression phenotypes are similarly improved by ESX in GAERS.
An intriguing question posed by the effects of ESX treatment relates to the mechanism by which it induces disease-modifying effects. ESX is widely believed to act as an antagonist at low threshold T-type calcium channels, and there is evidence from pilocarpine induced status epilepticus studies that T-type calcium channels play a role in limbic acquired epileptogenesis (Becker, et al. 2008), suggesting involvement of these ion channels. However, recent studies suggest that ESX may not be working as a pure antagonist at T-type channels (Goren and Onat 2007), so the role of T-type channels in these effects is not clear. It is also feasible that the effect of ESX represents the inhibition of a ‘kindling-like’ phenomenon, whereby a cycle of seizures begetting seizures is interrupted by the ESX treatment. Supporting evidence comes from observations that chronic treatment with other anti-absence drugs (eg: leveteracitam and zonisamide) induce similar sustained effects on epilepsy development in WAG/Rij rats (Russo, et al. 2011). However, chronic treatment with carbamazepine, which is recognized to acutely increase seizures in models of absence epilepsy (Liu, et al. 2006), did not induce the anticipated sustained increase in seizure activity (Russo, et al. 2011).
It is recognized that many changes in gene expression occur in GAERS over the course of disease development (Jones, et al. 2011), potentially representing causal or contributory drivers of the epileptic and behavioural phenotypes. Epigenetic mechanisms represent methods of controlling gene expression, and are typically mediated by changes in the structure of chromatin and other DNA binding proteins which modify the accessibility of transcription factors to their binding domains (Borrelli, et al. 2008). Here we demonstrate that chronic ESX treatment in GAERS results in alterations in the expression levels of the DNMT enzymes which catalyse DNA methylation; the most widely studied epigenetic modification. Indeed, such alterations in DNA methylation enzyme expression may also be relevant to other types of epilepsy, since a recent report identified increases in DNMT1 and DNMT3a in brain samples from human temporal lobe epilepsy patients (Zhu, et al. 2012). The expression changes identified here would be expected to modify the DNA methylation landscape and influence expression of many downstream genes, some of which may be relevant to the long-term disease-modifying effects of ESX. The attraction of this explanation lies in the prevailing assumption that DNA methylation is a stable and enduring mark, and therefore able to continue to mediate an ongoing gene expression profile long after the intervention has ceased (in this case, ESX treatment). DNMT1 acts as the maintenance methyltransferase during DNA replication, adding methyl groups onto the new DNA strand in appropriate positions (Leonhardt, et al. 1992). In addition to its role in cell division, it is highly expressed in post-mitotic cells (Veldic, et al. 2004), suggesting it also plays a role in regulating DNA methylation patterns in mature neurons, which may be relevant to neurological function and dysfunction. DNMT3A and DNMT3B are de novo methyltranferases, acting during development to dramatically alter the DNA methylation landscape (Okano, et al. 1998), and DNMT3A has also been shown to impact neuronal function in adulthood (LaPlant, et al. 2010). We found a significant gene versus drug interaction for DNMT3A expression, such that this enzyme was upregulated in GAERS only if the animals received ESX treatment. A similar trend, but without a statistical interaction, was observed for DNMT1. These effects did not appear to be acute effects of ESX, since a single drug injection did not affect DNMT expression. This is intriguing, and suggests that these changes may act to alter DNA methylation patterns to mediate the effects of ESX on disease development and severity. It may also explain why the ESX had no effect on anxiety-like behaviour in the NEC rats. Elevated levels of DNMT3B in both treated and untreated GAERS compared to NEC rats indicates that, while this enzyme may play roles in any DNA methylation differences between the strains, it is unlikely to be a mediator of any long-term beneficial effects of ESX. Further studies are required to demonstrate how ESX treatment might influence DNMT expression in GAERS, whether the changes in DNMT levels are relevant to the effects of ESX, and by extension whether this biological process can be targeted to induce disease-modifying effects in epilepsy (Qureshi and Mehler 2010).
Another point of interest raised from this study is whether there is a critical developmental window where treatment must be initiated for effects on disease progression to be realized. The ESX treatment in this study, and the previous studies in WAG/Rij rats, was started in young rats (3 weeks of age) prior to the onset of spontaneous recurrent seizures. This is relevant to any potential translation of this research to humans, where in most circumstances patients present to the clinic after their first seizure. An important next step will be to examine whether chronic ESX treatment initiated after the onset of epilepsy also produces disease-modifying and anxiolytic effects observed in this study. Overall, the current work supports an increasing literature base suggesting that targeting epileptogenic pathways is a feasible therapeutic strategy, and perhaps brings closer the prospect of identifying the holy grail of epilepsy research: a cure.
Acknowledgments
This work was supported by an NHMRC project grant to NJ (#566544) and an NHMRC CDA Fellowship to NJ (#628466), and by NIH R01 NS049307 and R01 NS066974 to HB.
Footnotes
Disclosures:
None of the authors has any conflict of interest, financial or otherwise, to disclose.
Ethical publication:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Attwood JT, Yung RL, Richardson BC. DNA methylation and the regulation of gene transcription. Cell Mol Life Sci. 2002;59:241–257. doi: 10.1007/s00018-002-8420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AJ, Pitsch J, Sochivko D, Opitz T, Staniek M, Chen CC, Campbell KP, Schoch S, Yaari Y, Beck H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J Neurosci. 2008;28:13341–13353. doi: 10.1523/JNEUROSCI.1421-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008;49:400–409. doi: 10.1111/j.1528-1167.2007.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouilleret V, Hogan RE, Velakoulis D, Salzberg MR, Wang L, Egan GF, O’Brien TJ, Jones NC. Morphometric abnormalities and hyperanxiety in genetically epileptic rats: a model of psychiatric comorbidity? Neuroimage. 2009;45:267–274. doi: 10.1016/j.neuroimage.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Caplan R, Arbelle S, Magharious W, Guthrie D, Komo S, Shields WD, Chayasirisobhon S, Hansen R. Psychopathology in pediatric complex partial and primary generalized epilepsy. Dev Med Child Neurol. 1998;40:805–811. doi: 10.1111/j.1469-8749.1998.tb12357.x. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Gurbani S, Hanson R, Sankar R, Shields WD. Depression and anxiety disorders in pediatric epilepsy. Epilepsia. 2005;46:720–730. doi: 10.1111/j.1528-1167.2005.43604.x. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R, Shields WD. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49:1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55:27–57. doi: 10.1016/s0301-0082(97)00091-9. [DOI] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, Buckmaster PS, Staley KJ, Moshe SL, Perucca E, Engel J, Jr, Loscher W, Noebels JL, Pitkanen A, Stables J, White HS, O’Brien TJ, Simonato M. Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia. 2012;53:571–582. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauguier D, van Luijtelaar G, Bihoreau MT, Wilder SP, Godfrey RF, Vossen J, Coenen A, Cox RD. Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia. 2004;45:908–915. doi: 10.1111/j.0013-9580.2004.13104.x. [DOI] [PubMed] [Google Scholar]
- Goren MZ, Onat F. Ethosuximide: from bench to bedside. CNS Drug Rev. 2007;13:224–239. doi: 10.1111/j.1527-3458.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O’Brien TJ, Pinault D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4:e6755. doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: can a natural history be developed? Lancet Neurol. 2008;7:151–160. doi: 10.1016/S1474-4422(08)70018-8. [DOI] [PubMed] [Google Scholar]
- Jones JE, Watson R, Sheth R, Caplan R, Koehn M, Seidenberg M, Hermann B. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–497. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- Jones NC, Martin S, Megatia I, Hakami T, Salzberg MR, Pinault D, Morris MJ, O’Brien TJ, van den Buuse M. A genetic epilepsy rat model displays endophenotypes of psychosis. Neurobiol Dis. 2010;39:116–125. doi: 10.1016/j.nbd.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Jones NC, O’Brien TJ. Stress, epilepsy and psychiatric comorbidity: how can animal models inform the clinic? Epilepsy and Behavior. 2012 doi: 10.1016/j.yebeh.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Jones NC, O’Brien TJ, Powell KL. Morphometric changes and molecular mechanisms in rat models of idiopathic generalized epilepsy with absence seizures. Neurosci Lett. 2011;497:185–193. doi: 10.1016/j.neulet.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Jones NC, Salzberg MR, Kumar G, Couper A, Morris MJ, O’Brien TJ. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp Neurol. 2008;209:254–260. doi: 10.1016/j.expneurol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52(Suppl 1):21–27. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt H, Page AW, Weier HU, Bestor TH. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 1992;71:865–873. doi: 10.1016/0092-8674(92)90561-p. [DOI] [PubMed] [Google Scholar]
- Liu L, Zheng T, Morris MJ, Wallengren C, Clarke AL, Reid CA, Petrou S, O’Brien TJ. The mechanism of carbamazepine aggravation of absence seizures. J Pharmacol Exp Ther. 2006;319:790–798. doi: 10.1124/jpet.106.104968. [DOI] [PubMed] [Google Scholar]
- McClelland S, Flynn C, Dube C, Richichi C, Zha Q, Ghestem A, Esclapez M, Bernard C, Baram TZ. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70:454–465. doi: 10.1002/ana.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- Ott D, Caplan R, Guthrie D, Siddarth P, Komo S, Shields WD, Sankar R, Kornblum H, Chayasirisobhon S. Measures of psychopathology in children with complex partial seizures and primary generalized epilepsy with absence. J Am Acad Child Adolesc Psychiatry. 2001;40:907–914. doi: 10.1097/00004583-200108000-00012. [DOI] [PubMed] [Google Scholar]
- Ott D, Siddarth P, Gurbani S, Koh S, Tournay A, Shields WD, Caplan R. Behavioral disorders in pediatric epilepsy: unmet psychiatric need. Epilepsia. 2003;44:591–597. doi: 10.1046/j.1528-1157.2003.25002.x. [DOI] [PubMed] [Google Scholar]
- Polack PO, Guillemain I, Hu E, Deransart C, Depaulis A, Charpier S. Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J Neurosci. 2007;27:6590–6599. doi: 10.1523/JNEUROSCI.0753-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KL, Cain SM, Ng C, Sirdesai S, David LS, Kyi M, Garcia E, Tyson JR, Reid CA, Bahlo M, Foote SJ, Snutch TP, O’Brien TJ. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizure expression in a polygenic rat model of absence epilepsy. J Neurosci. 2009;29:371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KL, Kyi M, Reid CA, Paradiso L, D’Abaco GM, Kaye AH, Foote SJ, O’Brien TJ. Genetic absence epilepsy rats from Strasbourg have increased corticothalamic expression of stargazin. Neurobiol Dis. 2008;31:261–265. doi: 10.1016/j.nbd.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis. 2010;39:53–60. doi: 10.1016/j.nbd.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf G, Bihoreau MT, Godfrey RF, Wilder SP, Cox RD, Lathrop M, Marescaux C, Gauguier D. Polygenic control of idiopathic generalized epilepsy phenotypes in the genetic absence rats from Strasbourg (GAERS) Epilepsia. 2004;45:301–308. doi: 10.1111/j.0013-9580.2004.50303.x. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, De Sarro G. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia. 2010;51:1560–1569. doi: 10.1111/j.1528-1167.2009.02400.x. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Perrota I, Di Paola ED, Constanti A, De Sarro G. Effects of early long-term treatment with antiepileptic drugs on development of seizures and depressive-like behavior in a rat genetic absence epilepsy model. Epilepsia. 2011;52:1341–1350. doi: 10.1111/j.1528-1167.2011.03112.x. [DOI] [PubMed] [Google Scholar]
- Sarkisova KY, Kuznetsova GD, Kulikov MA, van Luijtelaar G. Spike-wave discharges are necessary for the expression of behavioral depression-like symptoms. Epilepsia. 2010;51:146–160. doi: 10.1111/j.1528-1167.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Patten SB, Jette N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- Tringham E, Powell KL, Cain SM, Kuplast K, Mezeyova J, Weerapura M, Eduljee C, Jiang X, Smith P, Morrison JL, Jones NC, Braine E, Rind G, Fee-Maki M, Parker D, Pajouhesh H, Parmar M, O’Brien TJ, Snutch TP. T-type calcium channel blockers that attenuate thalamic burst firing and suppress absence seizures. Sci Transl Med. 2012;4:121ra119. doi: 10.1126/scitranslmed.3003120. [DOI] [PubMed] [Google Scholar]
- Vega C, Guo J, Killory B, Danielson N, Vestal M, Berman R, Martin L, Gonzalez JL, Blumenfeld H, Spann MN. Symptoms of anxiety and depression in childhood absence epilepsy. Epilepsia. 2011;52:e70–74. doi: 10.1111/j.1528-1167.2011.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Marescaux C, Boehrer A, Depaulis A. Are rats with genetic absence epilepsy behaviorally impaired? Epilepsy Res. 1991;9:97–104. doi: 10.1016/0920-1211(91)90019-c. [DOI] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: From tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng TW, O’Brien TJ, Morris MJ, Reid CA, Jovanovska V, O’Brien P, van Raay L, Gandrathi AK, Pinault D. Rhythmic neuronal activity in S2 somatosensory and insular cortices contribute to the initiation of absence-related spike-and-wave discharges. Epilepsia. 2012 doi: 10.1111/j.1528-1167.2012.03720.x. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Wang L, Zhang Y, Zhao FH, Luo J, Xiao Z, Chen GJ, Wang XF. Increased expression of DNA methyltransferase 1 and 3a in human temporal lobe epilepsy. J Mol Neurosci. 2012;46:420–426. doi: 10.1007/s12031-011-9602-7. [DOI] [PubMed] [Google Scholar]