Abstract
Purpose
To assess inter-observer agreement between two corneal specialists grading Fuchs dystrophy clinically, and to determine if the corneal central to peripheral thickness ratio (CPTR) might be an alternative and objective metric of disease severity.
Design
Cross-sectional study.
Participants
Forty-five eyes (26 subjects) with mild and moderate Fuchs dystrophy, 73 eyes (60 subjects) with advanced Fuchs dystrophy, and 267 eyes (142 subjects) with normal corneas.
Methods
Corneas with Fuchs dystrophy were graded by two corneal specialists based on the confluence and area of guttae, and the presence or absence of edema. Central corneal thickness (CCT) and peripheral corneal thickness at 4 mm from the center (PCT4) were measured by using scanning-slit pachymetry. CPTR4 was the quotient of CCT and PCT4.
Main Outcome Measures
Inter-observer agreement for clinical grade; CPTR4.
Results
Inter-observer agreement for clinical grading of Fuchs dystrophy was moderate (κ=0.32, 95% confidence interval, 0.19-0.45). In normal corneas, CCT was not correlated with age (r= -0.10, p=0.28, n=267), PCT4 decreased with age (r= -0.33, p<0.001, n=254), and CPTR4 increased with age (r= 0.59, p<0.001, n=254). CCT was higher in Fuchs dystrophy (652 ± 61 μm, n=118) than in normal corneas (559 ± 31 μm, n=267, p<0.001). PCT4 was higher in Fuchs dystrophy (650 ± 51 μm, n=107) than in normal corneas (643 ± 43 μm, n=254, p<0.001 after adjusting thickness for age). CPTR4 was higher in advanced Fuchs dystrophy (1.03 ± 0.07, n=65) than in mild and moderate Fuchs dystrophy (0.95 ± 0.07, n=42, age-adjusted p<0.001), which in turn was higher than in normal corneas (0.87 ± 0.05, n=254, age-adjusted p<0.001). CPTR4 was highly correlated with clinical grade of Fuchs dystrophy (r=0.77, p<0.001, n=361). CPTR4 was repeatable (median coefficient of variation, 1.3%), and provided excellent discrimination between Fuchs dystrophy and normal (area under the receiver operator characteristic curve, 0.93).
Conclusions
Agreement between corneal specialists for the subjective and morphologic clinical grading of Fuchs dystrophy is only moderate. The corneal CPTR is an objective, repeatable, and possibly functional, metric of severity of Fuchs dystrophy that warrants further investigation to determine its role in monitoring disease progression and predicting the need for keratoplasty.
Fuchs endothelial dystrophy is a corneal endothelial dysfunction characterized by guttae of Descemet membrane, attrition of endothelial cells, and corneal edema. The result is poor vision that requires treatment by corneal transplantation. The disease has often been categorized into stages involving the presence of guttae without edema, the presence of guttae with stromal or epithelial edema, and corneal scarring or neovascularization caused by chronic edema.1 This has occasionally led to clinicians distinguishing between “Fuchs dystrophy” and “cornea guttata” based on the presence or absence of corneal edema.2,3 Similarly, in clinical research of Fuchs dystrophy, disease severity has been graded at the slit-lamp by assessing the confluence and area of guttae, with corneal edema only present at the most severe grade.4,5 While these classification and grading methods split Fuchs dystrophy into stages with and without corneal edema, subjective determination of the presence of corneal edema is poorly defined, and in fact, subclinical corneal edema is present even in mild clinical grades of the disease.6 In addition, as new treatments are developed that enable earlier intervention in the course of Fuchs dystrophy,7,8 objective grading methods will be required to consistently determine the timing of intervention.
The central cornea thickens because of corneal edema in Fuchs dystrophy, and clinicians frequently measure central corneal thickness (CCT) to help in clinical decision making.9 Nevertheless, while a CCT greater than 640 µm usually indicates corneal edema, normal corneas may rarely be as thick or even thicker.10,11 Similarly, many eyes with visually-significant Fuchs dystrophy can have a CCT less than 600 μm if their pre-edematous CCT were thinner than the average normal CCT.6,12 Thus, CCT is not always helpful in clinical management and detecting mild, subclinical corneal edema by measuring CCT alone often could be impossible.
Our anecdotal clinical experience suggests the central cornea in Fuchs dystrophy swells more than the peripheral cornea, and a recent imaging study confirmed this observation.13 This suggested that peripheral corneal thickness (PCT) might serve as an internal reference when measuring central thickness in the same cornea. If PCT were unaffected early in the course of Fuchs dystrophy, the ratio between CCT and PCT could be an objective and functional metric to assess disease severity.
The goals of this study were to assess inter-observer agreement between the subjective, morphologic clinical grading of Fuchs dystrophy, and to evaluate whether the corneal central to peripheral thickness ratio (CPTR) might be an objective metric for assessing disease severity. We compared the CPTR between corneas with varying clinical grades of Fuchs dystrophy to normal corneas, and determined the relationship between CPTR and clinical grade.
METHODS
Subjects
Subjects with Fuchs endothelial dystrophy and subjects with normal corneas were recruited from patients of the Cornea and Refractive Surgery service at Mayo Clinic, Rochester, Minnesota. All subjects with Fuchs dystrophy were examined by a corneal specialist and were found to have central or paracentral guttae by slit-lamp examination; eyes were either phakic or pseudophakic with a posterior chamber intraocular lens. All subjects with normal corneas were also examined by a corneal specialist to confirm the absence of guttae; all included eyes were phakic only. Subjects with normal corneas were being evaluated for either keratorefractive or cataract surgery. Exclusion criteria for all subjects were the presence of any corneal disease (except Fuchs dystrophy in the Fuchs dystrophy group), previous intraocular surgery (except phacoemulsification in the Fuchs dystrophy group), previous keratorefractive surgery, contact lens wear, use of topical medications (except artificial tears) or systemic medications known to affect the cornea, diabetes, pregnancy, ocular hypertension, and glaucoma. This study was approved by the Mayo Clinic Institutional Review Board, and complied with the Health Insurance Portability and Accountability Act. The research adhered to the tenets of the Declaration of Helsinki; informed consent was obtained from all participants.
Clinical Grading
All subjects were examined by using slit-lamp biomicroscopy to determine normality versus grade of Fuchs dystrophy. Corneas without any guttae were considered normal, and corneas with central or paracentral guttae were considered to have Fuchs dystrophy. Corneas with Fuchs dystrophy were graded by using a modified scale based on the confluence and area of guttae, and the presence or absence of clinically detectable epithelial or stromal edema4,5 (Table 1). All corneas with Fuchs dystrophy were graded by one of two corneal specialists (KHB and SVP), and some of these corneas were independently graded by both corneal specialists in a masked manner to determine inter-observer agreement. Both observers had performed preliminary joint examinations of eyes to verify a similar understanding of the grading scale; corneal thickness or imaging data were not available to the investigator at the time of grading. For eyes graded by both investigators, the mean of the two grades was assigned for other statistical analyses. Corneas graded from 1 to 2.5 were considered to have mild Fuchs dystrophy, corneas graded from 3 to 4.5 were considered to have moderate Fuchs dystrophy, and corneas graded from 5 to 6 were considered to have advanced Fuchs dystrophy.
Table 1.
Clinical grading of Fuchs dystrophy was based on the confluence and area of guttae, and the presence or absence of corneal edema.4
| Grade | Central or paracentral Guttae |
|---|---|
| 1 | ≤12 scattered, non-confluent |
| 2 | > 12 scattered, non-confluent |
| 3 | 1-2 mm (widest diameter), confluent |
| 4 | 2-5 mm (widest diameter), confluent |
| 5 | >5 mm (widest diameter), confluent |
| 6 | > 5 mm, confluent, and with stromal or epithelial edema |
Corneal Thickness
Corneal thickness was first measured with a slit-scanning pachymeter (Orbscan II, Orbtek Inc., Salt Lake City, Utah) and then with an ultrasonic pachymeter (DGH Pachette, DGH Technology, Exton, Pennsylvania). During non-contact slit-scanning pachymetry, subjects self-fixated on a target to align the center of their corneas for measurement. The acoustic factor of this instrument was set to 0.92.14 From the numeric pachymetry map, CCT and PCTs along the horizontal and vertical meridians were recorded. PCT3, PCT3.6, and PCT4, were determined at approximately 3 mm, 3.6 mm, and 4 mm from the center of the cornea by calculating the mean of the respective 4 measurements superiorly, inferiorly, nasally, and temporally. We expected the farthest peripheral thickness to be least affected in Fuchs dystrophy, but measurements farther than 4 mm from the center could not be consistently obtained with the slit-scanning pachymeter. We also investigated the relationships at 3 mm and 3.6 mm from the center in the event that these might be appropriate for comparison to central thickness. Because of limitations with the numeric map of the slit-scanning pachymeter, the mean at 3 mm was calculated from thicknesses recorded at 2.9 mm superior and inferior to the center, and 3.1 mm nasal and temporal to the center. Similarly, the mean at 4 mm was calculated from thicknesses at 4 mm superior and inferior to the center, and 4.1 mm nasal and temporal to the center. Then mean at 3.6 mm was calculated from thicknesses at 3.6 mm from the center in all four locations. CPTR3, CPTR3.6, and CPTR4, were determined by dividing CCT by PCT3, PCT3.6, and PCT4, respectively.
After slit-scanning pachymetry, CCT was also measured with the ultrasonic pachymeter (CCTus). Topical anesthetic (proparacaine hydrochloride 0.5%) ophthalmic solution was instilled, and the ultrasound probe was placed on the central cornea. The pachymeter returned the mean of 25 measurements obtained in rapid succession.
Statistical analysis
We intended to recruit a minimum of 20 normal eyes per decade, and a minimum of 10 eyes per clinical grade of Fuchs dystrophy. Inter-observer agreement of clinical grades of Fuchs dystrophy was assessed by using the Kappa statistic and its 95% confidence interval (CI). Correlations between age and corneal thickness variables were illustrated by using Pearson correlation coefficients and significances of the correlations were determined by using generalized estimating equation (GEE) models to account for possible correlation between fellow eyes of the same subject.15 Corneal thickness variables were compared between Fuchs dystrophy and normal by using GEE models after adjusting thickness for age. Repeatability of the CPTR was determined in some eyes with Fuchs dystrophy by calculating the coefficient of variation (standard deviation/mean).
Receiver operating characteristic curves, which show the true positive rate (sensitivity) as a function of the false positive rate (1-specificity) for different cut-off values of a diagnostic test, were generated for CPTR and CCTus as discriminators between Fuchs dystrophy and normal; performance of the discriminators was assessed by calculating the area under the curve. The sensitivity and specificity of a logistic regression model incorporating CPTR and age for predicting Fuchs dystrophy versus normal was assessed for a range of cut-off scores.
RESULTS
Subjects
Corneal thickness was measured in 29 corneas of 16 subjects with mild Fuchs dystrophy, 16 corneas of 10 subjects with moderate Fuchs dystrophy, 73 corneas of 60 subjects with advanced Fuchs dystrophy, and 267 corneas of 142 normal subjects. Because the mean grade was assigned to Fuchs corneas that were examined by both observers, the number of eyes with moderate Fuchs dystrophy fell below the intended minimum of 10 eyes per grade. Age and lenticular status are summarized in Table 2.
Table 2.
Characteristics of subjects with Fuchs dystrophy and with normal corneas.
| Fuchs Dystrophy | Normal | |||
|---|---|---|---|---|
| Mild | Moderate> | Advanced | ||
| Number of eyes [subjects] | 29 [16] | 16 [10] | 73 [60] | 267 [142] |
| Age, Mean ± SD (years) [range] | 72 ± 10 [52-86] | 67 ± 14 [45-82] | 68 ± 12 [41-87] | 59 ± 16 [24-88] |
| Cornea Guttata (Grade) | 1-2.5 | 3-4.5 | 5-6 | No guttae |
| Lenticular Status | Phakic or PCIOL | Phakic or PCIOL | Phakic or PCIOL | Phakic only |
SD, standard deviation.
PCIOL, posterior chamber intraocular lens.
Inter-Observer Agreement for Clinical Grading
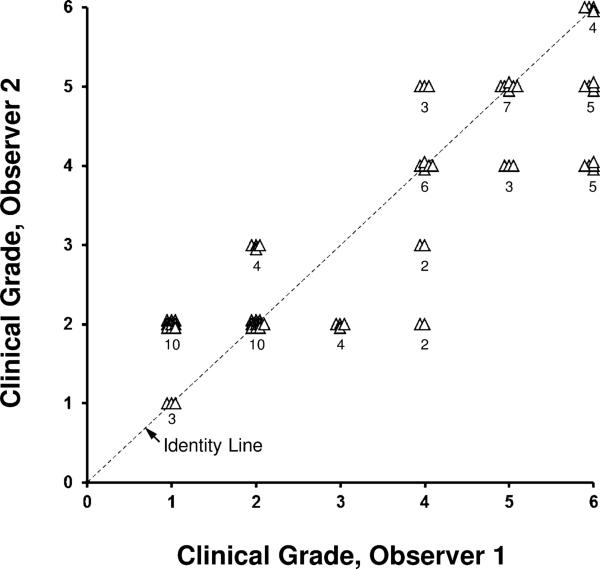
Inter-observer agreement of the clinical grade of Fuchs dystrophy between two corneal specialists was moderate, with a Kappa statistic of 0.32 (95% CI, 0.19-0.45; Figure 1). The two observers agreed exactly in 30 of 68 eyes and within one grade in 57 of 68 eyes.
Figure 1. Inter-observer variation for clinical grading of Fuchs dystrophy (68 eyes).
Agreement between two corneal specialists was only moderate (κ=0.32). The two observers agreed exactly for 30/68 eyes, and were within one grade for 57/68 eyes. Data are offset for clarity, and the number of observations is indicated.
Corneal Thickness
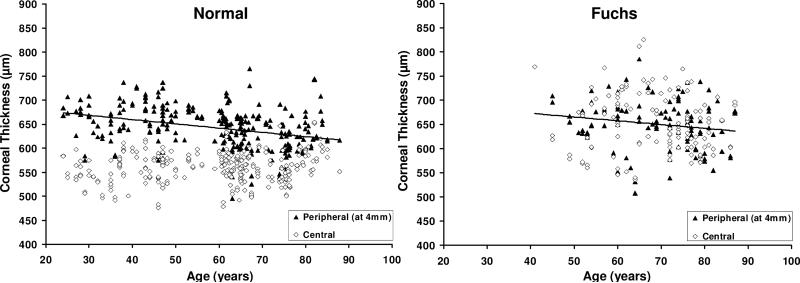
In normal corneas and all corneas with Fuchs dystrophy, CCT was not correlated with age whereas PCT decreased with age (Table 3, Figure 2). By regression analysis, the peripheral cornea thinned by 9 μm per decade in normal corneas. After adjusting thickness for age, central and peripheral corneas were thicker in Fuchs dystrophy compared to normal, but the difference for PCT4 was small and clinically insignificant (Table 4, Figure 2).
Table 3.
Relationship between corneal thickness variables and age.
| Fuchs Dystrophy (118 eyes)a | Normal (267 eyes)b | |||
|---|---|---|---|---|
| rc | pd | rc | pd | |
| CCT (μm) | ||||
| Ultrasound | -0.11 | 0.24 | -0.10 | 0.28 |
| Scanning-slit | -0.08 | 0.24 | 0.15 | 0.08 |
| PCT (μm) at stated distance from center (Scanning-slit) | ||||
| 3 mm (PCT3) | -0.18 | 0.02 | -0.18 | 0.04 |
| 3.6 mm (PCT3.6) | -0.21 | 0.01 | -0.31 | <0.001 |
| 4 mm (PCT4) | -0.17 | 0.04 | -0.33 | <0.001 |
| CPTR at stated distance from center (Scanning-slit) | ||||
| 3 mm (CPTR3) | 0.17 | 0.09 | 0.55 | <0.001 |
| 3.6 mm (CPTR36) | 0.18 | 0.10 | 0.58 | <0.001 |
| 4 mm (CPTR4) | 0.19 | 0.11 | 0.59 | <0.001 |
CCT, Central Corneal Thickness
PCT, Peripheral Corneal Thickness
CPTR, Central to peripheral thickness ratio
n=107 for PCT4 and CPTR4, because thickness could not be measured at 4 mm from the center in all subjects.
n=254 for PCT4 and CPTR4, because thickness could not be measured at 4 mm from the center in all subjects.
Pearson correlation coefficients to illustrate the relationship only.
Significances of the correlations were assessed by using generalized estimating equation models.
Figure 2. Relationship between corneal thickness and age in normal corneas and corneas with Fuchs dystrophy.
Left, In normal corneas, central corneal thickness (CCT) was not correlated with age (r=0.15, p=0.08, n=267), whereas peripheral corneal thickness at 4 mm from the center (PCT4) decreased with age (r= -0.33, p<0.001, n=254). Right, In corneas with Fuchs dystrophy, CCT was not correlated with age (r= -0.08, p=0.24, n=117), whereas PCT4 was correlated with age (r= -0.17, p=0.04, n=107) Although PCT4 was higher in Fuchs dystrophy (650 ± 51 μm) compared to normal corneas (643 ± 43 μm, p=0.002 after adjusting thickness for age), the difference was small and clinically insignificant. All data were measured with the scanning-slit pachymeter. Solid lines, regression lines for PCT4.
Table 4.
Corneal thickness and the central to peripheral thickness ratio in Fuchs dystrophy and normal corneas.
| Fuchs Dystrophy (118 eyes) | Normal (267 eyes) | pa | |
|---|---|---|---|
| CCT (μm) | |||
| Ultrasound | 614 ± 57 | 562 ± 30 | <0.001 |
| Scanning-slit | 652 ± 61 | 559 ± 31 | <0.001 |
| PCT (μm) at stated distance from center (Scanning-slit) | |||
| 3 mm (PCT3) | 650 ± 55 | 610 ± 35 | <0.001 |
| 3.6 mm (PCT3.6) | 654 ± 54 | 629 ± 39 | <0.001 |
| 4 mm (PCT4) | 650 ± 51b | 643 ± 43c | 0.002 |
| CPTR at stated distance from center (Scanning-slit) | |||
| 3 mm (CPTR3) | 1.01 ± 0.05 | 0.92 ± 0.03 | <0.001 |
| 3.6 mm (CPTR3.6) | 1.00 ± 0.06 | 0.89 ± 0.04 | <0.001 |
| 4 mm (CPTR4) | 1.00 ± 0.07b | 0.87 ± 0.05c | <0.001 |
Mean ± standard deviation.
CCT, Central Corneal Thickness
PCT, Peripheral Corneal Thickness
CPTR, Central to peripheral thickness ratio
Fuchs versus normal after adjusting thickness for age.
n=107 for PCT4 and CPTR4, because thickness could not be measured at 4 mm from the center in all subjects.
n=254 for PCT4 and CPTR4, because thickness could not be measured at 4 mm from the center in all subjects.
Central to Peripheral Thickness Ratio (CPTR)
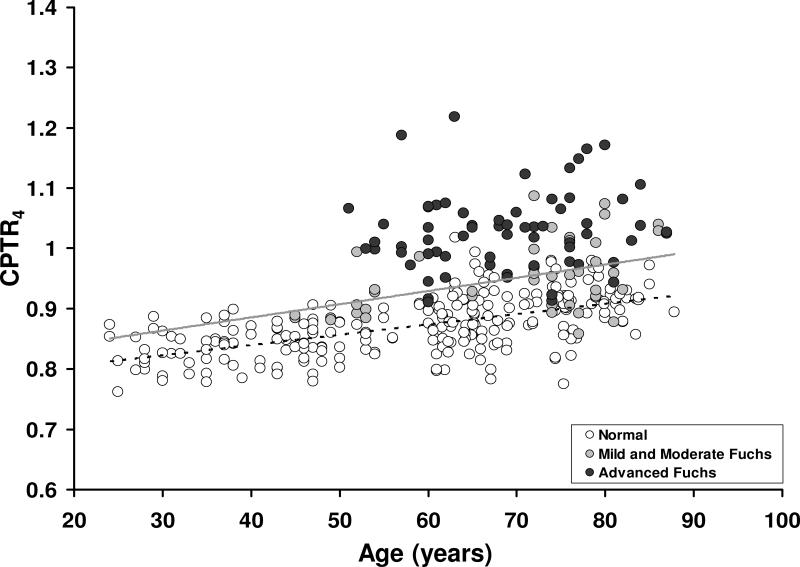
In normal corneas, the CPTR increased with age at 3.0 mm, 3.6 mm, and 4.0 mm from the center (Table 3, Figure 3). When all corneas with Fuchs dystrophy were combined, CPTR was not correlated with age (Table 3), but CPTR4 did increase with age in corneas with mild and moderate Fuchs dystrophy (Figure 3).
Figure 3. Relationship between the central-to-peripheral thickness ratio at 4 mm (CPTR4) and age in normal corneas and corneas with Fuchs dystrophy.
CPTR4 increased with age in normal corneas (r= 0.59, p<0.001, n=254) and corneas with mild and moderate Fuchs dystrophy (r= 0.48, p=.0008, n=42), but not in corneas with advanced Fuchs dystrophy (r= 0.14, p=0.26, n=65). After adjusting thickness ratios for age, CPTR4 was higher in advanced Fuchs dystrophy (1.03 ± 0.07, n=65) than in mild (0.96 ± 0.06, n=28, p<0.001) or moderate (0.94 ± 0.05, n=14, p<0.001), and higher in mild and moderate Fuchs dystrophy than normal (0.87 ± 0.05, n=254 p<0.001). Regression lines: gray, mild and moderate Fuchs dystrophy; dashed, normal.
After adjusting thickness ratios for age, CPTR4 was higher in advanced Fuchs dystrophy (1.03 ± 0.07, n=65) than in mild (0.96 ± 0.06, n=28, p<0.001) or moderate (0.94 ± 0.05, n=14, p<0.001) Fuchs dystrophy, and higher in mild and moderate Fuchs dystrophy than normal (0.87 ± 0.05, n=254 p<0.001). CPTR4 did not differ between mild and moderate Fuchs dystrophy (p=0.55). CPTR3 and CPTR3.6 had similar differences between groups. CPTR4 did not differ between phakic (0.99 ± 0.08, n=76) and pseudophakic (1.01 ± 0.07, n=31) eyes with Fuchs dystrophy (age-adjusted p=0.18).
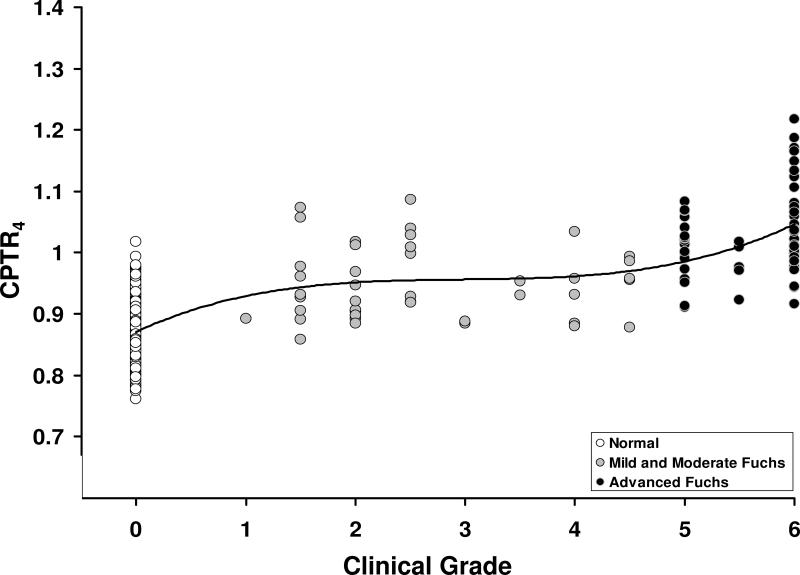
Relationship between CPTR and Clinical Grade
CPTR3, CPTR3.6, and CPTR4 were highly correlated with the clinical grade of Fuchs dystrophy. The data are shown for CPTR4 in Figure 4; the regression line spanning all grades of Fuchs dystrophy and normal corneas was best represented by a third-order polynomial (r= 0.77, p<0.001, n=359).
Figure 4. Relationship between the central-to-peripheral thickness ratio at 4 mm (CPTR4) and clinical grade of Fuchs dystrophy.
CPTR4 was correlated with the grade of Fuchs dystrophy (r= 0.77, p<0.001, n=359); grade 0 represents corneas without Fuchs dystrophy. The regression line is a third-order polynomial indicating that there are two phases of central corneal edema; the first phase was at grades 1 to 4.5, and the second phase was at grades 5 to 6.
Repeatability of the CPTR
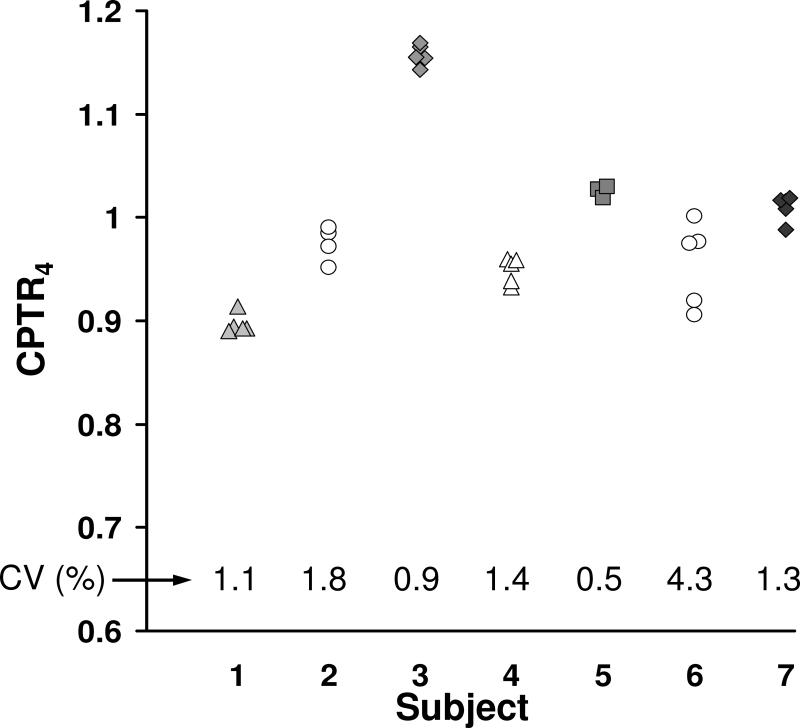
CPTR4 was determined from 3-5 repeated examinations with the scanning-slit pachymeter in 7 eyes with varying grades of Fuchs dystrophy. The median coefficient of variation (standard deviation/mean) was 1.3% (Figure 5).
Figure 5. Repeatability of the central-to-peripheral thickness ratio at 4 mm (CPTR4) in Fuchs dystrophy.
In 7 corneas with varying severity of Fuchs dystrophy, CPTR4 was determined from 3-5 repeated measurements. The median coefficient of variation (CV) was 1.3%. Each symbol represents a different cornea.
Predicting Fuchs Dystrophy versus Normal
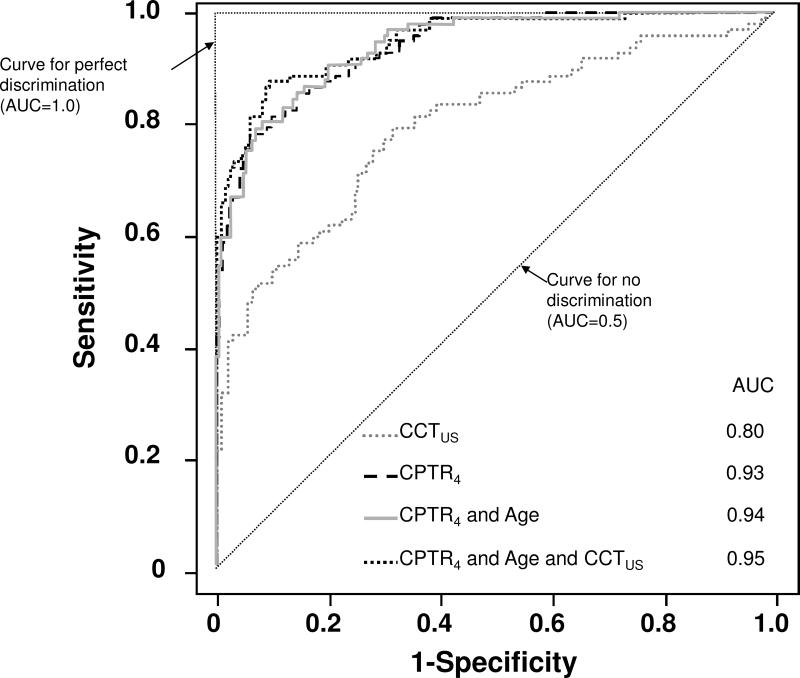
The area under receiver operating characteristic curves (i.e. the diagnostic performance) for CPTR3, CPTR3.6, and CPTR4 was 0.95, 0.94, and 0.93, respectively, higher than that for CCTus (0.80). Incorporating age, or age and CCTus, with CPTR minimally increased the areas under the receiver operating characteristic curves (Figure 6). The logistic regression model to predict Fuchs dystrophy versus normal based on age and CPTR4 was described by:
where a predictive score greater than a cut-off value of 64 predicted Fuchs dystrophy with a sensitivity of 79% and a specificity of 90% in our population, and a predictive score greater than a cut-off value of 70 predicted Fuchs dystrophy with a sensitivity of 66% and a specificity of 96%.
Figure 6. Receiver operating characteristic curves for Fuchs dystrophy versus normal.
The central-to-peripheral thickness ratio at 4 mm (CPTR4) provided excellent discrimination between corneas with Fuchs dystrophy and normal corneas, whereas central corneal thickness measured by ultrasound (CCTus) provided only moderate discrimination. AUC, area under curve.
DISCUSSION
The diagnosis of Fuchs dystrophy is rarely in doubt based on clinical examination, but stratifying disease severity has been subjective and may not represent the early stages of disease correctly.6 Fuchs dystrophy has traditionally been considered to have stages without and with clinically detectable corneal edema,1 and this is reflected in the clinical grading scheme, with edema present only at the most advanced grade.4 The definition of clinically detectable edema is subjective and could include epithelial bedewing or bullae, stromal thickening, Descemet folds, or merely subtle central thickening of the cornea relative to the periphery. Classifying guttae as confluent or non-confluent, which is the basis of current grading scales, can also be subjective and can vary if this determination is based on retroillumination16 versus specular reflection. We found only moderate agreement between two corneal specialists for the clinical grading of Fuchs dystrophy, suggesting that a more objective and reproducible measure of severity is needed to standardize the grading of Fuchs dystrophy for clinical research and to aid with making clinical decisions.
Corneas with Fuchs dystrophy become thicker because of edema, and changes in CCT can serve as an indicator of endothelial function. CCT greater than 640 μm in Fuchs dystrophy has been suggested to be a clinical indicator of endothelial dysfunction significant enough to warrant combined cataract and transplant surgery instead of cataract surgery alone.9 Unfortunately, using an isolated CCT to guide surgical planning may not be universally valid, because normal thickness varies widely.10,11 Ahmed et al. showed that the host thickness after endothelial keratoplasty of corneas with Fuchs dystrophy is similar to that of normal corneas,12 indicating that corneas with Fuchs dystrophy that began as thinner than average in a pre-edematous state could also be thinner than 600 μm when significantly edematous. Although consecutive long-term measurements of CCT could help monitor progression of disease, CCT measured at one point in time is not always a helpful indicator of disease severity, and the precision of CCT measured by ultrasonic pachymetry is limited by the ability to position the probe in the same location from one measurement to the next.14 Thus, CCT alone cannot always be used to determine the functional status of the endothelium or to grade Fuchs dystrophy because the nonedematous CCT is usually unknown.
In this study, we investigated the relationship between corneal thickness and age, and whether or not the CCT could be normalized to PCT to grade Fuchs dystrophy. In normal corneas, CCT was not associated with age, whereas the peripheral cornea thinned with age, confirming results by Martola and Baum,17 and Jonuscheit and Doughty.18 The decrease in PCT with age is best explained by a decrease in peripheral stromal thickness given the magnitude of corneal thinning (9 μm per decade), and that epithelial thickness is unrelated to age.19,20 In Fuchs dystrophy, the central cornea was significantly thicker than normal, as expected because of corneal edema. Although the cornea at 4 mm from the center was also statistically thicker than normal, the difference was small and clinically unimportant (650 μm versus 643 μm). Thus, corneal edema in Fuchs dystrophy is typically central, which corresponds to the central location of guttae, and thickness at 4 mm from the center can be an internal control for assessing central disease severity by determining the CPTR.
The CPTR increased with age in normal corneas because PCT decreased with age. CPTR4 in normal corneas was also similar to that found by Jonuscheit et al. using the same brand of scanning-slit pachymeter (they reported the inverse ratio, the peripheral to central thickness ratio).18 Our data indicate that the CPTR distinguished between the gross clinical classifications of Fuchs dystrophy. After adjusting for age, the mean CPTR was higher in corneas with mild or moderate Fuchs dystrophy than normal, and was higher in advanced Fuchs dystrophy than in mild or moderate Fuchs dystrophy. These data indicate that corneas with mild and moderate Fuchs dystrophy (Grades 1-4.5) were thicker than normal corneas centrally (Figure 3 and 4). Kopplin et al. compared CCT between large groups of subjects with and without Fuchs dystrophy and also found increased thickness in the early grades of Fuchs dystrophy.6 Because host thickness after endothelial keratoplasty of corneas with Fuchs dystrophy is similar to that of normal corneas,12 increased corneal thickness early in Fuchs dystrophy is most likely explained by edema. Although our study was smaller than that by Kopplin et al., we had sufficient power to make the same conclusion because we determined the CPTR, which adjusted CCT to PCT.
The relationship between the CPTR and clinical grade as a third-order polynomial (Figure 4) is most likely explained by non-linearity of the clinical grading scale, specifically that there might be only a small functional difference between morphologically mild and moderate Fuchs dystrophy. Another possible explanation is that there are two phases of increasing corneal edema in Fuchs dystrophy because multiple pathophysiologic processes have been implicated in the disease, including endothelial pump dysfunction, endothelial cell loss, and endothelial barrier disruption.2,21 Whether corneal edema in Fuchs dystrophy is biphasic or not, our data show that edema is present from the earliest clinical grades and progresses gradually, and does not support the notion that edema is present only in the advanced stages. The chronic state of corneal edema early in the disease probably contributes to the corneal ultrastructural changes that persist after restoration of endothelial function and resolution of corneal edema.22,23 The relationship between CPTR and clinical grade should be interpreted with caution, because the clinical grade may not be the best method for assessing disease severity, and it is conceivable that corneas with mild clinical grades of disease could be on the brink of requiring keratoplasty.
Alternative methods of objectively grading Fuchs dystrophy have been described. Zoega et al. graded the disease by assessing the area occupied by guttae in endothelial photographs acquired by specular microscopy24; they found no relationship between their grade and central corneal thickness, although their sample of subjects might have been biased toward the mildest cases of Fuchs dystrophy. Hatou et al. were able to classify Fuchs dystrophy by combining subject age and endothelial cell density measured from specular microscopy images.25 While the latter is relatively simple, measuring endothelial cell density in Fuchs dystrophy can be subject to sampling errors because images represent a very small area of endothelium and regional variation between visible cells and guttate areas can be high. Further studies are needed to determine the repeatability of endothelial cell density in Fuchs dystrophy, and to determine the relationship between endothelial cell density and the CPTR. Corneal volume is another parameter that been used to measure changes in corneal edema,26,27 but an isolated measurement of corneal volume has the same limitations as an isolated measurement of CCT.
Based on the area under the receiver operating characteristic curves, CPTR4 provided excellent discrimination between Fuchs dystrophy and normal whereas CCTus provided only moderate discrimination. The logistic regression equation, which incorporated CPTR4 and subject age, was very specific, but less sensitive, for predicting Fuchs dystrophy in our sample when using cut-off scores ranging from 64 to 70. From the logistic regression equation and the receiver operating characteristic curves, it was apparent that age had a small effect compared to CPTR4 when predicting Fuchs dystrophy.
Our study was limited by the distance from the center of the cornea at which PCT could be measured. With the scanning-slit pachymeter, thickness could only be measured as far as 4 mm in most, but not all, corneas. While PCT4 was similar between normal corneas and corneas with Fuchs dystrophy, enabling it to be the best reference of the three PCTs determined in this study, it is possible that measuring farther into the periphery might generate a more stable reference for calculating the CPTR. Alternative imaging modalities, such as anterior segment optical coherence tomography or Scheimpflug photography, might enable measurements farther into the peripheral cornea. It is important to note that the calibration, or acoustic factor, of Orbscan scanning-slit pachymeters can be changed, and that this can result in differences in absolute thickness measured within and between laboratories or measured by using other methods of corneal pachymetry.14 However, when using the scanning-slit pachymeter to determine thickness ratios, the need for consistent calibration between laboratories is less critical because thickness is normalized within the same cornea, and thus the CPTR could be a robust metric in multicenter studies.
In summary, the CPTR is an objective and repeatable metric that assesses the severity of Fuchs dystrophy by indicating endothelial function, in contrast to clinical grading which is a subjective and variable assessment of morphology. The CPTR was excellent at distinguishing between normal corneas and corneas with Fuchs dystrophy, unlike CCT measured by ultrasound, and could be an objective measure of disease severity in research studies. Prospective long-term studies are needed to demonstrate the utility of the CPTR as a metric for monitoring disease progression and predicting when corneas with Fuchs dystrophy might require keratoplasty. Additional studies are required to determine if the CPTR is a useful metric for assessing causes of corneal edema other than Fuchs dystrophy.
Acknowledgments
Financial Support: Mayo Clinic CTSA Grant UL1 TR000135 from the National Center for Research Resources and the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, Bethesda, Maryland; Research to Prevent Blindness (an unrestricted department grant and Dr. Patel as Olga Keith Wiess Special Scholar), New York, New York; Mayo Foundation, Rochester, Minnesota. The funding organizations had no role in the design or conduct of this research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None (all authors), except Dr. Baratz, who has an application in process for a patent entitled “Assessing the risk of developing Fuchs dystrophy” but for which no financial compensation has been received.
REFERENCES
- 1.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 2.Burns RR, Bourne WM, Brubaker RF. Endothelial function in patients with cornea guttata. Invest Ophthalmol Vis Sci. 1981;20:77–85. [PubMed] [Google Scholar]
- 3.Chiou AG, Kaufman SC, Beuerman RW, et al. Confocal microscopy in cornea guttata and Fuchs’ endothelial dystrophy. Br J Ophthalmol. 1999;83:185–9. doi: 10.1136/bjo.83.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krachmer JH, Purcell JJ, Jr, Young CW, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol. 1978;96:2036–9. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 5.Louttit MD, Kopplin LJ, Igo RP, Jr, et al. FECD Genetics Multi-Center Study Group. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: baseline characteristics and heritability. Cornea. 2012;31:26–35. doi: 10.1097/ICO.0b013e31821c9b8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopplin LJ, Przepyszny K, Schmotzer B, et al. Fuchs’ Endothelial Corneal Dystrophy Genetics Multi-Center Study Group. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol. 2012;130:433–9. doi: 10.1001/archophthalmol.2011.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okumura N, Koizumi N, Ueno M, et al. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br J Ophthalmol. 2011;95:1006–9. doi: 10.1136/bjo.2010.194571. [DOI] [PubMed] [Google Scholar]
- 8.Patel SV. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp Eye Res. 2012;95:40–7. doi: 10.1016/j.exer.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs’ corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology. 2005;112:441–6. doi: 10.1016/j.ophtha.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 10.Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408. doi: 10.1016/s0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 11.Prasad A, Fry K, Hersh PS. Relationship of age and refraction to central corneal thickness. Cornea. 2011;30:553–5. doi: 10.1097/ICO.0b013e3181fb880c. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed KA, McLaren JW, Baratz KH, et al. Host and graft thickness after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Am J Ophthalmol. 2010;150:490–7. doi: 10.1016/j.ajo.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Brunette I, Sherknies D, Terry MA, et al. 3-D characterization of the corneal shape in Fuchs dystrophy and pseudophakic keratopathy. Invest Ophthalmol Vis Sci. 2011;52:206–14. doi: 10.1167/iovs.09-4101. [DOI] [PubMed] [Google Scholar]
- 14.McLaren JW, Nau CB, Erie JC, Bourne WM. Corneal thickness measurement by confocal microscopy, ultrasound, and scanning slit methods. Am J Ophthalmol. 2004;137:1011–20. doi: 10.1016/j.ajo.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 16.Gottsch JD, Sundin OH, Liu SH, et al. Inheritance of a novel COL8A2 mutation defines a distinct early-onset subtype of Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2005;46:1934–9. doi: 10.1167/iovs.04-0937. [DOI] [PubMed] [Google Scholar]
- 17.Martola EL, Baum JL. Central and peripheral corneal thickness: a clinical study. Arch Ophthalmol. 1968;79:28–30. doi: 10.1001/archopht.1968.03850040030009. [DOI] [PubMed] [Google Scholar]
- 18.Jonuscheit S, Doughty MJ. Evidence for a relative thinning of the peripheral cornea with age in white European subjects. Invest Ophthalmol Vis Sci. 2009;50:4121–8. doi: 10.1167/iovs.08-3298. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, McLaren J, Hodge D, Bourne W. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–9. [PubMed] [Google Scholar]
- 20.Reinstein DZ, Archer TJ, Gobbe M, et al. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24:571–81. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SE, Bourne WM, O'Brien PC, Brubaker RF. Endothelial function and aqueous humor flow rate in patients with Fuchs’ dystrophy. Am J Ophthalmol. 1988;106:270–8. doi: 10.1016/0002-9394(88)90360-1. [DOI] [PubMed] [Google Scholar]
- 22.Baratz KH, McLaren JW, Maguire LJ, Patel SV. Corneal haze determined by confocal microscopy 2 years after Descemet stripping with endothelial keratoplasty for Fuchs corneal dystrophy. Arch Ophthalmol. doi: 10.1001/archophthalmol.2012.73. In press. [DOI] [PubMed] [Google Scholar]
- 23.Patel SV, Baratz KH, Hodge DO, et al. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–60. doi: 10.1001/archophthalmol.2008.581. [DOI] [PubMed] [Google Scholar]
- 24.Zoega GM, Fujisawa A, Sasaki H, et al. Prevalence and risk factors for cornea guttata in the Reykjavik Eye Study. Ophthalmology. 2006;113:565–9. doi: 10.1016/j.ophtha.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Hatou S, Shimmura S, Shimazaki J, et al. Mathematical projection model of visual loss due to Fuchs corneal dystrophy. Invest Ophthalmol Vis Sci. 2011;52:7888–93. doi: 10.1167/iovs.11-8040. [DOI] [PubMed] [Google Scholar]
- 26.Lam AK, Wong YZ, Cheng SY. Corneal volume measures for monitoring contact lens induced corneal swelling: a pilot study. Clin Exp Optom. 2011;94:93–7. doi: 10.1111/j.1444-0938.2010.00517.x. [DOI] [PubMed] [Google Scholar]
- 27.Li YJ, Kim HJ, Joo CK. Early changes in corneal edema following torsional phacoemulsification using anterior segment optical coherence tomography and Scheimpflug photography. Jpn J Ophthalmol. 2011;55:196–204. doi: 10.1007/s10384-011-0007-5. [DOI] [PubMed] [Google Scholar]