Abstract
Background Information
Interleukin 1β is a major pro-inflammatory cytokine that plays a crucial role in the regulation of inflammation and wound healing in the cornea. Elucidation of Interleukin1β signaling may help identify therapeutic targets for corneal wound healing; however, mechanisms such as cell migration, a component of Interleukin 1β induced wound healing response in human corneal endothelial cells, have not been well characterized.
Results
Stimulation of human corneal endothelial cells with Interleukin 1β activated expression of fibroblast growth factor 2 and resulted in enhanced cell migration. This, in turn, was abolished by treatment with either Interleukin-1 receptor antagonist or SU-5402, a pan fibroblast growth factor signaling inhibitor. Phosphatidyl inositol 3-kinase or interleukin receptor-associated kinase 1/4 antagonists demonstrated that interleukin receptor-associated kinase 1/4 activates phosphatidyl inositol 3-kinase, which in turn phosphorylates p38 and inhibitor κB kinase α/β, leading to fibroblast growth factor 2 expression through activation of activator protein 1 and nuclear factor kappa-light-chain-enhancer of activated B cells in human corneal endothelial cells. Treatment of interleukin 1β stimulated human corneal endothelial cells with either activator protein 1 or nuclear factor kappa-light-chain-enhancer of activated B cells antagonists decreased fibroblast growth factor 2 expression and resulted in reduced interleukin 1β enhanced cell migration. Co-treatment of interleukin 1β stimulated human corneal endothelial cells with both inhibitors completely blocked fibroblast growth factor 2 expression and interleukin 1β enhanced cell migration. Chromatin immunoprecipitation assays demonstrated that activator protein 1 and nuclear factor kappa-light-chain-enhancer of activated B cells directly bind to the fibroblast growth factor 2 promoter following interleukin 1β stimulation.
Conclusion
The results show that binding of interleukin 1β to its receptor in human corneal endothelial cells leads to parallel activation of activator protein 1 and nuclear factor kappa-light-chain-enhancer of activated B cells pathways, leading, in turn, to fibroblast growth factor 2 expression and enhanced cell migration.
Keywords: cell migration, humans, inflammation
Introduction
The cornea, the anterior-most tissue of the eye, is the only tissue in the human body that is completely transparent. Maintenance of corneal transparency, critical for clear vision, depends on the coordinated function of the epithelium, stroma and endothelium. Corneal endothelial cells (CEC) regulate tissue hydration by pumping fluid from the corneal stroma into the anterior chamber of the eye. Dysfunction of the corneal endothelial pump leads to corneal edema, with a subsequent decrease in transparency that results in vision loss. A distinctive feature of adult human CEC in vivo is that they are arrested in the G1 phase of the cell cycle (Joyce et al., 1996; Senoo and Joyce, 2000); however, they can be induced to undergo endothelial-mesenchymal transition (EMT) in response to severe inflammation or injury. Human CEC that undergo EMT show enhanced migration, proliferation and secretion of collagen type I, resulting in the formation of retrocorneal fibrous membranes (Waring, 1982; Chiou et al., 1998; Leung et al., 2000). Our previous studies using rabbit CEC demonstrated that fibroblast growth factor 2 (FGF2) is the direct mediator for such EMT. FGF2 signaling directly regulates cell cycle progression through degradation of p27Kip1 mediated by phosphatidyl inositol (PI) 3-kinase activation (Lee and Kay, 2007, 2011), facilitates synthesis and secretion of type I collagen into the extracellular space (Ko and Kay, 2005), and induces morphological change and migration through regulation of the Rho family of small GTPases (Lee and Kay, 2006a, 2006b). In human CEC, FGF2 treatment also stimulated cell proliferation through the PI 3-kinase - ERK1/2 pathway leading to phosphorylation of p27 in vitro (Lee et al., 2011). Although the formation of a retrocorneal fibrous membrane represents an end-stage ocular pathology in which lasting restoration of vision is no longer possible, some features of EMT, such as enhanced cell migration and proliferation, might be beneficial if they could be modulated.
Interleukin-1β (IL-1β) is a major mediator of corneal inflammation and wound healing (Moore et al., 2002; Djalilian et al., 2006). Binding of IL-1β to its receptor in cell types such as synovial fibroblasts (Yang et al., 2010) and periodontal ligament cells (Dudás et al., 2011; Tang et al., 2011) results in the formation of receptor-associated complexes, including myeloid differentiation primary response protein 88, interleukin receptor-associated kinase (IRAK) 1, IRAK4, and tumor necrosis factor receptor-associated factor (TRAF) 6 (Neumann et al., 2002; Yamazaki et al., 2009). This, in turn, results in the activation of both activator protein 1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), leading to transcriptional activation of various downstream targets, including FGF2 (Qian et al., 2001; Yang et al., 2010; Murayama et al., 2011; Lee and Kay 2012). Our previous studies reported the role of NF-κB in IL-1β induced FGF2 production in rabbit CEC (Lee and Kay, 2009, 2012). IRAK and TRAF6, temporally expressed by IL-1β stimulation, activate their downstream effectors of the canonical NF-κB pathway through PI 3-kinase. Activation of PI 3-kinase signaling involves phosphorylation of inhibitor κB (IκB) kinase (IKK) a/β, leading to degradation of IκB and activation of NF-κB. Activated NF-κB works as the transcription factor for the FGF2 gene by directly binding to its promoter. IL-1β has been shown to induce cell migration by activating AP-1 through p38 and the c-Jun N-terminal kinase pathway to activate expression of migration-related genes such as metalloproteinase-1, 9 and 13 (Lin et al., 2009; Kook et al., 2011; Lim and Kim, 2011). We also previously showed that p38 is the downstream effector molecule in IL-1β stimulated activation of PI 3-kinase pathway in rabbit CEC, both in vitro and ex vivo (Lee and Kay, 2009; Song et al., 2010).
Prior to our study, the effects of inhibiting various components of IL-1β signaling on migration of human CEC were not known. Herein, we present evidence showing that IL-1β mediated migration of human CEC is dependent on FGF2 signaling: IL-1β binding to its receptor recruits IRAK to activate PI 3-kinase, which subsequently leads to parallel activation of AP-1 and NF-κB, leading to FGF2 expression and enhanced cell migration. We further show that both AP-1 and NF-κB bind directly to the FGF2 promoter in human CEC. Activation of both AP-1 and NF-κB and their binding to the FGF2 promoter following IL-1β stimulation are critical for FGF2 expression and enhanced cell migration in human CEC.
Results
Effect of IL-1β on expression of FGF2
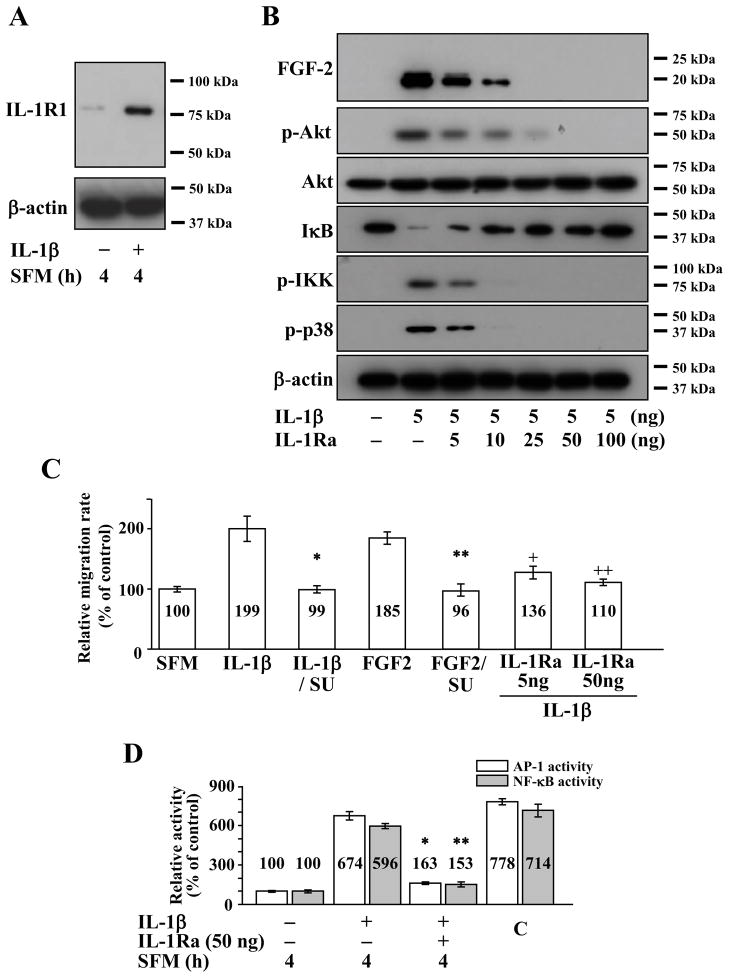
In our previous study (Lee and Kay, 2009), we reported that IL-1β stimulation of rabbit CEC resulted in activation of FGF2 expression, and treatment with FGF2 resulted in enhanced cell migration. To investigate the kinetics of cell migration following IL-1β stimulation in human CEC, we examined whether IL-1β receptor was expressed in human CEC under the same experimental conditions that we used for rabbit CEC in previous studies. In human CEC, a low level of constitutive interleukin 1 receptor type 1 (IL-1R1) expression could be observed, and its expression could be enhanced by IL-1β stimulation (Figure 1A). We previously reported on the roles of PI 3-kinase, p38 and NF-κB in IL-1β induced FGF2 expression in rabbit CEC (Lee and Kay, 2009, 2012), and in this study, we tested roles of the same candidates in human CEC. IL-1β stimulation resulted in activation of FGF2 expression in human CEC, and this effect could be decreased in a dose-dependent manner by treatment with IL-1 receptor antagonist (IL-1Ra) (Figure 1B). Treatment with 25 ng of IL-1Ra could completely inhibit IL-1β induced expression of FGF2 in human CEC (Figure 1B). Treatment with IL-1β dramatically increased phosphorylation of Akt, p38, and IKK with decreased level of IκB; and these effects could be inhibited in a dose-dependent manner by treatment with IL-1ra (Figure 1B). Correspondingly, migration of human CEC was dramatically enhanced following treatment with either IL-1β or FGF2, and this could be completely abolished by co-treatment with SU5402, a pan FGF antagonist, or IL-1Ra (Figure 1C). Because p38 works as an upstream regulator for AP-1 (Lin et al., 2009; Kook et al., 2011; Zhang and Bowden, 2012) and because activation of NF-κB is mediated by IKK phosphorylation (Kim et al., 2006; Hayden and Ghosh, 2008) in various cell types, we used ELISA to investigate whether IL-1β stimulation results in AP-1 and NF-κB activation in human CEC. Both AP-1 and NF-κB activities were greatly induced by IL-1β, and co-treatment with IL-1Ra nearly extinguished the IL-1β dependent activation of AP-1 and NF-κB in human CEC (Figure 1D).
Figure 1.
Effect of interleukin 1β (IL-1β) stimulation on human corneal endothelial cells (CEC). (A) Treatment with IL-1β resulted in increased expression of interleukin 1 receptor 1 (IL-1R1). (B) Treatment of human CEC with IL-1β resulted in expression of fibroblast growth factor-2 (FGF2); phosphorylation of Akt (p-Akt), inhibitor κB kinase (p-IKK) and p38 (p-p38); and decrease in inhibitor κB (IκB) levels. This effect could be attenuated in a dose-dependent manner using interleukin 1 receptor antagonist (IL-1Ra). (C) Treatment of human CEC with IL-1β or FGF2 resulted in enhanced cell migration as measured using scratch-induced directional migration assay. Co-treatment with SU5402, a pan FGF signaling inhibitor, abolished the IL-1β (* p = 0.005) and FGF2 (** p = 0.002) induced migration in human CEC. Co-treatment of human CEC with IL-1Ra also abolished the IL-1β induced migration (+ p = 0.2, ++ p < 0.001). Data represent the mean ± S.D. of three independent experiments. (D) Treatment of human CEC with IL-1β resulted in activation of activator protein-1 (AP-1) and nuclear factor κB (NF-κB). Activation of AP-1 and NF-κB by IL-1β could be blocked with IL-1Ra in human CEC (* p < 0.001, ** p < 0.001). SFM, serum free media; SU, SU5402; C, control.
Roles of IRAK and PI 3-kinase in AP-1 and NF-κB activation and FGF2 expression
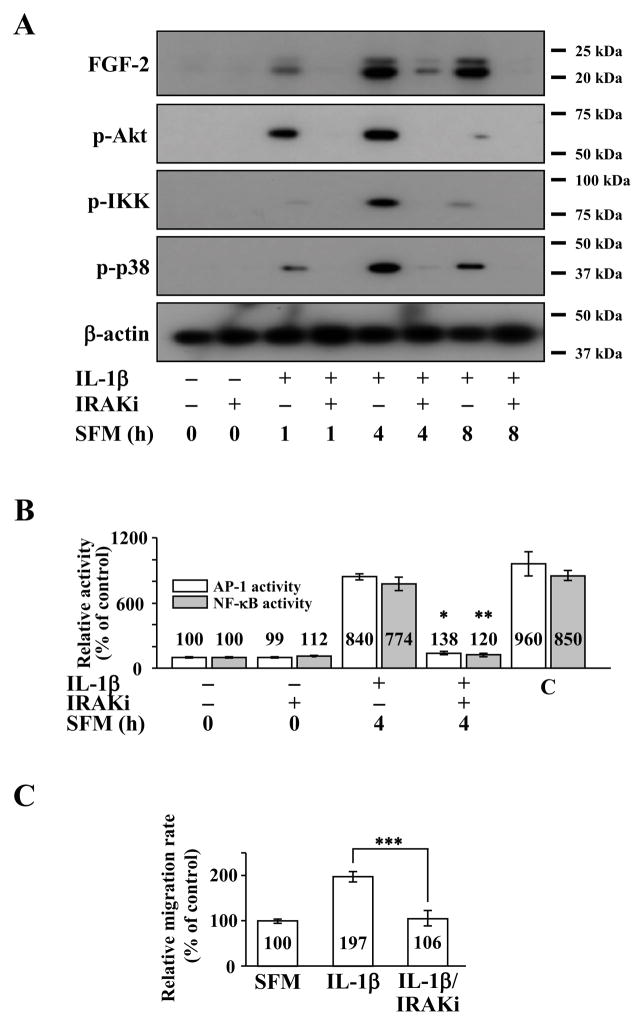
Because IL-1R1 signal transduction depends on the downstream activation of IRAK and TRAF6 (Neumann et al., 2002; Yamazaki et al., 2009), we investigated their roles in the IL-1β signaling human CEC using IRAK 1/4 inhibitor. Pretreatment of human CEC with IRAK 1/4 inhibitor prior to IL-1β stimulation resulted in decreased FGF2 expression and phosphorylation of Akt, IKK, and p38, with the maximum response being observed at 4 hours post IL-1β stimulation (Figure 2A). Pretreatment with IRAK 1/4 inhibitor also resulted in decreased AP-1 and NF-κB activities in human CEC (Figure 2B). Similar to SU5402 and IL-1Ra, IRAK 1/4 inhibitor was also able to abolish IL-1β enhanced cell migration in human CEC (Figure 2C).
Figure 2.
Effect of interleukin receptor-associated kinase (IRAK) 1/4 inhibition on interleukin 1β (IL-1β)-stimulated human corneal endothelial cells (CEC). (A) Fibroblast growth factor-2 (FGF2) starved human CEC were pretreated with IRAK 1/4 inhibitor (IRAKi) for 2 h and then treated with IL-1β for 10 min. The IL-1β ± IRAKi treated human CEC were maintained in serum-free medium (SFM) for indicated times. Pretreatment with IRAKi blocked IL-1β dependent phosphorylation of Akt (p-Akt), inhibitor κB kinase(p-IKK), and p38 (p-p38). (B) Nuclear fractions were prepared from FGF2-starved human CEC pretreated with IRAKi for 2 h during FGF2 starvation before stimulation with IL-1β for 10 min followed by 4 h incubation in SFM. Lamin B was used to normalize the nuclear protein concentration in activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) activity assays (data not shown). Pretreatment with IRAKi blocked both AP-1 and NF-κB activities in IL-1β stimulated human CEC. Data represent the mean ± S.D. of three independent experiments (* p < 0.001, ** p < 0.001). (C) Treatment with IRAKi blocked the IL-1β dependent migration of human CEC (*** p < 0.001). Data represent the mean ± S.D. of three independent experiments. IRAKi, IRAK 1/4 inhibitor; C, positive control.
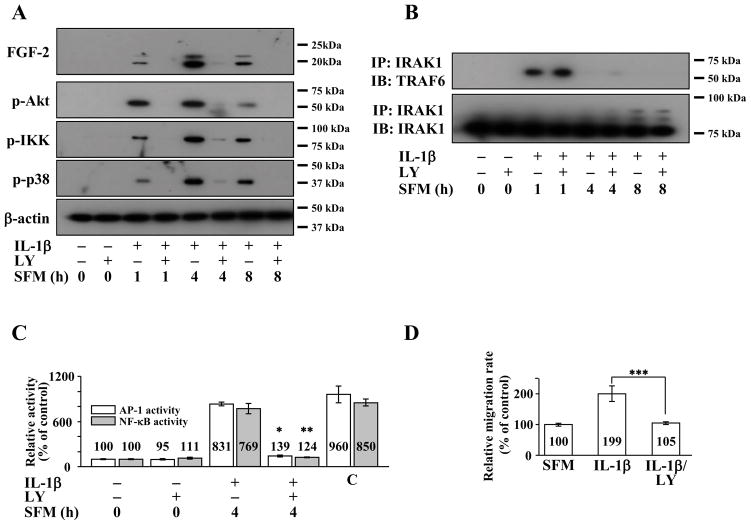
We previously reported on the role of PI 3-kinase in IL-1β mediated FGF2 expression and cell migration in rabbit CEC (Lee and Kay, 2009). We therefore investigated the role of PI 3-kinase in IL-1β-induced FGF2 expression and cell migration in human CEC using LY294002, a PI 3-kinase inhibitor. Pretreatment with LY294002 completely inhibited FGF2 expression and phosphorylation of Akt, IKK, and p38 in human CEC stimulated with IL-1β (Figure 3A). The similar inhibition profiles of IRAK 1/4 inhibitor and LY294002 led us to explore whether IRAK and PI 3-kinase were acting in parallel or in sequence in IL-1β-induced FGF2 expression and cell migration in human CEC. In human CEC stimulated with IL-1β, IRAK1 could be co-immunoprecipitated with TRAF6 and vice versa, and pretreatment with LY294002 did not interfere with IL-1β mediated IRAK1 - TRAF6 interaction (Figure 3B). Of note, pretreatment with IRAK 1/4 inhibitor led to decreased levels of Akt phosphorylation (Figure 2A). Pretreatment of human CEC with LY294002 prior to IL-1β stimulation also resulted in decreased AP-1 and NF-κB activities (Figure 3C). Similar to SU5402, IL-1Ra and IRAK1/4 inhibitor, LY294002 was also able to abolish IL-1β enhanced cell migration in human CEC (Figure 3D).
Figure 3.
Effect of phosphatidyl inositol (PI) 3-kinase inhibition on interleukin-1β (IL-1β) stimulated human corneal endothelial cells (CEC). (A) Cell lysates were prepared from fibroblast growth factor-2 (FGF2) starved cells pretreated with PI 3-kinase inhibitor LY294002 for 2 h and then treated with IL-1β for 10 min followed by incubation in serum-free medium (SFM) for indicated times. Pretreatment with LY294002 resulted in decreased FGF2 expression along with decreased phosphorylation of Akt (p-Akt), inhibitor κB kinase (p-IKK), and p38 (p-p38) in IL-1β stimulated human CEC. (B) Cell lysates were prepared from human CEC treated as described in (A). Pretreatment with LY294002 did not interfere with interleukin receptor-associated kinase (IRAK) 1 and tumor necrosis factor receptor associated factor (TRAF) 6 interaction in IL-1β stimulated human CEC. (C) Nuclear fractions were prepared from FGF2-starved human CEC pretreated LY294002 for 2 h during FGF2 starvation before stimulation with IL-1β for 10 min followed by 4 h incubation in SFM. Lamin B was used to normalize the nuclear protein concentration in activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) activity assays (data not shown). Pretreatment with LY294002 blocked both AP-1 and NF-κB activities in IL-1β stimulated human CEC. Data represent the mean ± S.D. of three independent experiments (* p < 0.001, ** p < 0.001). (D) Pretreatment with LY294002 abolished the IL-1β induced migration in human CEC (*** p = 0.005). Data represent the mean ± S.D. of three independent experiments. IP, immunoprecipitation; IB, immunoblotting; LY, LY294002; C, positive control.
Cell migration facilitated by IL-1β-induced FGF2 via parallel and independent activation of AP-1 and NF-κB
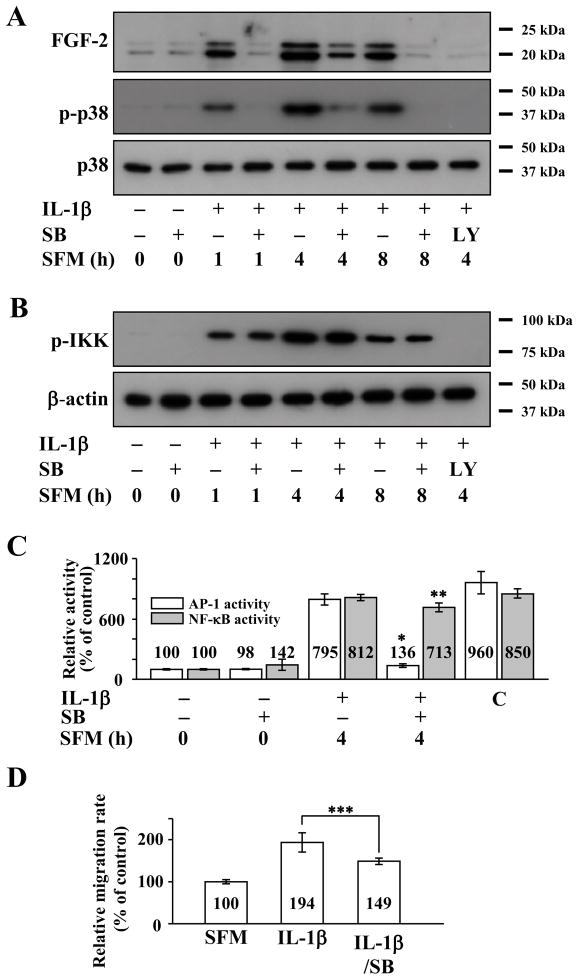
Because p38, an upstream activator of AP-1, is a major signaling molecule for cell migration in various cell types including rabbit CEC (Sharma et al., 2003; Frey et al., 2006; Shahabuddin et al., 2006; Lee and Kay, 2009), we examined whether p38 participates in IL-1β enhanced cell migration through FGF2 expression in human CEC using SB203580, a p38 inhibitor. Pretreatment of human CEC with SB203580 decreased, but did not abolish, IL-1β-induced FGF2 expression even though phosphorylation of p38 was severely inhibited (Figure 4A). SB203580 pretreatment had no effect on IKK phosphorylation (Figure 4B). Congruently, SB203580 was able to abolish IL-1β induced AP-1 activity but had no effect on NF-κB activity (Figure 4C). SB203580 was able to attenuate, but not completely inhibit, IL-1β enhanced cell migration in human CEC (Figure 4D).
Figure 4.
Effect of activator protein-1 (AP-1) inhibition on interleukin-1β (IL-1β) stimulated human corneal endothelial cells (CEC). (A) Cell lysates prepared from FGF2-starved cells pretreated with SB203580, an AP-1 inhibitor, for 2 h and then treated with IL-1β for 10 min followed by incubation in serum-free medium (SFM) for indicated times. Pretreatment with SB203580 resulted in decreased fibroblast growth factor-2 (FGF2) expression along with decreased phosphorylation of p38 (p-p38) in IL-1β stimulated human CEC. (B) Pretreatment with SB203580 had no effect on inhibitor κB kinase phosphorylation (p-IKK) in IL-1β stimulated human CEC. (C) Nuclear fractions were prepared from FGF2-starved human CEC that were pretreated with SB203580 for 2 hours during FGF2 starvation, followed by stimulation with IL-1B for 10 min and then incubated with SFM for 4 h. Lamin B was used to normalize the nuclear protein concentration in AP-1 and nuclear factor-κB (NF-κB) activity assays (data not shown). Pretreatment with SB203580 blocked AP-1 but not NF-κB activities in IL-1β stimulated human CEC. Data represent the mean ± S.D. of three independent experiments (* p < 0.001, ** p = 0.264). (D) Pretreatment with SB203580 decreased but did not completely block IL-1β enhanced migration in human CEC (*** p = 0.03). Data represent the mean ± S.D. of three independent experiments. SB, SB203580; LY, LY294002; C, positive control.
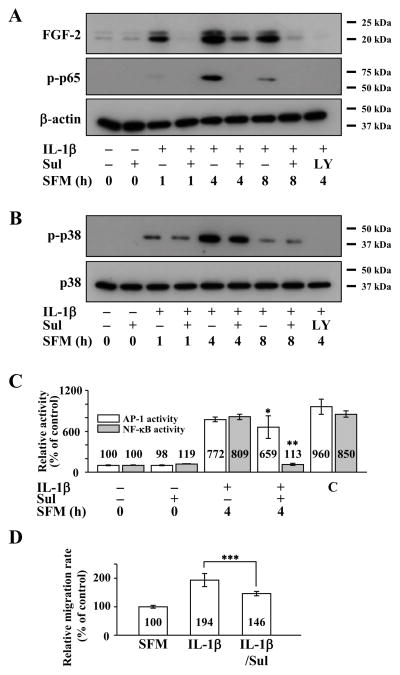
It has previously been reported that IL-1β activates NF-κB (Qian et al., 2001; Murayama et al., 2011) and IL-1β induced FGF2 expression in rabbit CEC is mediated through NF-κB (Lee and Kay, 2012). We therefore examined whether NF-κB also has a role in IL-1β-induced FGF2 expression and migration in human CEC using sulfasalazine, a NF-κB inhibitor. Pretreatment of human CEC with sulfasalazine decreased, but did not completely block, FGF2 expression, and it also completely blocked phosphorylation of p65 (Figure 5A). Sulfasalazine had no effect on phosphorylation of p38 (Figure 5B). Sulfasalazine also inhibited NF-κB but not AP-1 activity in IL-1β stimulated human CEC (Figure 5C). Treatment with sulfasalazine attenuated, but did not completely abolish, enhancement of cell migration in IL-1β stimulated human CEC (Figure 5D).
Figure 5.
Effect of nuclear factor -κB (NF-κB) inhibition on interleukin 1β (IL-1β) stimulated human corneal endothelial cells (CEC). (A) Cell lysates prepared from serum-starved cells pretreated with sulfasalazine, a NF-κB inhibitor, for 2 h and then treated with IL-1β for 10 min followed by incubation in serum-free medium (SFM) for indicated times. Pretreatment with sulfasalazine resulted in decreased FGF2 expression along with decreased phosphorylation of p65 (p-p65) in IL-1β stimulated human CEC. (B) Pretreatment with sulfasalazine had no effect on p38 phosphorylation (p-p38) following IL-1β stimulation in human CEC. (C) Nuclear fractions were prepared from FGF2-starved human CEC that were pretreated with sulfasalazine for 2 hours during FGF2 starvation, followed by stimulation with IL-1B for 10 min and then incubated with SFM for 4 h.Lamin B was used to normalize the nuclear protein concentration in activator protein-1 (AP-1) and NF-κB activity assays (data not shown). Pretreatment with sulfasalazine blocked NF-κB but not AP-1 activities in IL-1β stimulated human CEC. Data represent the mean ± S.D. of three independent experiments (* p = 0.31, ** p < 0.001). (D) Pretreatment with sulfasalazine decreased but did not completely block IL-1β enhanced migration in human CEC (*** p = 0.054). Data represent the mean ± S.D. of three independent experiments. Sul, sulfasalazine; C, positive control.
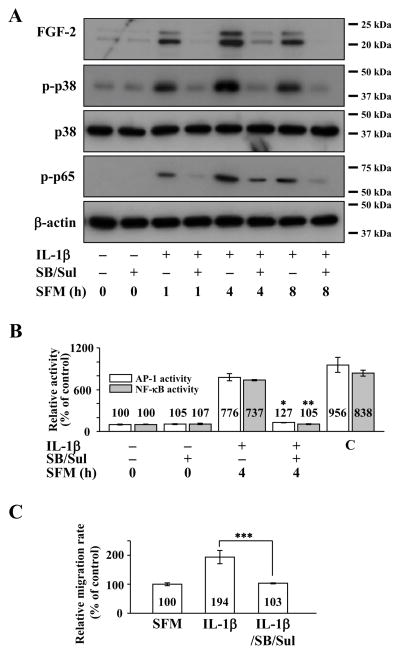
Co-treatment of human CEC with SB203580 and sulfasalazine resulted in severely decreased FGF2 expression, more so over single treatment, along with inhibition of p38 and p65 phosphorylation (Figure 6A). It also resulted in simultaneous inhibition of AP-1 and NF-κB activities in IL-1β stimulated human CEC (Figure 6B). Simultaneous use of both inhibitors also abolished IL-1β enhanced migration in human CEC (Figure 6C).
Figure 6.
Effect of simultaneous activator protein-1 (AP-1) and nuclear factor -κB (NF-B) inhibition on interleukin 1β(IL-1β) stimulated human corneal endothelial cells (CEC).(A) Cell lysates prepared from FGF2-starved cells were pretreated with SB203580 and sulfasalazine for 2 h and then treated with IL-1β for 10 min followed by incubation in serum-free medium (SFM) for indicated times. Pretreatment with SB203580 and sulfasalazine resulted in severely decreased fibroblast growth factor 2 (FGF2) expression along with decreased phosphorylation of p38 (p-p38) and p65 (p-p65) in IL-1β stimulated human CEC. (B) Nuclear fractions were prepared from FGF2-starved human CEC that were pretreated with SB203580 and sulfasalazine for 2 hours during FGF2 starvation, followed by stimulation with IL-1B for 10 min and then incubated with SFM for 4 h. Lamin B was used to normalize the nuclear protein concentration in activator protein (AP-1) and nuclear factor-κB (NF-κB) activity assays (data not shown).Pretreatment with SB203580 and sulfasalazine blocked both AP-1 and NF-κB activities in human CEC that were stimulated by IL-1β (* p < 0.001, ** p < 0.001). Data represent the mean ± S.D. of three independent experiments. (C) Pretreatment with SB203580 and sulfasalazine completely blocked the IL-1β enhanced migration in human CEC (*** p = 0.001). Data represent the mean ± S.D. of three independent experiments. C, positive control; SB, SB203580; Sul, sulfasalazine.
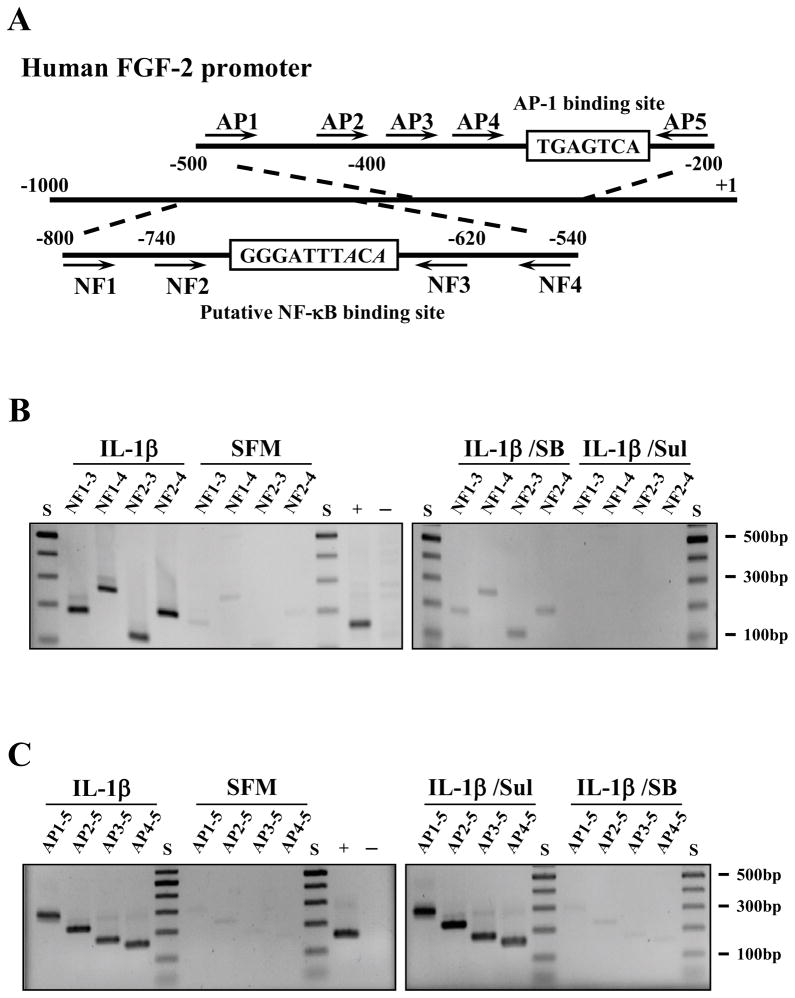
We reported previously that FGF2 is a NF-κB target gene of NF-κB in human retinal pigment epithelial cells (Lee and Kay, 2012). In the human FGF2 promoter sequence, there also is a potential AP-1 binding site within the core promoter region (Shibata et al., 1991). To determine whether FGF2 is a direct target of NF-κB and/or AP-1 in human CEC, we performed chromatin immunoprecipitation (ChIP) assays using anti-NF-κB and AP-1 antibodies. The polymerase chain reaction primers used to detect NF-κB and AP-1 binding are shown in figure 7A. Human CEC stimulated with IL-1β showed the expected PCR products of 120, 180, 200, and 260 base pairs in the ChIP assay using an anti-NF-κB antibody (Figure 7B). A similar pattern could be seen in IL-1β stimulated human CEC treated with SB203580; but NF-κB binding to DNA could be abolished by treating IL-1β stimulated human CEC with sulfasalazine (Figure 7B). Likewise, human CEC stimulated with IL-1β showed the expected PCR products of 156, 180, 234, and 282 base pairs in the ChIP assay using an anti-AP-1 antibody (Figure 7C). A similar pattern could be seen in IL-1β stimulated human CEC treated with sulfasalazine; but AP-1 binding to DNA could be abolished by treating IL-1β stimulated human CEC with SB203580 (Figure 7C).
Figure 7.
Binding of activator protein-1 (AP-1) and nuclear factor -κB (NF-κB) to the FGF2 promoter in human corneal endothelial cells (CEC). (A) Schematic illustration of putative AP-1 and NF-κB binding sites in the human fibroblast growth factor-2 (FGF2) promoter. The DNA sequence that is different from conserved NF-κB binding site (GGGATTTCCC) is italicized. The location of each primer used in ChIP assays is indicated.(B) FGF2-starved human CEC were pretreated with either SB203580 or sulfasalazine for 2 h and then stimulated with IL-1β for 10 min, followed by incubation in serum-free medium (SFM) for 4 h. At the end of treatment, cross-linked cell lysates were treated with nuclease and immunoprecipitated with an anti-NF-κB antibody. The purified DNA samples were subjected to PCR using the shown primer sets. The expected PCR product sizes are: NF1-NF3, 180 bp; NF1-NF4, 260 bp; NF2-NF3, 120 bp; NF2-NF4, 200 bp. Simulation with interleukin-1β (IL-1β) resulted in NF-κB binding to the FGF2 promoter in human CEC. Pretreatment with sulfasalazine inhibited NF-κB binding to the promoter while SB203580 pretreatment had no effect on NF-κB binding. (C) The cross-linked cell lysates from SB203580 pretreated human CEC were treated with nuclease and immunoprecipitated with an anti-AP-1 antibody. The purified DNA samples were subjected to PCR using the shown primer sets. The expected PCR product sizes are: AP1-AP5, 282 bp; AP2-AP5, 234 bp; AP3-AP5, 180 bp; AP4-AP5, 156 bp. IL-1β stimulation resulted in AP-1 binding to the FGF2 promoter in human CEC. Pretreatment with SB203580 inhibited AP-1 binding to the promoter while sulfasalazine pretreatment had no effect on AP-1 binding. Anti-histone H3 antibody was used for positive control (+) and normal rabbit IgG was used for negative control (-) in the ChIP assays. SB, SB203580; Sul, sulfasalazine.
Discussion
The corneal endothelium plays a critical role in maintaining corneal transparency by regulating corneal hydration and facilitating the passage of nutrients (Kreutziger, 1976; Geroski and Edelhauser 1984). Adult human CEC are arrested at the G1 phase of the cell cycle in vivo, and except in severe cases of inflammation, they do not reenter the cell cycle even in response to various insults such as injury or infection (Joyce et al., 1996; Senoo and Joyce, 2000). Loss of CEC due to aging, infection, or injury leads to a decrease in endothelial cell density, and a decrease below approximately 500 cells/mm2 leads to corneal edema and subsequent vision loss. Despite the progressive decrease in cell density, human CEC have the ability to maintain an intact endothelial monolayer, even in light of their state of cell cycle arrest, which is critical for maintenance of the endothelial pump function. When human CEC are injured, wound healing occurs through migration, enlargement, and spreading of existing cells, resulting in polymegethism and pleomorphism of CEC (Van Horn et al., 1977; Matsuda et al., 1985). Vision loss secondary to endothelial dysfunction is a common indication for corneal transplantation in developed countries. The current surgical standard of care for endothelial vision loss is Descemet’s stripping endothelial keratoplasty (DSEK), where the dysfunctional endothelium and Descemet’s membrane from the host are replaced by a posterior corneal lenticule from a donor (Melles et al., 1999). Compared with penetrating keratoplasty, DSEK offers faster visual rehabilitation and an improved safety profile with decreased risk of graft rejection (Price and Price, 2007; Kuo et al., 2008). Graft dislocation associated with severe endothelial cell loss and poor wound healing following DSEK have been reported (Cheng et al., 2007; Prakash et al., 2007). Investigating mechanisms of human CEC wound healing, including cell migration, may provide strategies for delaying DSEK and decreasing the risk of DSEK graft dislocation in patients with endothelial dysfunction.
Cell migration induced by cytokines plays important roles in many physiologic and pathologic processes, such as organogenesis, neovascularization, inflammatory responses, and wound repair (Ridley et al., 2003; Kuwano et al., 2004; Nakao et al., 2007). Despite the wide-ranging roles of cytokines in cell migration, the molecular mechanisms underlying cytokine-induced cell migration in human CEC are poorly understood. In this study, we investigated the intracellular signaling pathway by which IL-1β induces FGF2 expression and examined the effects of targeting specific components of the IL-1β signaling on cell migration in human CEC. To minimize the effects of cell proliferation confounding cell migration results, the wound-healing assays were performed in the presence of mitomycin C.
Robust enhancement in cell migration could be elicited by treating human CEC with IL-1β or FGF2. The elimination of the effects of IL-1β and FGF2 on cell migration by SU5402 indicated FGF2 was the direct downstream mediator of IL-1β induced cell migration in human CEC. Expression of IL-1R1 and dose-dependent inhibition of IL-1β signaling by IL-1Ra showed that IL-1β signaling proceeded through the IL-1R1 in human CEC. The signaling pathway in human CEC utilized IRAK, PI 3-kinase, AP-1 and NF-κB as described in other model systems. Inhibition of AP-1 and NF-κB activities by IRAK 1/4 inhibitor and LY294002 showed that IRAK and PI 3-kinase were upstream of AP-1 and NF-κB. Inhibition of Akt phosphorylation by IRAK 1/4 inhibitor and the inability of LY294002 to inhibit IRAK1 - TRAF6 interaction suggested that there was a sequential activation of IRAK followed by PI 3-kinase activation in IL-1β signaling in human CEC. Activated IRAK1 is known to be degraded through ubiquitination (Yamin and Miller, 1997; Qian et al., 2001). The time-dependent decrease in IRAK1 and increase in its ubiquitinated form in LY294002-treated cells following IL-1β stimulation further shows that IL-1β induces IRAK1 activation prior to PI 3-kinase activation in human CEC (Figure 3B).
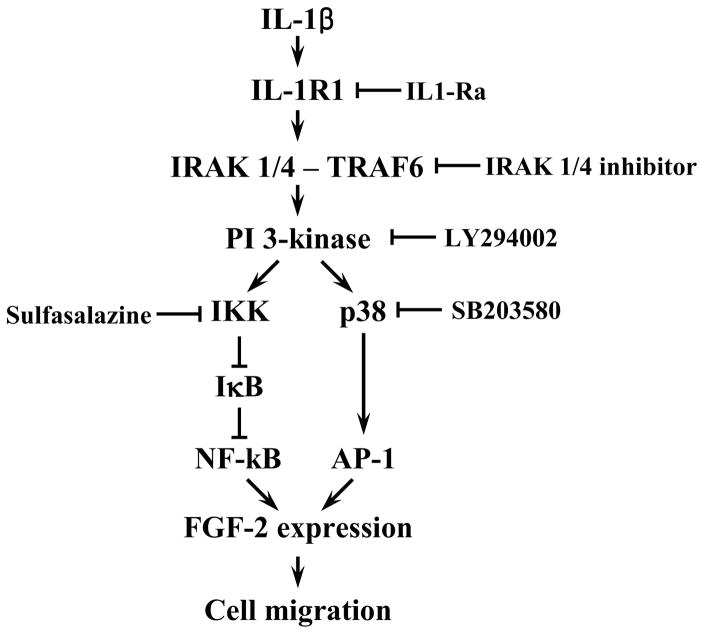
Attenuation but not complete elimination of IL-1β induced FGF2 expression and enhanced cell migration by sulfasalazine, a NF-κB inhibitor, and SB203580, an AP-1 inhibitor, showed that IL-1β binding to IL-1R1 led to a parallel activation of AP-1 and NF-κB in human CEC. There was a decrease in AP-1 activity, while NF-κB activity was preserved, along with a corresponding attenuation of FGF2 expression and cell migration in human CEC treated with SB203580. A similar pattern of observation was made in human CEC treated with sulfasalazine: NF-κB activity was inhibited while AP-1 activity was preserved along with a corresponding attenuation in FGF2 expression and cell migration. Simultaneous inhibition of AP-1 and NF-κB activities with the corresponding elimination of FGF2 expression and enhanced cell migration in IL-1β stimulated human CEC treated with sulfasalazine and SB203580 suggest that AP-1 and NF-κB are the main immediate downstream mediators of IL-1β signaling in human CEC. Involvement of AP-1 and NF-κB in these responses is consistent with previous reports indicating that p38 and NF-κB play important roles in the FGF2 expression induced by IL-1β in rabbit CEC (Lee and Kay, 2009, 2012). Cellular responses to stimulation with cytokines such as IL-1β are highly dependent on transcription factors such as AP-1 and NF-κB, and this has been reported in different cell types: promotion of matrix metalloproteinase-9 expression in A549 cells (Lin et al., 2009); induction of intercellular adhesion molecule-1 expression in synovial fibroblasts (Yang et al., 2010); and expression of brain expressed X-linked gene-2 in breast cancer cells (Naderi et al., 2010). Here we provide evidence of parallel AP-1 and NF-κB activation in human CEC. Lastly, ChIP showing that AP-1 and NF-κB bind directly to the FGF2 promoter, and data showing correlation between AP-1 and NF-κB activities with FGF2 expression, strongly support that AP-1 and NF-κB act as transcriptional activators for FGF2 expression in IL-1β stimulated human CEC. Taken together, our findings indicate that FGF2 expression, mediated by parallel activation of AP-1 and NF-κB, is the major downstream mediator of IL-1β induced cell migration in human CEC (Figure 8).
Figure 8.
Schematic presentation of the pathway for interleukin-1β (IL-1β) induced human corneal endothelial cell (CEC) migration that is mediated by fibroblast growth factor-2 (FGF2) expression dependent on parallel activator protein-1 (AP-1) and nuclear factor -κB (NF-κB) activation. Binding of IL-1β to interleukin 1 receptor 1 results in assembly of the canonical signaling components, including interleukin receptor-associated kinase (IRAK) 1/4 and tumor necrosis factor receptor associated factor (TRAF) 6, that in turn activates PI 3-kinase. This leads to parallel activation of NF-κB and AP-1 resulting in FGF2 expression and enhanced human CEC migration.
Elucidation of the regulatory mechanisms for cell migration in human CEC may lead to alternative treatment strategies for patients with corneal endothelial injury or infection. Moreover it may also offer a means to shorten the postoperative rehabilitation following DSEK in patients with endothelial dysfunction.
Materials and Methods
Materials
Anti-Akt, phospho-Akt (Ser473), IκB, phospho-IKKa/β, p38, phospho-p38 (Thr180/Tyr182) antibodies and anti-phospho-p65 (Ser236) antibody were purchased from Cell Signaling Technology (Danvers, MA). Sulfasalazine, SB203580 and IRAK1/4 inhibitor were obtained from Calbiochem (San Diego, CA). Anti-IL-1R1 antibody was purchased from Abgent (San Diego, CA). IL-1β, LY294002, anti-β-actin antibody and peroxidase conjugated secondary antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Recombinant human IL-1ra was obtained from R&D systems (Minneapolis, MN). SU5402 was purchased from Tocris Bioscience (Minneapolis, MN). Anti-FGF2 antibody was purchased from Upstate Biotechnology (Charlottesville, VA). Anti-lamin B1 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
Immortalized human CEC line hCEC-B4G12 (DSMZ, Braunschweig, Germany) was cultured as previously described (Valtink et al., 2007; Götze et al., 2008). Briefly, hCEC-B4G12 was cultured in human endothelial-serum-free medium (SFM) supplemented with 10 ng/ml human recombinant FGF2 without antibiotics (SFM-F). Cells were grown in a humidified atmosphere containing 5% CO2 at 37 °C. For subculture, confluent cultures were treated with 0.05% trypsin and 5 mM EDTA in phosphate-buffered saline (PBS) for 5 min. Cells were plated in 100-mm tissue culture dishes coated with 1 mg/ml chondroitin-6-sulfate and 10 mg/ml lamin at a concentration of 1 × 106 cells. Second passage human CEC maintained in SFM-F were used for all experiments. Medium was changed twice a week. In some experiments, pharmacologic inhibitors were used in the presence of IL-1β (5 ng/ml) stimulation: SU5402 (FGF receptor inhibitor: 20 μM), LY294002 (PI 3-kinase inhibitor: 20 μM), IRAK1/4 inhibitor (5 μM), SB203580 (p38 inhibitor: 20 μM) or sulfasalazine (inhibitor for IκB degradation: 2 mM).
Scratch-induced directional migration assay
Cell migration assay was performed using our published protocols with some modification (Lee and Kay, 2006a). Cells were plated in 24-well tissue culture dishes at a concentration of 4 × 104 cells and maintained in SFM-F until the cells reached greater than 90% confluency. After FGF2 starvation for 24 h, the tip of a micropipette was used to wound the cells, creating linear, cross-stripe scrapes, 2 mm apart. Cells were washed with PBS to remove floating cellular debris and re-fed for an additional 16 hours with either SFM (as a negative control) or experimental medium. Wound closure or cell migration was photographed when the scrape wound was introduced and at 16 h after wounding, using an inverted EVOS microscope equipped with a digital camera (Advanced Microscopy Group, Bothell, WA). The individual gaps were measured in each culture condition and at each time point using the SPOT program (Diagnostic Instruments, Inc., version 2.1.2). The residual gap between the migrating cells from the opposing wound edge was expressed as a percentage of migrating cells maintained with SFM. All experiments were conducted in the presence of 5 μg/ml of mitomycin-C to inhibit cell proliferation.
Protein preparation, protein assay, SDS PAGE, western blotting analysis, co-immunoprecipitation and nuclear fractionation
All details of methods and procedures have been presented previously (Lee and Kay, 2007, 2009, 2011; Lee et al., 2011; Lee and Kay, 2012). The following gel concentrations were used to separate proteins: 15% gel for FGF2, 12% gel for IκB, p38, p-p38, 10% gel for Akt, p-Akt, p-p65, lamin B and β-actin, 8% gel for p-IKK and IL-1R1.
NF-κB ELISA assay
The nuclear fractions from the cells maintained in each culture condition were used to measure the NF-κB activity. NF-κB ELISA assay was performed using NF-κB ELISA assay kit (Rockland Immunochemicals Inc., Gilbertsville, PA) according to previously described protocol (Lee and Kay, 2012). Briefly, the 50 μg of nuclear fraction was mixed with transcription factor binding buffer supplied by the manufacturer and then applied to each well of a 96-well plate covered with oligo-DNA fragment containing consensus NF-κB binding sequence. After incubation for 16 h at 4°C without agitation, the wells were washed 5 times with 200 μl PBS containing 0.05% Tween 20. After the final wash, 100 μl of diluted anti-NF-κB (p65) antibody (1:100) solution was added to each well except the blank wells and the plate was incubated for 1 h at room temperature without agitation. Each well was washed again using PBS and then incubated with 100 μl of diluted peroxidase conjugated secondary antibody (1:100) for 1 h at room temperature without agitation. Each well was then treated with chemiluminescence developing solution. After a 30-min incubation at room temperature with gentle agitation, protected from light, 100 μl of the stop solution was added to each well and absorbance was measured at a wavelength of 450 nm using a spectrophotometric plate reader (Benchmark Plus Microplate Spectrophotometer, Bio-Rad Laboratories, Inc., Hercules, CA). Cell extract of tumor necrosis factor-a-stimulated HeLa cell extract was also used as positive control for this assay.
AP-1/c-Jun ELISA assay
The nuclear fractions from the cells maintained in each culture condition were used to measure the AP-1 activity. AP-1 ELISA assay was performed using AP-1 activity assay kit (GeneCopoeia Inc., Rockville, MD) according to the manufacturer’s protocol. Briefly, the 50 μg of nuclear fraction was mixed with transcription factor binding buffer supplied by the manufacturer and then applied to the each well of 96-well plate covered with oligo-DNA fragment containing consensus AP-1 binding sequence. After incubation for 1 h at room temperature with gentle rocking, the wells were washed with 200 μl washing buffer supplied by manufacturer for 1 min with gentle rocking. After the final wash, 100 μl of diluted anti-AP-1 antibody (1:1000) solution was added to each well except the blank wells, and the plate was incubated for 1 h at room temperature with gentle rocking. Each well was washed 2 more times using washing buffer and then incubated with 100 μl of diluted peroxidase conjugated secondary antibody (1:1000) for 1 h at room temperature with gentle rocking. After two washes, each well was then treated with chemiluminescence developing solution. After a 30-min incubation at room temperature with gentle agitation, protected from light, 100 μl of the stop solution was added to each well and absorbance was measured at a wavelength of 450 nm using a spectrophotometric plate reader. Nuclear extract of MCF-7 cells was also used as positive control for this assay.
Chromatin immunoprecipitation assay
FGF2-starved human CEC were pre-treated with SB203580 or sulfasalazine for 2 h, maintained with or without IL-1β for 10 min, and then maintained in SFM for 16 h. After 16 h, 3 × 107 cells were washed with PBS and cross-linked in 1% (v/v) formaldehyde for 10 min at room temperature. Chromatin immunoprecipitation experiments were performed using the SimpleCHIP enzymatic ChIP kit (agarose bead) from Cell Signaling according to the manufacturer’s protocol. Briefly, nuclei were isolated by lysis of the cytoplasmic fraction, and chromatin was digested into fragments of 150–900 bp by micrococcal nuclease for 30 min at 37 °C, followed by ultrasonic disruption of the nuclear membrane using a standard microtip and a Branson Digital Sonifier (Danbury, CT) with four pulses and 70% amplitude. For immunoprecipitation, four aliquots (10 μg each: 7 × 106 cell equivalents) of sheared and cross-linked chromatin were incubated with 5 μg of the anti-p65 antibody, anti-c-Jun antibody, normal rabbit IgG or anti-histone H3 antibody at 4°C overnight, respectively. The normal rabbit IgG and anti-histone H3 antibody were used as negative and positive controls. After incubation with 30 μl of ChIP grade protein G-agarose beads for 2 h at 4 °C, antibody-DNA complexes were eluted from the beads and digested by 40 μg of proteinase K for 2 h at 65 °C, followed by spin column-based purification of the DNA. Transcription factor binding to FGF2 promoter was finally assessed by PCR using the following primers: for NF-κB forward, 5′-TAGGT-ACTCAATACATGCAA-3′ (NF1: −800) and 5′-GCTATAT-CCTACTGAAAATT-3′ (NF2: −740); for NF-κB reverse, 5′-GACCTGGCATTTGCCCTAGC-3′ (NF3: ′620) and 5′-AATTAGACGAC-GCAGAAAGA-3′ (NF4:−540); for AP-1 forward, 5′-CTCTCCTTTTGTTGGTAGACG-3’ (AP1:−471), 5′-AAGTTTATGCCCCACTTGTAC-3′ (AP2:−424); 5′-GCCTGCTCTGACACAGACTCT-3′ (AP3:−370) and 5′-CTTGGATTGCAACTTCTCTAC-3′ (AP4:−347); for AP-1 reverse, 5′-TTTAGGCTTTCTCCACACTGC-3′ (AP5:−211). PCR conditions were as follows: for NF-κB, 5 min at 94°C followed by 33 cycles of 30 sec at 94°C, 30 sec at 55°C, 30 sec at 72°C, and a final extension for 2 min at 72°C; for AP-1, 5 min at 94°C followed by 33 cycles of 30 secs at 94°C, 60 sec at 53°C, 30 sec at 72°C, and a final extension for 2 min at 72°C. PCR products were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining.
Acknowledgments
Funding: Baxter Foundation, Research to Prevent Blindness, and National Institutes of Health [EY03040, EY021485]
Abbreviations used
- AP-1
activator protein 1
- CEC
corneal endothelial cell
- FGF2
fibroblast growth factor 2
- IKK
inhibitor κB kinase
- IL-1β
interleukin 1β IL-1, IL-1R1, receptor 1
- IL-1Ra
IL-1 receptor antagonist
- IRAK
interleukin receptor-associated kinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PI
phosphatidyl inositol
References
- Cheng YYY, Pels E, Cleutjens J, van Suylen RJ, Hendrikse F, Nuijts RMMA. Corneal endothelial viability after femtosecond laser preparation of posterior lamellar discs for Descemet-stripping automated endothelial keratoplasty. Cornea. 2007;26:1118–1122. doi: 10.1097/ICO.0b013e31814531d1. [DOI] [PubMed] [Google Scholar]
- Chiou AG, Chang C, Kaufman SC, Ohta T, Maitchouk D, Beuerman RW, Kaufman HE. Characterization of fibrous retrocorneal membrane by confocal microscopy. Cornea. 1998;17:669–671. doi: 10.1097/00003226-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Djalilian AR, Nagineni CN, Mahesh SP, Smith JA, Nussenblatt RB, Hooks JJ. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea. 2006;25:709–714. doi: 10.1097/01.ico.0000208815.02120.90. [DOI] [PubMed] [Google Scholar]
- Dudás J, Fullár A, Bitsche M, Schartinger V, Kovalszky I, Sprinzl GM, Riechelmann H. Tumor-produced, active interleukin-1β regulates gene expression in carcinoma-associated fibroblasts. Exp Cell Res. 2011;317:2222–2229. doi: 10.1016/j.yexcr.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J. 2006;25:5683–5692. doi: 10.1038/sj.emboj.7601457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geroski DH, Edelhauser HF. Quantitation of Na/K ATPase pump sites in the rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 1984;25:1056–1060. [PubMed] [Google Scholar]
- Götze T, Valtink M, Nitschke M, Gramm S, Hanke T, Engelmann K, Werner C. Cultivation of an immortalized human corneal endothelial cell population and two distinct clonal subpopulations on thermo-responsive carriers. Graefes Arch Clin Exp Ophthalmol. 2008;246:1575–1583. doi: 10.1007/s00417-008-0904-6. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle-associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–1575. [PubMed] [Google Scholar]
- Kim HJ, Hawke N, Baldwin AS. NF-κB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- Ko MK, Kay EP. Regulatory role of FGF2 on type I collagen expression during endothelial mesenchymal transformation. Invest Ophthalmol Vis Sci. 2005;46:4495–4503. doi: 10.1167/iovs.05-0818. [DOI] [PubMed] [Google Scholar]
- Kook SH, Jang YS, Lee JC. Involvement of JNK-AP-1 and ERK-NF- B signaling in tension-stimulated expression of type I collagen and MMP-1 in human periodontal ligament fibroblasts. J Appl Physiol. 2011;111:1575–1583. doi: 10.1152/japplphysiol.00348.2011. [DOI] [PubMed] [Google Scholar]
- Kreutziger GO. Lateral membrane morphology and gap junction structure in rabbit corneal endothelium. Exp Eye Res. 1976;23:285–293. doi: 10.1016/0014-4835(76)90129-9. [DOI] [PubMed] [Google Scholar]
- Kuo AN, Harvey TM, Afshari NA. Novel delivery method to reduce endothelial injury in Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2008;145:91–96. doi: 10.1016/j.ajo.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M, Yamamoto T, Kuwano M, Ono M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18:300–310. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- Lee JG, Kay EP. FGF2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Invest Ophthalmol Vis Sci. 2006a;47:1376–1386. doi: 10.1167/iovs.05-1223. [DOI] [PubMed] [Google Scholar]
- Lee JG, Kay EP. Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF2. Invest Ophthalmol Vis Sci. 2006b;47:2358–2368. doi: 10.1167/iovs.05-1490. [DOI] [PubMed] [Google Scholar]
- Lee JG, Kay EP. Two populations of p27 use differential kinetics to phosphorylate Ser-10 and Thr-187 via phosphatidylinositol 3-Kinase in response to fibroblast growth factor-2 stimulation. J Biol Chem. 2007;282:6444–6454. doi: 10.1074/jbc.M607808200. [DOI] [PubMed] [Google Scholar]
- Lee JG, Kay EP. Common and distinct pathways for cellular activities in FGF2 signaling induced by IL-1beta in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:2067–2076. doi: 10.1167/iovs.08-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Kay EP. PI 3-kinase/Rac1 and ERK1/2 regulate FGF2-mediated cell proliferation through phosphorylation of p27 at Ser10 by KIS and at Thr187 by Cdc25A/Cdk2. Invest Ophthalmol Vis Sci. 2011;52:417–426. doi: 10.1167/iovs.10-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Kay EP. NF-κB is the transcription factor for FGF2 that causes endothelial mesenchymal transformation in cornea. Invest Ophthalmol Vis Sci. 2012;53:1530–1538. doi: 10.1167/iovs.11-9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Song JS, Smith RE, Kay EP. Human corneal endothelial cells employ phosphorylation of p27(Kip1) at both Ser10 and Thr187 sites for FGF2-mediated cell proliferation via PI 3-kinase. Invest Ophthalmol Vis Sci. 2011;52:8216–8223. doi: 10.1167/iovs.11-8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung EW, Rife L, Smith RE, Kay EP. Extracellular matrix components in retrocorneal fibrous membrane in comparison to corneal endothelium and Descemet’s membrane. Mol Vis. 2000;6:15–23. [PubMed] [Google Scholar]
- Lim H, Kim HP. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch Pharm Res. 2011;34:109–117. doi: 10.1007/s12272-011-0113-4. [DOI] [PubMed] [Google Scholar]
- Lin CC, Kuo CT, Cheng CY, Wu CY, Lee CW, Hsieh HL, Lee IT, Yang CM. IL-1 beta promotes A549 cell migration via MAPKs/AP-1- and NF-kappaB-dependent matrix metalloproteinase-9 expression. Cell Signal. 2009;21:1652–1662. doi: 10.1016/j.cellsig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Sawa M, Edelhauser HF, Bartels SP, Neufeld AH, Kenyon KR. Cellular migration and morphology in corneal endothelial wound repair. Invest Ophthalmol Vis Sci. 1985;26:443–449. [PubMed] [Google Scholar]
- Melles GR, Lander F, Beekhuis WH, Remeijer L, Binder PS. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy. Am J Ophthalmol. 1999;127:340–341. doi: 10.1016/s0002-9394(98)00324-9. [DOI] [PubMed] [Google Scholar]
- Moore JE, McMullen TC, Campbell IL, Rohan R, Kaji Y, Afshari NA, Usui T, Archer DB, Adamis AP. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Invest Ophthalmol Vis Sci. 2002;43:2905–2915. [PubMed] [Google Scholar]
- Murayama R, Kobayashi M, Takeshita A, Yasui T, Yamamoto M. MAPKs, activator protein-1 and nuclear factor- B mediate production of interleukin-1β-stimulated cytokines, prostaglandin E3 and MMP-1 in human periodontal ligament cells. J Periodontal Res. 2011;46:568–575. doi: 10.1111/j.1600-0765.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- Naderi A, Liu J, Hughes-Davies L. BEX2 has a functional interplay with c-Jun/JNK and p65/RelA in breast cancer. Mol Cancer. 2010;9:111. doi: 10.1186/1476-4598-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao S, Hata Y, Miura M, Noda K, Kimura YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, Dana MR, Kuwano M, Ono M, Ishibashi T, Hafezi-Moghadam A. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis. Am J Pathol. 2007;171:1058–1065. doi: 10.2353/ajpath.2007.070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Lienenklaus S, Rosati O, Martin MU. IL-1-induced phosphorylation of PKB/AKT depends on the presence of IRAK-1. Eur J Immunol. 2002;32:3689–3698. doi: 10.1002/1521-4141(200212)32:12<3689::AID-IMMU3689>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Prakash G, Jhanji V, Titiyal JS. Will Descemet’s stripping with automated endothelial keratoplasty (DSAEK) lower the rates of allograft rejection in corneal transplants for endothelial failure? Med Hypotheses. 2007;69:1117–1119. doi: 10.1016/j.mehy.2007.01.083. [DOI] [PubMed] [Google Scholar]
- Price MO, Price FW. Descemet’s stripping endothelial keratoplasy. Curr Opin Ophthalmol. 2007;18:290–294. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFkappa B. J Biol Chem. 2001;276:41661–41667. doi: 10.1074/jbc.M102262200. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–667. [PubMed] [Google Scholar]
- Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, Aksoy MO, Kelsen SG. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–C39. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- Shibata F, Baird A, Florkiewicz RZ. Functional characterization of the human basic fibroblast growth factor gene promoter. Growth Factors. 1991;4:277–287. doi: 10.3109/08977199109043913. [DOI] [PubMed] [Google Scholar]
- Song JS, Lee JG, Kay EP. Induction of FGF2 synthesis by IL-1beta in aqueous humor through PI3-kinase and p38 in rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 2010;51:822–829. doi: 10.1167/iovs.09-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Zhou XD, Wang Q, Zhang L, Wang Y, Li XY, Huang DM. Expression of TRAF6 and pro-inflammatory cytokines through activation of TLR2, TLR4, NOD1, and NOD2 in human periodontal ligament fibroblasts. Arch Oral Biol. 2011;56:1064–1072. doi: 10.1016/j.archoralbio.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Valtink M, Gruschwitz R, Funk RH, Engelmann K. Two clonal cell lines of immortalized human corneal endothelial cells show either differentiated or precursor cell characteristics. Cells Tissues Organs. 2008;187:286–94. doi: 10.1159/000113406. [DOI] [PubMed] [Google Scholar]
- Van Horn DL, Sendele DD, Seideman S, Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977;16:597–613. [PubMed] [Google Scholar]
- Waring GO., III Posterior collagenous layer of the cornea. Ultrastructural classification of abnormal collagenous tissue posterior to Descemet’s membrane in 30 cases. Arch Ophthalmol. 1982;100:122–134. doi: 10.1001/archopht.1982.01030030124015. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Gohda J, Kanayama A, Miyamoto Y, Sakurai H, Yamamoto M, Akira S, Hayashi H, Su B, Inoue J. Two mechanically and temporally distinct NF-B activation pathways in IL-1 signaling. Sci Signal. 2009;2:ra66. doi: 10.1126/scisignal.2000387. [DOI] [PubMed] [Google Scholar]
- Yamin TT, Miller DK. The interleukin-1 receptor-associated kinase is degraded by proteasomes following its phosphorylation. J Biol Chem. 1997;272:21540–21547. doi: 10.1074/jbc.272.34.21540. [DOI] [PubMed] [Google Scholar]
- Yang CM, Luo SF, Hsieh HL, Chi PL, Lin CC, Wu CC, Hsiao LD. Interleukin-1beta induces ICAM-1 expression enhancing leukocyte adhesion in human rheumatoid arthritis synovial fibroblasts: involvement of ERK, JNK, AP-1, and NF-kappaB. J Cell Physiol. 2010;224:516–526. doi: 10.1002/jcp.22153. [DOI] [PubMed] [Google Scholar]
- Zhang J, Bowden GT. Activation of p38 MAP kinase and JNK pathways by UVA irradiation. Photochem Photobiol Sci. 2012;11:54–61. doi: 10.1039/c1pp05133d. [DOI] [PMC free article] [PubMed] [Google Scholar]