Abstract
Members of many species tend to congregate, a behavioral strategy known as local enhancement. Selective advantages of local enhancement range from efficient use of resources to defense from predators. While previous studies have examined many types of social behavior in fruit flies, few have specifically investigated local enhancement. Resource-independent local enhancement has recently been described in the fruit fly using a measure called social space index, although the neural mechanisms remain unknown. Here we analyze resource-independent local enhancement of Drosophila under conditions that allow us to elucidate its neural mechanisms. We have investigated the effects of general volatile anesthetics, compounds that compromise higher order functioning of the type typically required for responding to social cues. We exposed Canton-S flies to non-immobilizing concentrations of halothane and found that flies had a significantly decreased social space index compared to flies tested in air. Narrow abdomen (na) mutants, which display altered responses to anesthetics in numerous behavioral assays, also have a significantly reduced social space index, an effect that was fully reversed by restoring expression of na by driving a UAS-NA rescue construct with NA-GAL4. We found that na expression in cholinergic neurons fully rescued the behavioral defect, whereas expression of na in glutamatergic neurons did so only partially. Our results also suggest a role for na expression in the mushroom bodies, since suppressing na expression in the mushroom bodies of NA-GAL4 rescue flies diminishes social space index. Our data indicate that resource-independent local enhancement, a simple behavioral strategy, requires complex neural processing.
Keywords: cholinergic, Drosophila, general volatile anesthetics, halothane, local enhancement, mushroom bodies, narrow abdomen, social behavior
Introduction
All social behavior requires conspecifics to be close enough to interact with one another. In many species, the proximity of conspecifics appears to result from a tendency to assemble into groups. This behavior, which is called local enhancement, has been observed in a wide range of species and can aid in defense against predators (Rohlfs & Hoffmeister, 2004), utilization of natural resources (Stamps, 1991), and learning (Zentall, 2006). More broadly, local enhancement may be a prerequisite for the initiation of competitive or cooperative interactions among conspecifics, depending on the particular configuration of social cues in a given context. Despite a possible central role in modulating complex social interactions, neither genetic nor neural determinants of local enhancement are known. However, recent studies of local enhancement in Drosophila suggest that this genetically tractable organism, which has been successfully used to study the neural circuits underlying behaviors including sleep (Harbison et al., 2009), learning and memory (Pitman et al., 2009; Waddell & Quinn, 2001), and courtship and mating (Villella & Hall, 2008), may be a useful model for elucidating the genetic and neural bases of local enhancement.
Previous studies have shown that Drosophila group in the presence of a resource or in response to pheromones. These studies have focused primarily on aggregation in response to cis-vaccenyl acetate (cVA) (Bartelt et al., 1985; Ha & Smith, 2006) and in the presence of resources such as potential mates (Chen et al., 2002), egg-laying substrates (Battesti et al., 2012; Mery & Kawecki, 2002; Prokopy & Duan, 1998; Sarin & Dukas, 2009) and food (Hoffmann, 1990; Lefranc et al., 2001; Saltz & Foley, 2011). While such gatherings of conspecifics are examples of local enhancement, it is not clear whether these animals are responding primarily to the presence of conspecifics or to other cues.
Although some studies provide evidence that grouping is not simply governed by an individual’s attraction to the resource (Tinette et al., 2004), few have examined local enhancement in the absence of environmental resources. An early study by Navarro and del Solar (1975) provided evidence for gregarious behavior in Drosophila in such conditions, but only recently has rigorous analysis established local enhancement as a robust behavior in Drosophila under such conditions (Simon et al., 2012). Simon et al (2012) eliminated possible environmental confounds associated with most other fly behavioral studies, and were thus able to characterize a simple, resource-independent form of local enhancement for the first time. Another recent study performed in the absence of a food source demonstrated that flies within groups similar to those described by Simon et al (2012) form social networks (Schneider et al., 2012). To distinguish this form of local enhancement from local enhancement observed in the presence of environmental resources (and often accompanied by more complex behaviors), we refer to it here as resource-independent local enhancement (RILE). This study elucidates for the first time neural and genetic mechanisms involved in Drosophila RILE and paves the way for a better understanding of the mechanisms involved in local enhancement.
Materials and Methods
Fly Culture
All flies were reared on cornmeal-molasses medium and were maintained at 25°C in a 12:12 hr light–dark cycle. All strains used were in a Canton-S genetic background to eliminate the confounding effect of genetic background on behavior. The following fly strains were used in this study: Canton-S (lab stock); trp301; trpl302; trpl302 trp301 (Cheng & Nash, 2008); nahar38 (Krishnan & Nash, 1990); nahar38;+;UAS-NA; w1118;dv-Glut-GAL4/SM5 (Daniels et al., 2008); w1118; Cha-GAL4/SM5CyO (Bloomington Stock Center, Bloomington, IL); nahar38/FM7;+;NA-GAL4/TM6B; nahar38/FM7a;Cha-GAL4/CyO; w1118; MB-GAL80/CyO; UAS-NA/TM6B (Krashes et al., 2007); w1118; +; UAS-NA; and w1118;UAS-RedStinger/CyO; NA-GAL4/TM6B (RedStinger flies originally from Bloomington Stock Center, Bloomington, IN). Flies containing either the UAS-NA or NA-GAL4 inserts originated with Lear et al (Lear et al., 2005), and were crossed to balanced and/or Cantonized lab stocks and kept as stocks as indicated above.
Behavioral Testing
Chamber
Our testing chamber was engineered and fabricated by the Section on Instrumentation (NIMH, Bethesda, MD). The inside dimensions were 12.5 cm × 11.5 cm × 3 mm, and airflow through box was 149 ml/min, allowing about 3.5 changeovers of air per minute. This flow rate was tested and determined not disturb fly behavior. The ceiling and sides of the box were acrylic, while the floor was constructed of 3/8″ thin-walled square brass tubing covered in a thin layer of epoxy, sanded smooth and spray painted white. Our circular chamber was identical in design except that the inside diameter was 11.5 cm. Although flies were unable to jump or fly in the low-ceilinged chamber, we found that they could walk on the ceiling after flipping themselves over; thus, we coated the ceiling (made of acrylic) with a thin layer of Fluon (Whitford Corporation, Elverson, PA) to restrict flies to a monolayer. Metal needles through holes on one side of the box top allowed airflow into the chamber, while a corresponding set of holes on the other side was for a vacuum line. Humidifying air or adding halothane (Sigma-Aldrich) to breathing air was accomplished by flowing air through a gas washing bottle filled with either water or halothane.
All air flows were controlled with gas flowmeters (Cole-Parmer Instrument Company, Vernon Hills, IL). Concentrations of GVA were monitored with a Miran infrared gas analyzer (Foxboro Company, East Bridgewater, MA). For tests in dim red light, we used a Kodak LED Safelight (Electron Microscopy Sciences, Hatfield, PA) as the only light source in the testing room.
While Simon et al (2012) used a vertical test chamber for most experiments, they observed similar local enhancement in their horizontal chambers. We chose to focus on an horizontally oriented arena to minimize the effects of both negative geotaxis and the increased burden of walking against gravity in the presence of anesthetics. Additionally, since we wanted to be able to test the effects of GVA on Drosophila behavior, we flowed a gentle current of air through our chamber for all experiments. In agreement with Simon et al (2012), we also show that the behavior is both independent of arena shape and dependent on visual cues.
Fly Handling
Young male flies were sorted and collected under CO2 anesthesia 48 hours before testing. Flies were between 2–6 days old when tested. We tested males to eliminate possible behavioral complexity due to ovulation status of females. On the day of testing, flies were loaded into perforated 50mL Falcon tubes (80 flies per tube) without further anesthetic. Flies were equilibrated in these tubes in a large closed chamber with a constant flow of either humidified air or a fixed concentration of halothane for 30 min. For flies exposed to halothane, this period allowed the anesthetic to reach steady state within the nervous system; the flies tested in humidified air alone were similarly equilibrated to control pre-testing conditions. At the end of this equilibration period, all 80 flies were gently tapped through a funnel into the testing chamber, through which air (with or without anesthetic) had been streaming during equilibration. Between trials, flies were CO2 anesthetized to remove them and the platform floor cleaned with 70% ethanol to clear traces (feces, pheromones, etc.) of previously tested flies.
Data Acquisition and Analysis
Images of flies in the testing chamber were acquired with a digital still camera (Nikkon Coolpix P100, Nikon) every 4 min after introducing flies into the arena. Although wild-type flies tested under ambient light in humidified air (control conditions) start settling into groups relatively quickly and are stationary after 7–10 min, other testing conditions led to an increased time until flies stopped walking. Flies in some test conditions walked up to 24 min, but all flies tested in all conditions had stopped walking between 24 and 28 min; thus, we used this time-point (28 min) for all analyses.
Still photos were imported into ImageJ software (NIH, rsbweb.nih.gov/ij/) and analyzed for nearest neighbor distances. Briefly, flies were rendered as particles and assigned 2-D coordinates. These coordinates were used to determine the distance from each fly to its nearest neighbor using a modified version of the Nearest Neighbor macro developed by Wayne Rasband (NIMH); these data were imported into SigmaPlot 11 (Systat Software, San Jose, CA) for further analysis. Data were divided into 5-mm bins, and the percentage of flies in each bin was calculated. Social Space Index (SSI) was calculated as the difference between the percentage of flies in the first bin (0–5 mm from the nearest neighbor) and the percentage of flies in the second bin (5–10 mm from the nearest neighbor) (Simon et al., 2012). Over all 116 local enhancement trials performed in this study, the first two bins of the nearest neighbor histograms account for a vast majority of flies (88.21 ± 6.67; mean ± standard deviation); thus, this metric was considered sufficient to describe the behavior we were observing.
Wholemount immunostaining of adult brains
Flies were incubated in high-octane fixative (12.5% formaldehyde, 50% octane, pH 6.8) for 20 minutes before being briefly washed once with octane and resuspended in PBS. Brains were dissected in PBS, incubated in anti-DC0 antibody (alias PKA-C1, catalytic subunit of Drosophila protein kinase A; gift of Daniel Kalderon; 1:1000) (Skoulakis et al., 1993) overnight. Alexa Fluor 488 goat anti-rabbit secondary antibody was used (1:150) and brains were mounted in Vectashield (Vector Labs, Burlingame, CA). Confocal imaging was performed using a Nikon C-1 confocal microscope. Optical sections (3μm) were made with a 20x objective, using the 543 nm laser emission lines for RedStinger excitation and the 488 nm laser emission lines for DC0 labeling. Images were generated using Nikon’s EZ-C1 Freeviewer software.
Statistical Analysis
All data are presented as mean ± SEM unless otherwise noted. For two group comparisons, Student’s t-test was used. For multi-group comparisons to a control, we used ANOVA with the Holm-Sidak posthoc test for multiple comparisons to a control group. For all data, differences were considered significant when p < 0.05.
Results
Local enhancement in D. melanogaster has been described to a) occur in the absence of environmental stimuli; b) be dependent on vision; and c) be independent of chamber geometry and orientation (Simon et al., 2012). To study RILE in Drosophila under conditions in which we could conveniently test animals exposed to general anesthetics, we built an apparatus similar to that described by Simon et al (2012), modified to allow laminar airflow through horizontally oriented chambers.
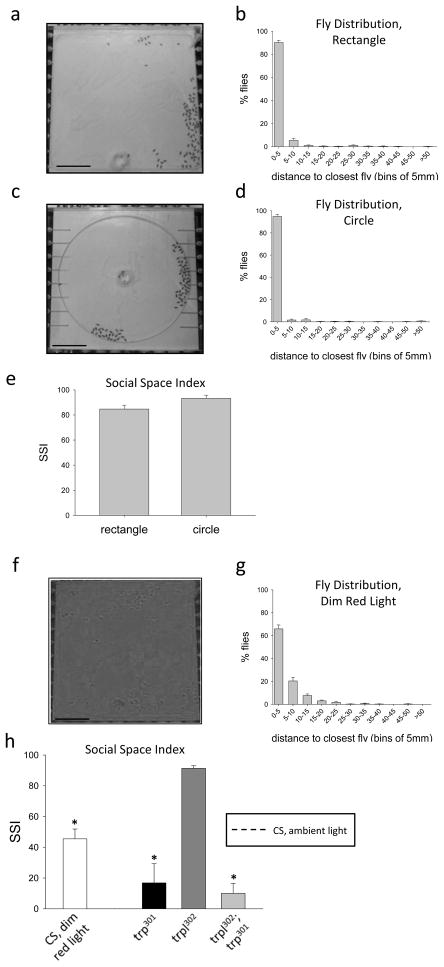
To validate that flies in this chamber exhibited local enhancement, we first tested Canton-S male flies in our rectangular testing chamber. We observed that after an initial period of vigorous walking lasting approximately 5 min, Canton-S flies began to settle into groups that were stationary for up to 45 min. In our rectangular chamber, most (80.8 ± 3.3%, n=9 trials) young male Canton-S flies are within 1 mm of at least two other flies (data not shown). A greater percentage of flies (90.3 ± 1.7%, n=9) settle within 5 mm of their nearest neighbor (Fig 1a, b). Chamber geometry is known to affect exploratory and spontaneous activity of flies (Liu et al., 2007). To test whether chamber geometry affects local enhancement under our assay conditions, we tested behavior in a circular chamber and found that 94.9 ± 1.8% flies (n=8 trials) are within 5 mm of their nearest neighbor (Fig 1c, d). In order to provide a simpler measure of local enhancement, we calculated the social space index (SSI) which is obtained from the histograms of fly distributions by subtracting the second bin from the first (Simon et al., 2012). The SSI of male Canton-S flies in our rectangular and circular chambers (84.7 ± 3.2, n=9 and 93.3 ± 2.5, n=8, respectively) did not statistically differ from one another (Student’s t-test, Fig 1e). Young female Canton-S flies demonstrate behavior similar to the males in our rectangular arena (SSI=84.4 ± 3.8, n=4, data not shown), consistent with observations by Simon et al (2012). As expected, based on previous studies that have shown flies in an arena will spend a large part of the time near the arena walls (Soibam et al., 2012; Valente et al., 2007), flies in each of our arenas demonstrated a preference for settling in corners or along edges after their initial exploratory behavior.
Figure 1. Resource-independent local enhancement of flies in different chambers or under conditions of visual impairment.
a, b. Wild-type Canton-S (CS) males in humidified air in rectangular arena. A representative image is shown (a). Scale bar represents 30 mm. The histogram (b) represents the mean percentage of flies (± SEM) at the indicated distance from their nearest neighbors (n=9). c, d. CS males in humidified air in circular arena. A representative image is shown (c). Scale bar represents 30 mm. The histogram (d) represents the mean percentage of flies (± SEM) at the indicated distance from their nearest neighbors (n=8). e. Social space indices (SSI) for CS flies in the two different chambers shown in a and c (error bars represent ± SEM; no significant difference, Student’s t-test). f, g. A representative image of CS males tested in humidified air in dim red light (f). Scale bar represents 30 mm. The histogram (g) shows the percentage of flies at given distance intervals from their nearest neighbor (mean ± SEM, n=7). h. Summarized mean SSI (± SEM) data for CS flies tested in dim red light (white bar) and for the three visual mutants tested in ambient light: trp301 (n=6), trpl302 (n=5), and trpl302;trp301 double mutants (n=6). The dashed horizontal line represents the mean SSI of CS flies tested in ambient light and is shown for comparison. *p <0.05 compared to CS control.
We also tested whether social space index under our testing conditions was vision-dependent, as reported by Simon et al (2012). SSI for flies tested under dim red light is 45.5 ± 6.4 (n=7; p<0.05 compared with flies tested under ambient light) (Fig 1f, g and 1h). In dim red light, Canton-S males introduced into our chamber walk for a considerably longer time (~20 min) than flies tested under ambient light (~5 min). However, flies finally settle between 22 and 25 minutes. Interestingly, the strong preference flies exhibit for corners and edges is notably diminished, with flies often settling down in the middle area of the arena.
To further examine the dependence of RILE on vision, we examined the behavior of visual mutant flies with partial or complete impairment of the phototransduction machinery. trp mutants (trp301), which are nearly completely blind, carry perturbations in the transient receptor potential (trp) gene product, a subunit of the cation channel that carries a major component of the light-induced current in fly eyes (Montell, 2005). A separate minor component of light-induced current is mediated by Trpl protein (Niemeyer et al., 1996; Reuss et al., 1997). In our arena, trp flies tested under ambient light and in humidified air demonstrate significantly reduced RILE compared to wild-type flies (SSI=16.8 ± 12.5, n=6, p<0.05; Fig 1h). In contrast, the SSI of trpl flies (trpl302) (SSI=91.3 ± 1.7, n=5), which have relatively minor visual impairments, did not differ from that of wild-type flies (Fig 1h). Given the difference in visual impairment between trp and trpl flies, the difference in their behavior is not surprising. Flies carrying both the trp and trpl mutations (trpl302;trp301) did not statistically differ in SSI from trp flies, indicating that the trp mutation itself accounts for the behavioral deficit in the double mutants.
Overall, our data, which demonstrate that RILE in flies is independent of chamber geometry, occurs in the absence of environmental cues and is vision dependent, are consistent with those of Simon et al (2012).
Apart from its dependence on visual cues, little is known about the neural mechanisms that mediate RILE in Drosophila. Because social interactions require responses to diverse cues, we were interested in compromising the fly’s ability to coordinate such neural processing. To determine whether the mechanisms that govern RILE are sensitive to the effects of general anesthetics, which, at non-immobilizing concentrations, target neural circuits involved in central processing and decouple higher-order brain processes from simpler neural functions (van Swinderen, 2006; van Swinderen et al., 2004), we examined RILE of wild-type flies exposed to a low concentration of the GVA halothane. It has been shown previously both that flies are sensitive to clinical doses of GVA and that simple reflexes in Drosophila are preserved at subclinical doses (Allada & Nash, 1993; Campbell & Nash, 1994; Campbell & Nash, 1998; Gamo et al., 1981). We exposed flies to halothane for 30 min before testing to allow the anesthetic to reach equilibrium in their nervous systems. Flies were equilibrated and tested at 0.15% halothane, a concentration that was low enough to allow flies to walk unhindered on horizontal surfaces (data not shown).
When introduced into the testing arena with halothane, flies exhibit an initial exploratory period lasting 15–20 min, which was longer than the period of exploration in Canton-S flies tested in humidified air alone. By 25 minutes, most flies have stopped walking. In these conditions, flies settle into a dispersed array with substantially reduced clustering (Fig 2). Compared with flies tested in humidified air, significantly fewer flies were found within 5 mm of their nearest neighbor (62.6 ± 2.6% in halothane, n=6; p<0.001) and about 25% of flies are 5–10 mm from their nearest neighbor (Fig 2b), resulting in a significantly lower SSI compared to flies tested in humidified air alone (SSI= 37.3 ± 4.1, n=6; p < 0.001, Fig 2b). Thus, exposure to the GVA halothane at low doses disrupts normal RILE in Canton-S flies.
Figure 2. Resource-independent local enhancement of flies tested in the presence of halothane.
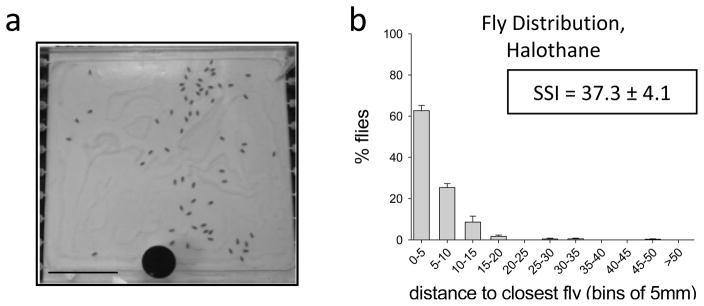
a. A representative image of flies in the testing chamber in the presence of 0.15% (volume) halothane is shown. Scale bar represents 30 mm. b. The histogram shows the mean percentage of Canton-S flies (± SEM) at the indicated distance from their nearest neighbors (n=6) during testing in halothane. The SSI (± SEM) is also indicated.
Given the above results, we hypothesized that the neural circuitry that governs RILE employs signaling molecules that are sensitive to halothane. Halothane sensitivity is thought to be mediated, at least in part, by the ion channel encoded by the na gene, and the har38 allele (nahar38) causes pronounced changes in sensitivity to this anesthetic (Guan et al., 2000; Krishnan & Nash, 1990; Nash et al., 1991). We therefore tested the behavioral phenotype of nahar38 flies.
Although nahar38 flies are deficient in their climbing ability in ambient air and will alternate between hesitant walking and freezing (Humphrey et al., 2007), we did not observe this locomotor phenotype during the exploratory activity on our horizontal testing surface. However, nahar38 flies are significantly deficient in RILE with an SSI of only 22.2 ± 4.1 (n=9, Fig 3a), an effect that is fully rescued by expression of a UAS-NA construct under the control of NA-GAL4 (Lear et al., 2005), which drives transgene expression in cells that normally express na (nahar38; +; NA-GAL4/UAS-NA, SSI=74.4 ± 4.5, n=7, Fig 3a). Neither the NA-GAL4 driver line by itself nor the UAS-NA rescue construct by itself, each in the nahar38 mutant background, provide behavioral rescue (Fig 3a).
Figure 3. Social space indices of nahar38 mutant and rescue flies.
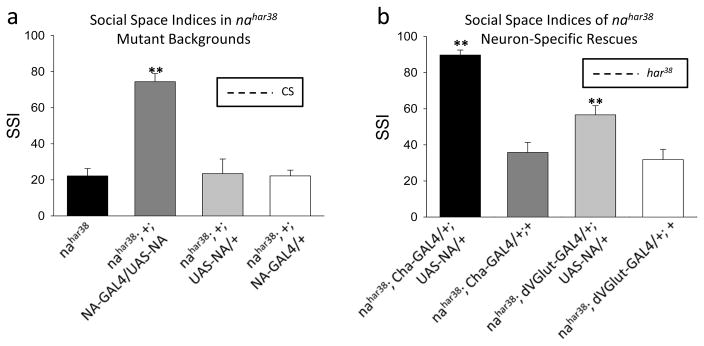
a. SSI are shown for nahar38(n=9); nahar38;+;NA-GAL4/UAS-NA rescue (n=7); and control lines nahar38; +; UAS-NA/+ (n=7) and nahar38; +; NA-GAL4/+ (n=7) flies. CS SSI (mean) represented by dashed horizontal line for reference. **p < 0.001 compared with har38. b. SSI are shown for nahar38; Cha-GAL4/+; UAS-NA/+ (black, n=7); Cha-GAL4 driver line by itself (dark grey, n=9); nahar38; dVGlut-GAL4/+; UAS-NA/+ (light grey, n=9); and dVGlut-GAL4 driver line by itself (white, n=7). Mean SSI of nahar38 flies represented by dashed horizontal line for reference. **p < 0.001 compared with nahar38 flies.
Our data suggests that the NA ion channel is involved in mediating RILE. To begin to elucidate its neuronal site of action, we selectively restored na gene expression in various neuronal subtypes in nahar38 mutants using specific GAL4 drivers. We tested these flies for RILE under ambient light in humidified air. We found that expression of na in cholinergic neurons (nahar38; Cha-GAL4/+; UAS-NA/+) completely rescues the behavioral defect (SSI=89.7 ± 2.8, n=7, **p < 0.01 compared to nahar38, Fig 3b), whereas restoration of na expression in glutamatergic neurons (nahar38; dVGlut-GAL4/+; UAS-NA/+) only partially rescues the RILE phenotype (SSI=43.9 ± 5.9, n=6, **p < 0.01 compared to nahar38, Fig 3b). While both cholinergic and glutamatergic rescue flies have SSI that significantly differ from that of the mutant nahar38 flies (Fig 3b), the glutamatergic rescue flies’ SSI also differs from that of the control CS flies, thus suggesting a behavioral rescue midway between wild-type and nahar38 flies. Neither of the driver lines expressed by itself in the nahar38 background affected the phenotype (Fig 3b).
In addition to determining in what type of neurons na expression is needed for normal RILE behavior, we were also interested in determining in what central brain structure(s) na expression is needed. NA-GAL4 has a strong expression pattern in the MB. Additionally, RILE may depend in part on learning, as socially isolated flies have a significantly lower SSI than socially enriched flies (Simon et al., 2012). Various forms of learning in Drosophila, including context generalization in visual learning (Liu et al., 1999; Zars, 2000), have been shown to require the MB. Thus, we hypothesized that inhibition of na expression in the MB in the NA-GAL4 rescue flies would prevent behavioral rescue.
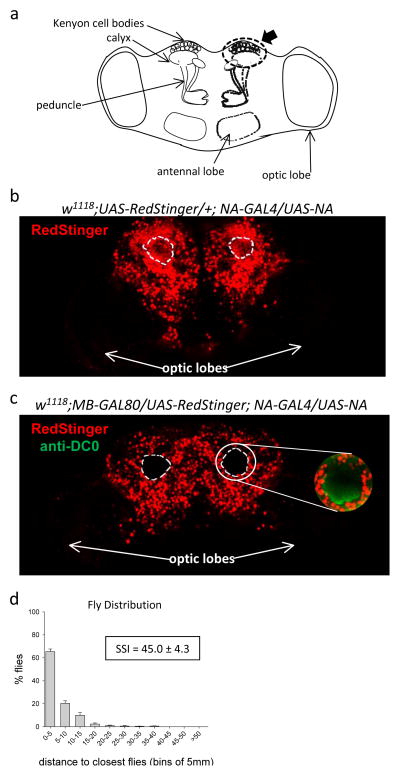
We used an MB-GAL80 line (Krashes et al., 2007) to selectively decrease na expression in the MB of nahar38; +; NA-GAL4/UAS-NA rescue flies. We first verified the effectiveness of the MB-GAL80 line by comparing the expression of a nuclear marker, RedStinger, in the MB in the presence (w1118; MB-GAL80/UAS-RedStinger; NA-GAL4/UAS-NA) or absence (w1118; UAS-RedStinger/+; NA-GAL4/UAS-NA) of the MB-GAL80 transgene by examining wholemount brains with confocal imaging (Fig 4). The nerve fibers of the Kenyon cells of the MB form the peduncle and calyces, which appear as bilaterally symmetric holes toward the dorsal side of the brain (Fig 4a). We labeled the MB with anti-DC0 antibody (Skoulakis et al., 1993), thus providing a readily identifiable landmark by which to identify distally located MB cell bodies. In the control fly brains (from flies without MB-GAL80), we saw robust expression of RedStinger (under control of the NA-GAL4 driver) in the MB calyces (Fig 4b; dashed lines denote circumference of anti-DC0 labeling). However, in the presence of MB-GAL80, the signal was reduced in the MB (Fig 4c; dashed lines denote circumference of anti-DC0 labeling). The functional effect of blocking na expression in the MB of the NA-GAL4 rescue flies was to reduce the SSI (SSI=45.0 ± 4.3, n=8) compared to that of the nahar38; +; NA-GAL4/UAS-NA control flies (Fig 4d). Thus, na expression in the MB plays a role in normal RILE behavior.
Figure 4. Social space indices of na-rescue flies in which na expression is blocked selectively in the mushroom bodies.
a. Schematic representation of the fly brain showing the relevant orientation and position of the MB calyces, peduncles and Kenyon cell bodies. The dashed oval around the Kenyon cell bodies and the calyx in the right hemisphere correspond to the area enclosed in dashed circles in (b) and (c). b, c. Expression of both UAS-RedStinger and anti-DC0 labeling was visualized in whole-mount adult brains. Representative images of whole brain from w1118; UAS-RedStinger/+;NA-GAL4/UAS-NA (b, n=4) and from w1118;MB-GAL80/UAS-RedStinger;NA-GAL4/UAS-NA (c, n=4) show the expression of RedStinger. The MB calyces, as marked by anti-DC0 labeling (green, as shown in inset in (c)), are outlined in white dashed lines. d. The histogram shows the mean percentage of nahar38; MB-GAL80/UAS-RedStinger; NA-GAL4/UAS-NA flies (± SEM) at the indicated distance from their nearest neighbors (n=8). The SSI (± SEM) is also indicated.
Discussion
Although local enhancement behavior is widespread among animal species, its neural basis is obscure. This is partly due to the fact that many studies on social grouping have used complex environmental conditions. Using a paradigm that excludes most environmental cues, we describe the genetic and neural basis of what we call resource-independent local enhancement (RILE). We show that in Drosophila, RILE is sensitive to the GVA halothane and that the anesthetic-sensitive mutant, nahar38, has impaired RILE. In addition, we show that RILE requires na expression in both cholinergic and MB neurons. We also confirm and extend the observation that RILE depends on visual cues (Simon et al., 2012). Taken together, our study represents a first step in mapping the genetic and neural substrates of RILE in Drosophila.
Defining Resource Independent Local Enhancement
The phenomenon of local enhancement, in which conspecifics assemble into groups, can be driven by a variety of factors, including pheromones and food. These stimuli can elicit behavioral interactions, such as aggression, and may obscure more basic mechanisms that bring animals together. To study such mechanisms, researchers have looked for local enhancement in the absence of such potential confounds. In Drosophila, local enhancement in the absence of resources was first observed in the mid-1970s (Navarro & del Solar, 1975), but focused largely on describing the behavior under different temperatures and between the two sexes. Recently, Simon et al described the behavior in greater detail (Simon et al., 2012).
Prior to that, studies of fly social behavior focused largely on such phenomena as pheromone-specific aggregation (Bartelt et al., 1985; Ha & Smith, 2006), mating (Villella & Hall, 2008), social learning about egg-laying substrates (Sarin & Dukas, 2009), or male aggression (Chen et al., 2002; Nilsen et al., 2004). The execution of these behaviors typically relies on multiple cues, and thus may obscure more basic mechanisms driving animal grouping. As an example of the complexities that may result from environmental interactions, female Drosophila will gather on food sources, whereas males demonstrate territoriality and avoidance (Hoffmann, 1990; Saltz & Foley, 2011). While this suggests a sexual dimorphism to local enhancement itself, sexually dimorphic responses to environmental cues must also be taken into account. For example, females may gather on a food source to oviposit in response to females that have laid eggs there already (Sarin & Dukas, 2009); however, males may avoid one another because they are defending limited resources (Shelly, 1987).
Given the confounds of complex environments on behavior, an investigation into local enhancement will rely on test conditions with minimum environmental cues. Using the paradigm first described by Simon et al (2012), which eliminates as many environmental cues as possible, our wild-type control males were often within one millimeter of their nearest neighbor and exhibited no aggressive behavior. This is in contrast to observations made in the presence of food or females, where male flies display aggressive behavior toward one another (Chen et al., 2002; Hoffmann, 1990; Saltz & Foley, 2011; Wang & Anderson, 2010). Although conspecific assembly in the presence of a resource is a manifestation of local enhancement, local enhancement in male flies is clearly a function of environmental context. Thus, we wish to refine the definition of the behavior presented here, and have called it resource-independent local enhancement (RILE).
Dependence of RILE on Visual Cues
Simon et al (2012) observed significantly reduced SSI in flies tested in dim red light (a result we confirm) and in visually impaired white mutants. Because white flies have additional behavioral phenotypes that are independent of visual impairment (Campbell & Nash, 2001; Diegelmann et al., 2006), we sought to confirm the vision requirement using the vision-specific mutant trp301, which is almost completely blind. Our finding that these mutants have impaired RILE is consistent with the hypothesis that RILE depends on visual cues. Further, Simon et al (2012) found that olfactory receptor mutants have normal RILE, thus excluding a role for the pheromone cVA. We have found that RILE in females (both virginal females and females isolated from males before testing to eliminate cVA from testing conditions) is similar to that of males, supporting the idea that RILE is not olfactory-dependent (unpublished results). Thus, the local enhancement behavior studied here is distinct from pheromonally-modulated aggregation (Bartelt et al., 1985; Ha & Smith, 2006; Wertheim et al., 2006). It is possible that neural processes leading to vision-dependent RILE and those leading to olfactory-dependent aggregation are simultaneously active and compete with one another in a manner dependent on environmental conditions. To focus explicitly on neurobiological mechanisms that generally promote conspecific gathering, we have investigated here the neural basis of RILE.
Effects of Anesthetics and Anesthesia-Sensitive Mutation on RILE
While GVA have been used to study simple reflexes (Allada & Nash, 1993; Campbell & Nash, 1994; Campbell & Nash, 1998; Gamo et al., 1981), they are also useful in studying more complex behavior, as they inhibit central brain processing when administered at sub-clinical doses (van Swinderen, 2006). We observed that both nahar38 flies and wild-type flies tested in halothane have a reduced SSI and take longer to stop walking when compared to wild-type flies. Because both phenotypes are reminiscent of flies tested in dim red light, it is possible that the decrease in RILE in the presence of GVA or in nahar38 flies could be due to a visual defect, a conclusion consistent with the observation that exposure to halothane alters the electroretinogram of wild-type flies (Rajaram & Nash, 2004). However, since our rescue data point to a role for the central brain in RILE, we think it is likely that the RILE phenotype caused by halothane and na mutation is the result of more than a simple sensory deficiency.
One possibility is that the defect in RILE stems from a lack of coordination between visual processing and motor behavior. The MB have been implicated in visual learning (Liu et al., 1999; Zars, 2000) and are involved in the organization of motor output (Heisenberg, 2003). Additionally, the MB have been shown to be involved in fly exploration (Besson & Martin, 2005) and in other aspects of locomotor behavior (Martin et al., 1998). Thus, it may be that abnormal RILE in both nahar38 flies and in flies exposed to halothane is due to a lack of coordination in the MB between sensory input and behavioral output. The restoration of normal RILE in nahar38 mutants seen when na expression is rescued using NA-GAL4 is consistent with this hypothesis insofar as NA-GAL4 expresses strongly in the MBs. Likewise, the lack of full rescue when na expression is rescued throughout the NA-GAL4 pattern less the MB also supports a role for the MB in RILE.
While the MB appear to be involved in normal RILE, our results suggest complexity. Rescue of na expression using Cha-GAL4, like rescue with NA-GAL4, restores normal RILE, but unlike NA-GAL4, Cha-GAL4 is not strongly expressed in the mushroom bodies (Mehren & Griffith, 2006). Further investigation of the expression of these two drivers is necessary, but it is possible that parallel neural pathways support RILE. Alternatively, na expression in Cha+ neurons may compensate for the lack of expression in the MB to produce normal RILE. The central complex, which has been implicated in visual orientation behavior both in flight (Ilius et al., 1994) and in walking (Strauss, 1999; Xiong et al., 2010), may also play a role in RILE and provides an opportunity for future investigation. While the neural circuitry involved in RILE is thus apparently complex, the na gene represents a necessary substrate for this behavior.
It is worth noting that the effect of GVA on RILE in Drosophila can be used as an anesthetic endpoint to study the mechanisms of GVA (Nash, 2002). Notably, the effect of halothane on RILE is reversible (unpublished data) and, unlike previous assays which have been used to study anesthetic sensitivity in flies (Guan et al., 2000; Leibovitch et al., 1995), our assay depends on a horizontal surface rather than a vertical one. Therefore, RILE will be a useful tool with which to probe anesthetic mechanisms.
RILE as an Organizing Focus of Behaviors
While RILE provides a simple model for studying the neural and genetic underpinnings of a rudimentary behavior, its simplicity is in part a consequence of the impoverished environment in which it occurs. The ethological status of a generalized tendency for conspecifics to associate is thus not necessarily obvious. One possibility is that RILE represents a permissive substrate for behavior, bringing animals into proximity so that environmental cues may trigger other social behaviors. In this sense, it is reminiscent of the behavioral “centers” posited by Tinbergen’s early model of behavioral sequences (Tinbergen, 1951) in which each “center” has specific motor programs that can be elicited in response to particular environmental and internal inputs. RILE might thus be viewed as a “center” for promoting social behavior. This possibility remains to be explored, but the emerging characterization of RILE suggest that this simple behavior will provide fertile ground for ethological and neurobiological studies into mechanisms of behavior.
Acknowledgments
The authors would like to thank Drs. David Sandstrom, Anne F. Simon and Shuying Gao for helpful discussions. We would also like to thank Dr. Bridget Lear for flies carrying the UAS-NA and NA-GAL4 inserts; Dr. Jens Rister for the MB-GAL80 flies; Dr. Aaron DiAntonio for the dVGlut-GAL4 flies; and Dr. Daniel Kalderon for the anti-DC0 antibody. The authors acknowledge Mr. Tom Talbot of the Section on Instrumentation at NIMH for designing and fabricating the assay arena and Dr. Wayne Rasband for writing our data analysis macro. We also extend our appreciation to Mr. Robert Scott for performing the whole mount brain dissections and imaging, to Dr. Dongyu Guo for assistance with behavior experiments and to Mrs. Erin Kollins for help with data analysis. Special thanks to Dr. Benajamin White for support and guidance in all aspects of this work after the death of our mentor and co-author, Dr. Howard Nash. Finally, we wish to dedicate this manuscript to the memory of Howard Nash, whose incisive mind, encyclopedic memory and critical acumen will be sorely missed, not only by those of us who worked with him, but also by those who benefitted indirectly from these qualities through his seminal contributions to science, first in the area of DNA recombination and more recently in the fields of anesthetic action and Drosophila neuroscience. The work was supported entirely by the DIRP of NIMH, NIH (MH 002228-27).
References
- Allada R, Nash HA. Drosophila melanogaster as a model for study of general anesthesia: the quantitative response to clinical anesthetics and alkanes. Anesth Analg. 1993;77:19–26. doi: 10.1213/00000539-199307000-00005. [DOI] [PubMed] [Google Scholar]
- Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. Journal of Chemical Ecology. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- Battesti M, Moreno C, Joly D, Mery F. Spread of Social Information and Dynamics of Social Transmission within Drosophila Groups. Curr Biol. 2012;22:309–313. doi: 10.1016/j.cub.2011.12.050. [DOI] [PubMed] [Google Scholar]
- Besson M, Martin JR. Centrophobism/thigmotaxis, a new role for the mushroom bodies in Drosophila. J Neurobiol. 2005;62:386–396. doi: 10.1002/neu.20111. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Nash HA. Use of Drosophila mutants to distinguish among volatile general anesthetics. Proc Natl Acad Sci U S A. 1994;91:2135–2139. doi: 10.1073/pnas.91.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Nash HA. The visually-induced jump response of Drosophila melanogaster is sensitive to volatile anesthetics. J Neurogenet. 1998;12:241–251. doi: 10.3109/01677069809108561. [DOI] [PubMed] [Google Scholar]
- Campbell JL, Nash HA. Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J Neurobiol. 2001;49:339–349. doi: 10.1002/neu.10009. [DOI] [PubMed] [Google Scholar]
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Nash HA. Visual mutations reveal opposing effects of illumination on arousal in Drosophila. Genetics. 2008;178:2413–2416. doi: 10.1534/genetics.107.085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol. 2008;508:131–152. doi: 10.1002/cne.21670. [DOI] [PubMed] [Google Scholar]
- Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn Mem. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo S, Ogaki M, Nakashima-Tanaka E. Strain differences in minimum anesthetic concentrations in Drosophila melanogaster. Anesthesiology. 1981;54:289–293. doi: 10.1097/00000542-198104000-00006. [DOI] [PubMed] [Google Scholar]
- Guan Z, Scott RL, Nash HA. A new assay for the genetic study of general anesthesia in Drosophila melanogaster: use in analysis of mutations in the X-chromosomal 12E region. J Neurogenet. 2000;14:25–42. doi: 10.3109/01677060009083475. [DOI] [PubMed] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison ST, Mackay TF, Anholt RR. Understanding the neurogenetics of sleep: progress from Drosophila. Trends Genet. 2009;25:262–269. doi: 10.1016/j.tig.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA. The influence of age and experience with conspecifics on territorial behavior in Drosophila melanogaster. Journal of Insect Behavior. 1990;3:1–12. [Google Scholar]
- Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Ilius M, Wolf R, Heisenberg M. The central complex of Drosophila melanogaster is involved in flight control: studies on mutants and mosaics of the gene ellipsoid body open. J Neurogenet. 1994;9:189–206. doi: 10.3109/01677069409167279. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KS, Nash HA. A genetic study of the anesthetic response: mutants of Drosophila melanogaster altered in sensitivity to halothane. Proc Natl Acad Sci U S A. 1990;87:8632–8636. doi: 10.1073/pnas.87.21.8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear BC, Lin JM, Keath JR, McGill JJ, Raman IM, Allada R. The ion channel narrow abdomen is critical for neural output of the Drosophila circadian pacemaker. Neuron. 2005;48:965–976. doi: 10.1016/j.neuron.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Lefranc A, Jeune B, Thomas-Orillard M, Danchin E. Non-independence of individuals in a population of Drosophila melanogaster: effects on spatial distribution and dispersal. C R Acad Sci III. 2001;324:219–227. doi: 10.1016/s0764-4469(00)01297-x. [DOI] [PubMed] [Google Scholar]
- Leibovitch BA, Campbell DB, Krishnan KS, Nash HA. Mutations that affect ion channels change the sensitivity of Drosophila melanogaster to volatile anesthetics. J Neurogenet. 1995;10:1–13. doi: 10.3109/01677069509083455. [DOI] [PubMed] [Google Scholar]
- Liu L, Davis RL, Roman G. Exploratory activity in Drosophila requires the kurtz nonvisual arrestin. Genetics. 2007;175:1197–1212. doi: 10.1534/genetics.106.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wolf R, Ernst R, Heisenberg M. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400:753–756. doi: 10.1038/23456. [DOI] [PubMed] [Google Scholar]
- Martin JR, Ernst R, Heisenberg M. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learn Mem. 1998;5:179–191. [PMC free article] [PubMed] [Google Scholar]
- Mehren JE, Griffith LC. Cholinergic neurons mediate CaMKII-dependent enhancement of courtship suppression. Learn Mem. 2006;13:686–689. doi: 10.1101/lm.317806. [DOI] [PubMed] [Google Scholar]
- Mery F, Kawecki TJ. Experimental evolution of learning ability in fruit flies. Proc Natl Acad Sci U S A. 2002;99:14274–14279. doi: 10.1073/pnas.222371199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Drosophila TRP channels. Pflugers Arch. 2005;451:19–28. doi: 10.1007/s00424-005-1426-2. [DOI] [PubMed] [Google Scholar]
- Nash HA. In vivo genetics of anaesthetic action. Br J Anaesth. 2002;89:143–155. doi: 10.1093/bja/aef159. [DOI] [PubMed] [Google Scholar]
- Nash HA, Campbell DB, Krishnan KS. New mutants of Drosophila that are resistant to the anesthetic effects of halothane. Ann N Y Acad Sci. 1991;625:540–544. doi: 10.1111/j.1749-6632.1991.tb33885.x. [DOI] [PubMed] [Google Scholar]
- Navarro J, del Solar E. Pattern of spatial distribution in Drosophila melanogaster. Behav Genet. 1975;5:9–16. doi: 10.1007/BF01067577. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman JL, DasGupta S, Krashes MJ, Leung B, Perrat PN, Waddell S. There are many ways to train a fly. Fly (Austin) 2009;3:3–9. doi: 10.4161/fly.3.1.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopy RJ, Duan JJ. Socially Facilitated Egglaying Behavior in Mediterranean Fruit Flies. Behavioral Ecology and Sociobiology. 1998;42:117–122. [Google Scholar]
- Rajaram S, Nash HA. A specific alteration in the electroretinogram of Drosophila melanogaster is induced by halothane and other volatile general anesthetics. Anesth Analg. 2004;98:1705–1711. doi: 10.1213/01.ANE.0000113548.27457.A3. table of contents. [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Rohlfs M, Hoffmeister TS. Spatial aggregation across ephemeral resource patches in insect communities: an adaptive response to natural enemies? Oecologia. 2004;140:654–661. doi: 10.1007/s00442-004-1629-9. [DOI] [PubMed] [Google Scholar]
- Saltz JB, Foley BR. Natural genetic variation in social niche construction: social effects of aggression drive disruptive sexual selection in Drosophila melanogaster. Am Nat. 2011;177:645–654. doi: 10.1086/659631. [DOI] [PubMed] [Google Scholar]
- Sarin S, Dukas R. Social learning about egg-laying substrates in fruitflies. Proc Biol Sci. 2009;276:4323–4328. doi: 10.1098/rspb.2009.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Dickinson MH, Levine JD. Social structures depend on innate determinants and chemosensory processing in Drosophila. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1121252109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly TE. Lek behaviour of a Hawaiian Drosophila: male spacing, aggression and female visitation. Animal Behaviour. 1987;35:1394–1404. [Google Scholar]
- Simon AF, Chou MT, Salazar ED, Nicholson T, Saini N, Metchev S, Krantz DE. A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav. 2012;11:243–252. doi: 10.1111/j.1601-183X.2011.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning and memory. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- Soibam B, Mann M, Liu L, Tran J, Lobaina M, Kang YY, Gunaratne GH, Pletcher S, Roman G. Open-field arena boundary is a primary object of exploration for Drosophila. Brain Behav. 2012;2:97–108. doi: 10.1002/brb3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps JA. The Effect of Conspecifics on Habitat Selection in Territorial Species. Behavioral Ecology and Sociobiology. 1991;28:29–36. [Google Scholar]
- Strauss R. Altered spatio-temporal orientation and course control in walking Drosophila mutants with structural central-complex defects in the brain. J Neurogenet. 1999;13:71. [Google Scholar]
- Tinbergen N. The Study of Instinct. 2. Oxford University Press; London: 1951. [Google Scholar]
- Tinette S, Zhang L, Robichon A. Cooperation between Drosophila flies in searching behavior. Genes Brain Behav. 2004;3:39–50. doi: 10.1046/j.1601-183x.2003.0046.x. [DOI] [PubMed] [Google Scholar]
- Valente D, Golani I, Mitra PP. Analysis of the trajectory of Drosophila melanogaster in a circular open field arena. PLoS One. 2007;2:e1083. doi: 10.1371/journal.pone.0001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen B. A succession of anesthetic endpoints in the Drosophila brain. J Neurobiol. 2006;66:1195–1211. doi: 10.1002/neu.20300. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal States in Drosophila. Curr Biol. 2004;14:81–87. [PubMed] [Google Scholar]
- Villella A, Hall JC. Neurogenetics of courtship and mating in Drosophila. Adv Genet. 2008;62:67–184. doi: 10.1016/S0065-2660(08)00603-2. [DOI] [PubMed] [Google Scholar]
- Waddell S, Quinn WG. What can we teach Drosophila? What can they teach us? Trends Genet. 2001;17:719–726. doi: 10.1016/s0168-9525(01)02526-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim B, Allemand R, Vet LEM, Dicke M. Effects of aggregation pheromone on individual behaviour and food web interactions: a field study on Drosophila. Ecological Entomology. 2006;31:216–226. [Google Scholar]
- Xiong Y, Lv H, Gong Z, Liu L. Fixation and locomotor activity are impaired by inducing tetanus toxin expression in adult Drosophila brain. Fly (Austin) 2010:4. doi: 10.4161/fly.12668. [DOI] [PubMed] [Google Scholar]
- Zars T. Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol. 2000;10:790–795. doi: 10.1016/s0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Zentall TR. Imitation: definitions, evidence, and mechanisms. Anim Cogn. 2006;9:335–353. doi: 10.1007/s10071-006-0039-2. [DOI] [PubMed] [Google Scholar]