Abstract
Injection of AAV into the cerebrospinal fluid (CSF) offers a means to achieve widespread transgene delivery to the central nervous system, where the doses can be readily translated from small to large animals. In contrast to studies with other serotypes (AAV2, AAV4, AAV5) in rodents, we report that a naturally-occurring capsid (AAV9) and rationally-engineered capsid (AAV2.5) are able to achieve broad transduction throughout the brain and spinal cord parenchyma following a single injection into the CSF (via cisterna magna or lumbar cistern) in non-human primates (NHP). Using either vector at a dose of ~2×1012 vg per 3-6 kg animal, approximately 2% of the entire brain and spinal cord was transduced, covering all regions of the CNS. AAV9 in particular displayed efficient transduction of spinal cord motor neurons. The peripheral organ biodistribution was highly reduced compared to intravascular delivery, and the presence of circulating anti-AAV neutralizing antibodies up to a 1:128 titer had no inhibitory effect on CNS gene transfer. Intra-CSF delivery effectively translates from rodents to NHPs, which provides encouragement for the use of this approach in humans to treat motor neuron and lysosomal storage diseases.
Keywords: AAV, cerebrospinal fluid, Brain, Spinal Cord, Gene Therapy, non-human primate
INTRODUCTION
Recently, the adeno-associated virus (AAV) serotype 9 capsid was identified as a vector capable of transducing neurons and glia throughout the CNS following intravenous delivery in adult rodents, cats, and NHPs [1-5]. However, the translational feasibility of an intravascular delivery approach for CNS disorders is problematic due to the high doses required, high biodistribution to peripheral tissues, reduced efficiency for CNS transduction in NHPs, and strong inhibition by low levels of circulating anti-AAV9 neutralizing antibodies (NAbs) [3]. Intracerebroventricular injection of AAV into the CSF has been utilized as an alternative approach to transduce the brain, but previously tested AAV serotypes (2, 4, and 5) are not able to efficiently penetrate into the brain parenchyma and transduce neurons without adjuvants such as mannitol; instead, AAV 4 and 5 efficiently transduce the ependymal cells that line the ventricles [6-8].
Intrathecal injection into the lumbar cistern or injection into the cisterna magna have been utilized as alternative approaches to deliver AAV to the central and peripheral nervous systems via the CSF. Although these studies have been conducted in rodents with AAV1, AAV2, AAV3, AAV5, AAV6, AAV8, and AAV9, the primary focus has been on spinal cord and/or DRG transduction. Conversely, in rodents intrathecal AAV delivery produces only minimal brain transduction [9-14]. For AAV9, transduction of the spinal cord in mice was reportedly efficient but mostly limited to the lower thoracic and lumbar spinal cord [13]. In contrast, when pigs received intrathecal injection of AAV9, the transduction pattern could be diffusely spread across the entire spinal cord if a catheter was used to inject the vector into the CSF directly at the cervical, thoracic, and lumbar regions [15]. Interestingly and in contrast to the results in mice, in the pigs there was a high degree of brain transduction present regardless of whether the vector was injected diffusely across the spinal cord or just in the lumbar cistern (S. Gray, unpublished observation). Very recently, Samaranch et al [5]reported that injection of AAV9 into the cisterna magna of NHPs resulted in widespread brain transduction similar to intravascular delivery. These studies suggest that injection into CSF allows diffuse delivery to large areas of the brain and spinal cord where the doses can be realistically scaled to larger animals and humans.
In this study, we sought to explore the translational potential of intra-CSF delivery of AAV for spinal cord and brain transduction. Based on previously published results [5, 13, 15, 16] and those presented here, AAV2.5 and AAV9 are capable of intraparenchymal neuronal transduction following intra-CSF delivery. AAV9 and AAV2.5 were compared 4 weeks following injection into the cisterna magna in NHPs, then compared to AAV9 injected into the lumbar intrathecal space. We assessed variables critical for the translation of this approach to humans, including the efficiency of brain and spinal cord transduction, dose response, biodistribution to peripheral organs, and evasion of naturally-occurring NAbs to the vector.
RESULTS
AAV2.5 is an engineered version of AAV2 that allows transduction of neurons in the brain parenchyma following intra-CSF injection
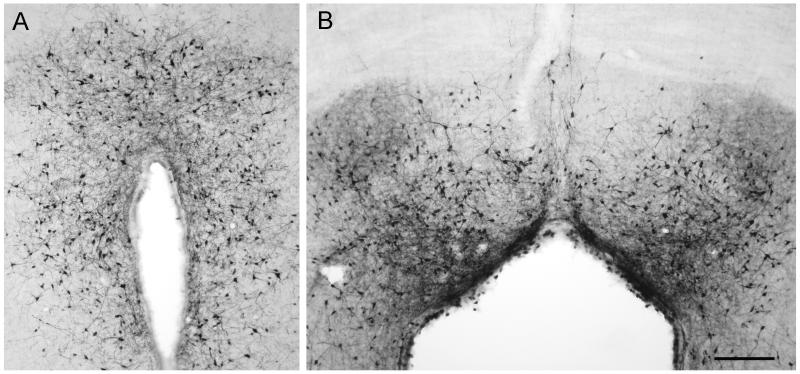
Following injection of AAV vectors into the ventricles of the brain, natural serotypes of AAV have only been successful at transducing ependymal cells lining the ventricles rather than neurons within the brain parenchyma [6]. AAV2.5 is a hybrid of AAV2 and AAV1, incorporating 6 amino acids from AAV1 into the AAV2 capsid [17]. These mutations confer enhanced muscle tropism to AAV2.5, and this capsid was used in a clinical trial for Duchenne’s Muscular Dystrophy [17]. AAV2.5 (10 uL, 6.6×1010 vg), was injected into the anterior portion of the right lateral ventricle of adult rats in order to investigate the potential to transduce neurons following intra-CSF administration. Two weeks later, the rats were perfused, and tissue sections were taken through the entire rostral-caudal extent of the brain for immunohistochemistry (IHC) and immunofluorescence (IF). As seen in Figure 1, substantial transduction was found in the hypothalamus along the extent of the third ventricle, as well as in the central gray surrounding the Sylvian aqueduct. Furthermore, extensive transduction was found in the subcommissural organ, located within the dorsal third ventricle (Supplemental Figure 1), while some GFP positive vestibular neurons were found near the fourth ventricle. Importantly, in our previous unpublished studies, this ability to transduce distal structures along the ventricular system was not seen with AAV2, AAV5, or AAV9 (for AAV9, see supplemental Figure 2).
Figure 1. AAV2.5 can cross the ependymal cell barrier and transduce neurons after ventricular administration.
AAV2.5/GFP (10 uL, 6.6×1010 vg) was injected into the anterior portion of the right lateral ventricle of adult rats, and after 2 weeks gene expression was assessed by anti-GFP IHC. (A) Transduction of cells with neuronal morphology in the hypothalamus along the third ventricle. (B) GFP-positive cells with neuronal morphology in the dorsal central gray. Scale bar is 50 microns.
Injection of AAV9 or AAV2.5 into the cisterna magna of NHPs results in widespread transduction of the entire brain and spinal cord
AAV2.5 showed a unique characteristic that separates it from known naturally-occurring AAV capsids; namely, it can cross the ependymal cell barrier and transduce neurons in the CNS following intra-CSF delivery. In our previous studies, AAV9 showed superior ability in transducing the spinal cord following intrathecal delivery in mice and pigs (Supplemental Figure 3 and [13, 15]). To compare the potential value of these vectors for gene delivery in human applications, we evaluated AAV2.5 and AAV9 in cynomolgus monkeys, when injected into the CSF of the cisterna magna. Table 1 provides a summary of all the NHPs used in this study. Two NHPs were injected with scAAV2.5 (2×1012 vg) and two with scAAV9 (1.83×1012 vg) in 1.5 mL of PBS containing 5% sorbitol. For direct comparison of the intracisternal versus intrathecal routes, 2 additional animals were injected via the lumbar cistern with scAAV9 (1.83×1012 vg in 1 mL). After 4 weeks the animals were sacrificed. Serum samples were analyzed for the prevalence of neutralizing antibodies against the injected capsid, at the time of injection and at the time of sacrifice (Table 2). All animals showed a rise in NAbs of the injected serotype after 4 weeks. After 4 weeks, two animals (101 injected with AAV9 and 104 injected with AAV2.5) produced NAbs against the other serotype while 2 animals (102 and 103) did not. The native GFP fluorescence signal was not reliably or readily detectable, so IHC was used to enhance the signal. GFP expression in the brain, spinal cord, and DRG was visualized and quantified by IHC, and vector biodistribution throughout the spinal cord and major peripheral organs was assessed by qPCR (Figure 2). The specificity of the IHC was validated by performing parallel IHC on uninjected NHP samples, on the injected NHP samples without addition of the primary anti-GFP antibody, and on injected versus uninjected rat brains (Supplemental Figure 4). The location of the coronal sections portrayed in Figure 2 is shown in Supplemental Figure 5. For the most part, AAV2.5 and AAV9 performed in a roughly equivalent manner throughout the brain and spinal cord, transducing a similar amount of tissue. The biodistribution data favored AAV2.5, which had approximately a 100-fold lower distribution to the spleen compared to AAV9. Both animals injected with AAV2.5 had pre-existing NAbs against the AAV2.5 capsid (1:8 and 1:128), so this could have affected the biodistribution of the vector to peripheral organs.
TABLE 1. Summary of NHPs in this study.
| Subject | Age (yrs) |
Weight (kg) |
Route | Capsid | Dose (vg × 1012) |
Volume (mL) |
|---|---|---|---|---|---|---|
| 101 | 3 | 2.8 | IC | 9 | 1.83 | 1.5 |
| 102 | 3 | 2.8 | IC | 9 | 1.83 | 1.5 |
| 103 | 3 | 2.6 | IC | 2.5 | 2.0 | 1.5 |
| 104 | 3 | 2.9 | IC | 2.5 | 2.0 | 1.5 |
| 303 | 3 | 6.3 | IT | 9 | 1.83 | 1.0 |
| 304 | 3 | 6.9 | IT | 9 | 1.83 | 1.0 |
| 201 | 4-7 | 4.8 | IT | 9 | 1.83 | 1.0 |
| 202 | 4-7 | 4.4 | IT | 9 | 1.83 | 1.0 |
| 203 | 4-7 | 5.1 | IT | 9 | 1.83 | 1.0 |
| 204 | 4-7 | 4.8 | IT | 9 | 1.83 | 1.0 |
| 205 | 4-7 | 4.1 | IT | 9 | 5.5 | 1.0 |
| 206 | 4-7 | 3.5 | IT | 9 | 5.5 | 1.0 |
Abbreviations: NHP = non-human primate, yrs = years, vg = vector genomes, NAb = neutralizing antibody, IC = intracisternal, IT = intrathecal
TABLE 2. Neutralizing Antibodies in Serum and CSF and Vector Persistence.
| Subject | Serum NAb Titer* | CSF NAb titer | CSF | CSF | |||
|---|---|---|---|---|---|---|---|
| pre- screen |
at injection | 4 weeks post inj. |
at injection |
4 weeks post-inj. |
vg at 2 hours post-injection (per mL) |
circulating vg at 2 hrs* |
|
| 101 | NT | <1:2 (<1:2) | 1:2048 (1:2048) |
NT | NT | NT | NT |
| 102 | NT | 1:32 (1:128) |
1:1024 (1:4) | NT | NT | NT | NT |
| 103 | NT | 1:32 (1:128) |
<1:2 (1:1024) | NT | NT | NT | NT |
| 104 | NT | 1:2 (1:8) | 1:1024 (>1:2048) |
NT | NT | NT | NT |
| 303 | <1:2 | <1:2 | 1:256 | <1:2 | <1:2 | NT | NT |
| 304 | <1:2 | <1:2 | >1:2048 | <1:2 | <1:2 | NT | NT |
| 201 | 1:32 | 1:32 | 1:1024 | <1:2 | <1:2 | <1×104 | <0.0001% |
| 202 | 1:128 | 1:128 | 1:1024 | <1:2 | 1:4 | 3.31×105 | <0.0001% |
| 203 | <1:2 | 1:4 | 1:1024 | <1:2 | <1:2 | 8.02×105 | <0.0001% |
| 204 | <1:2 | <1:2 | 1:256 | <1:2 | <1:2 | 3.30×108 | 0.0007% |
| 205 | <1:2 | 1:8 | 1:256 | <1:2 | <1:2 | 6.96×105 | <0.0001% |
| 206 | <1:2 | <1:2 | 1:64 | <1:2 | <1:2 | <1×104 | <0.0001% |
Abbreviations: CSF = cerebrospinal fluid, NAb = neutralizing antibody, inj. = injection, vg = vector genome, hrs = hours, NT = not tested (sample not available)
Values are for NAb titers against AAV9. NAb titers against AAV2.5 are indicated in parentheses when tested.
Assuming 12 mL of CSF per animal and an even distribution of AAV particles throughout the entire 12 mL, circulating vg in the CSF was calculated as the percentage of the total vg injected, per 12 mL (1.5×1011 vg per mL CSF for low dose, 4.6×1011 vg per mL CSF for high dose).
Figure 2. Comparison of AAV9 and AAV2.5 transduction after intracisternal administration in NHPs, versus AAV9 intrathecal administration.
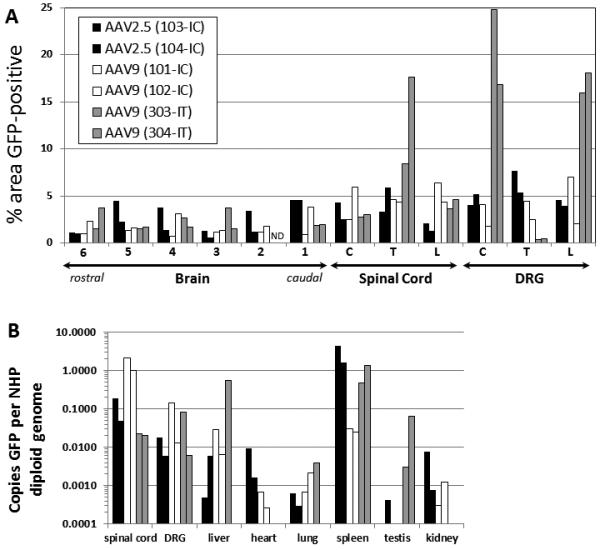
scAAV9 or scAAV2.5 vectors were injected into the cisterna magna (IC) or lumbar intrathecal space (IT) of NHPs, and the animals were sacrificed 4 weeks later. (A) Five to seven micron sections from the brain, spinal cord, and DRG were subjected to IHC against GFP, then quantified by histomorphometry. For the location of brain samples 1-6, see supplemental figure 5. (B) Total DNA was purified from the samples, and the copies of GFP relative to the NHP GAPDH locus were ascertained by qPCR. C = cervical, T = thoracic, L = lumbar, ND = no data. The legend for both panels is provided in (A).
The 2 NHPs injected via the lumbar cistern, for the most part, were comparable to these receiving an intracisternal injection. However, unlike the pattern seen following intracisternal injection, the DRG were highly transduced with approximately 15-20% of the area positive for GFP expression.
Intrathecal injection of AAV9 avoids pre-existing NAbs and transduces DRG at a higher efficiency than intracisternal injection
Our previous studies in mice by intrathecal injection showed that AAV9 was considerably better at transducing motor neurons than AAV2.5 ([13] and data not shown). For several disease applications (such as Spinal Muscular Atrophy, Giant Axonal Neuropathy, and Amyotrophic Lateral Sclerosis), motor neuron transduction is likely to be an important criteria for selecting the best vector, so we conducted further tests on AAV9 in NHPs to determine the translational feasibility of its use as a gene therapy vector. To test the efficacy of intrathecal gene transfer (relative to intracisternal) in a non-human primate model, 6 cynomolgus monkeys were injected with 1.83×1012 vg of scAAV9/GFP vectors in 1 mL of 1x PBS containing 5% sorbitol. In order to investigate a possible dose-response relationship, an additional 2 animals were injected with 5.5×1012 vg of the same vector, in 1 mL of PBS containing 5% sorbitol. All animals were pre-screened for NAbs against AAV9, and 2 of the “low dose” animals were specifically selected because they had naturally-occurring NAb titers of 1:32 and 1:128 (Table 2). The animals were sacrificed after 4 weeks. Serum samples were analyzed for prevalence of neutralizing antibodies against the injected capsid at the time of injection and at the time of sacrifice. Two of the animals had tested negative for NAbs in the prescreen 2 months prior to injection, but had (1:4 and 1:8) titers of NAbs at the time of injection. CSF was collected from the cisterna magna at the time of injection, 2 hours post-injection, and at the time of sacrifice in order to check for neutralizing factors in the CSF before and after injection, as well as to assess the persistence of the vector at 2 hours post-injection (Table 2). We found that at pre- and post-injection time points the CSF did not contain any factors that inhibited AAV transduction, except in one animal at the 4 week time point (1:4 dilution). This animal (202) had the highest pre-existing NAb titer (1:128) and an even higher titer (1:1024) at 4 weeks following injection. NHP 202 had a low but detectable amount of NHP DNA in the 4 week CSF sample, indicating some blood contamination of the collected CSF, which could explain the presence of NAbs in the CSF (See Supplemental Table 1). All animals examined had virtually no vector remaining in circulation in the CSF at 2 hours post-injection, at least in the cisterna magna (Table 2).
GFP expression in the brain, spinal cord, and DRG was visualized and quantified by IHC, and vector biodistribution throughout the brain, spinal cord, DRG, and major peripheral organs was assessed by qPCR. For most of the animals (3 low dose NAb+, 1 low dose NAb-, 2 high dose), IHC was performed on the liver and spleen to assess vector expression in these peripheral organs. To broadly compare how the experimental variables affected gene expression in an unbiased and quantitative manner, anti-GFP IHC was done on 5-7 micron sections from all samples and subjected to automated morphometric analysis to assess gene expression throughout the brain, spinal cord, DRG, optic nerve, liver, and spleen (Figure 3A and supplemental table 2, locations of coronal sections shown in supplemental table 6). Both the gray and white matter showed expression of GFP that was not evident in sections prepared in parallel without the primary anti-GFP antibody or on naïve tissue (Supplemental Figure 4). The percent area of each slice that is GFP positive does not necessarily correlate to the percentage of cells transduced. In all, we found no difference in the distribution of CNS or peripheral organ GFP expression between any groups, regardless of the presence of anti-AAV9 NAbs or the vector dose. The distribution of GFP expression was fairly even across the entire brain and spinal cord, with an average value of approximately 2% of the area positive for GFP in any given region of the CNS. The vector DNA biodistribution mostly followed the same pattern as GFP expression, with a few notable exceptions (Figure 3B). The spinal cord had more vector genomes than the brain, and these levels were comparable to the DRG except in the high dose group where the DRG were at least 10-fold higher. When compared to intravascular delivery of AAV9 [3], intrathecal delivery resulted in a much lower proportion of vector genomes in peripheral organs compared to the CNS. While high levels of vector genomes were detected in the liver, the presence of vector genomes did not correlate with a large portion of the liver being positive for GFP expression. Similarly, although over 50 times more vector genomes were detected in the spleen than the brain, only approximately 2-5% of the spleen was positive for GFP expression. Interestingly, the presence of pre-existing AAV9 NAbs did not correlate with lower biodistribution to peripheral organs.
Figure 3. Intrathecal AAV9 injection in NHPs provides widespread CNS transduction with reduced peripheral biodistribution and evasion of NAbs.
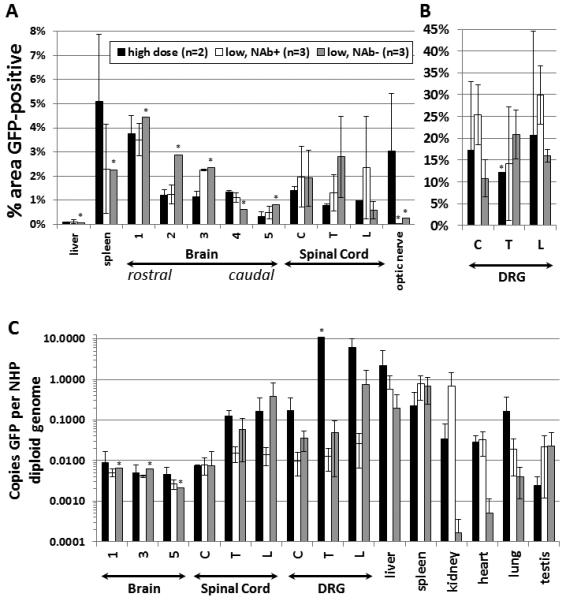
NHPs received a single intrathecal injection of 1.83×1012 vg (low dose) or 5.5×1012 vg (high dose) scAAV9/CBh-GFP. Four weeks later, the animals were sacrificed and the indicated samples from the CNS and peripheral organs were processed. (A and B) Samples were sectioned at 5-7 microns and subjected to IHC against GFP with histomorphometric quantification of GFP expression. (C) Total DNA was purified from the samples, and the copies of GFP relative to the NHP GAPDH locus were ascertained by qPCR. All brain measurements are the average of all quadrants per block analyzed. For the location of brain samples 1-5 and individual data points, see supplemental figure 6 and supplemental table 2. Brain IHC for animals 303 and 304 examined slightly different brain areas, so this data is portrayed only in Figure 2. C = cervical, T = thoracic, L = lumbar. Asterisks indicate tissues where a sample from only 1 animal was tested. NAb+ = NAb titer >1:2, and NAb− = NAb titer <1:2. All error bars are S.E.M. The legend for all panels is provided in (A).
To illustrate how the “percent of area GFP-positive” correlates to the percentage of positive cells, sample images of those used for the quantitative IHC portrayed in Figure 3A are provided in Figure 4 (spinal cord, DRG, liver, and spleen) and Figure 5 (brain). Manual counting of GFP-positive versus -negative cells was done across animals 201-206, focusing on specific brain and spinal cord structures, and this information is provided in Table 3 with representative photographs of each region provided in supplemental figure 7. Although there were modest differences in the overall transduction efficiency of specific brain structures, all areas were transduced regardless of their proximity to the CSF.
Figure 4. The NHP spinal cord and DRG are efficiently transduced following intrathecal AAV9 vector administration.
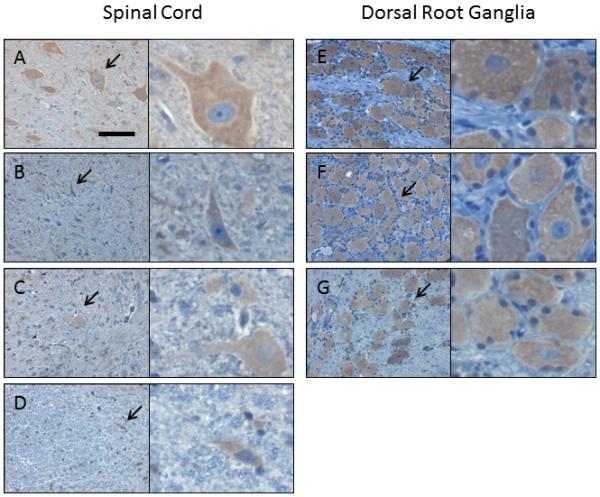
Four weeks following intrathecal injection of scAAV9/CBh-GFP vector, 5-7 micron sections from the spinal cord and DRG were subjected to IHC against GFP. Shown are sample images from those used for histomorphetric quantitation in Figure 3, and more information is available in Supplemental Figure 6 and Supplemental Table 2. These images are from NHP 203 and the specific areas are as follows, with spinal cord in panels A-D and DRG in panels E-G: (A) cervical, ventral gray matter, (B) thoracic, ventral white matter, (C) lumbar, dorsal gray matter, (D) lumbar, dorsal white matter, (E) cervical, (F) thoracic, and (G) lumbar. For each panel, the right image is a 4x enlargement of the area indicated by an arrow, and the total width of the right field is 55 microns. Slides are counterstained with cresyl violet (blue) to show nuclei, and brown (DAB) indicates GFP expression. Scale bar in (A) is the same for all panels and is 55 microns.
Figure 5. The NHP brain is transduced following intrathecal AAV9 vector administration.
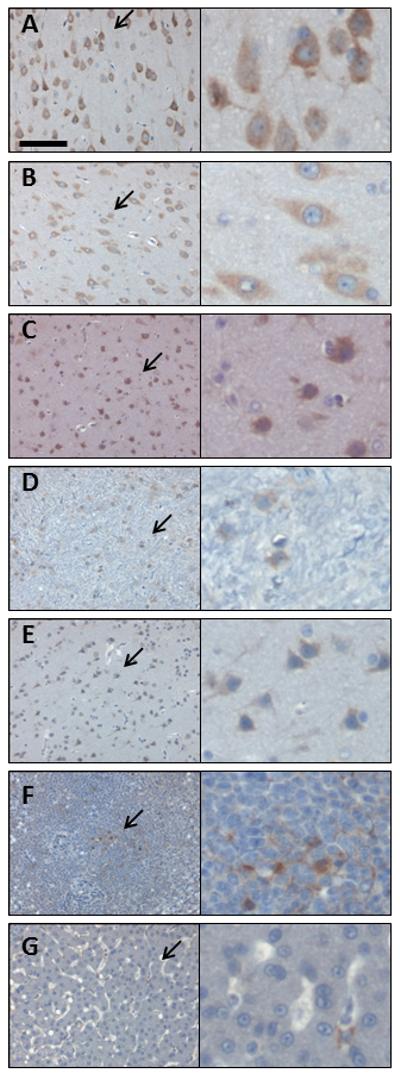
Four weeks following intrathecal injection of scAAV9/CBh-GFP vector, 5-7 micron sections from the spinal cord and DRG were subjected to IHC against GFP. Shown are sample images from those used for histomorphetric quantitation in Figure 3A, and more information is available in Supplemental Figure 6 and Supplemental Table 2. These images are from NHP 203 brain and the specific areas are as follows: (A) brain region 1C, (B) brain region 2B, (C) brain region 3C, (D) brain region 4, (E) brain region 5B, (F) liver, and (G) spleen. For each panel, the right image is a 4x enlargement of the area indicated by an arrow, and the total width of the right field is 55 microns. Slides are counterstained with cresyl violet (blue) to show nuclei, and brown (DAB) indicates GFP expression. Scale bar in (A) is the same for all panels and is 55 microns.
TABLE 3. GFP-positive cells in various brain and spinal cord regions.
| Brain area | NHP 201 | NHP 202 | NHP 203 | NHP 204 | NHP 205 | NHP 206 | Average | Standard Deviation |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1A caudate | 63.0 | 56.5 | 52.7 | 51.7 | 50.5 | 50.6 | 54.2 | 4.9 |
| 1A putamen | 54.7 | 52.2 | 51.2 | 50.5 | 50.3 | 50.1 | 51.5 | 1.8 |
| 1A thalamus | 64.0 | 45.1 | 58.6 | 43.5 | 57.4 | 43.1 | 51.9 | 9.1 |
| 1B amygdala | 58.7 | 58.9 | 49.9 | 54.1 | 48.0 | 53.0 | 53.8 | 4.5 |
| 1B lateral geniculate nucleus | 41.7 | 59.7 | 41.1 | 59.2 | 41.0 | 59.1 | 50.3 | 9.9 |
| 1B lateral globus pallidus | 62.7 | 50.8 | 55.2 | 65.4 | 45.8 | 58.8 | 56.5 | 7.4 |
| 2A corpus callusum | 63.4 | 60.0 | 51.4 | 53.9 | 48.8 | 52.5 | 55.0 | 5.6 |
| 2A motor cortex | 62.5 | 57.1 | 52.2 | 52.2 | 50.0 | 51.1 | 54.2 | 4.7 |
| 2B hippocampus | 50.6 | 61.8 | 45.0 | 57.9 | 43.7 | 57.0 | 52.7 | 7.4 |
| 2B hypothalamus | 56.3 | 69.7 | 44.7 | 60.9 | 42.3 | 59.0 | 55.5 | 10.3 |
| 2B thalamus | 37.9 | 54.2 | 41.1 | 56.9 | 42.0 | 57.5 | 48.3 | 8.9 |
| 3B midbrain | 40.6 | 65.4 | 38.3 | 63.0 | 37.8 | 62.5 | 51.3 | 13.6 |
| 5 cerebellum | 65.5 | 65.9 | 49.8 | 56.9 | 46.7 | 54.9 | 56.6 | 7.9 |
| 5 pons | 55.6 | 48.5 | 53.4 | 47.6 | 52.8 | 47.4 | 50.9 | 3.5 |
| cervical spinal cord | 45.1 | 26.2 | 63.2 | 29.3 | 68.3 | 30.0 | 43.7 | 18.4 |
| thoracic spinal cord | 25.0 | 38.0 | 39.7 | 48.9 | 44.8 | 52.2 | 41.4 | 9.7 |
| lumbar spinal cord | 74.6 | 52.6 | 58.6 | 100 | 37.0 | 73.0 | 66.0 | 21.7 |
GFP-positive cells were manually counted in 5-7 micron sections after anti-GFP IHC, and expressed as a percentage of the total number of cresyl violet-stained nuclei in the same section.
In the ventral horn of the spinal cord, an abundance of large diameter (25-50 micron) cells were GFP-positive, indicating efficient transduction of motor neurons. This is confirmed by the colocalization of GFP and ChAT seen in Supplemental Figure 8. Consistent with previous reports [5], we observed transduction of both neurons and astrocytes throughout the brain and spinal cord (Supplemental Figures 9 and 10). A similar degree of spread throughout the brain parenchyma, along with transduction of neurons and glia, was observed in mice (Supplemental Figure 3). Interestingly, in some sections we captured part of the choroid plexus within the lateral ventricle (Figure 6). The choroid plexus was very strongly GFP positive, in stark contrast to the lack of GFP expression in the ependymal cells that line the ventricles.
Figure 6. AAV9 strongly transduces the choroid plexus after intrathecal administration in NHPs.
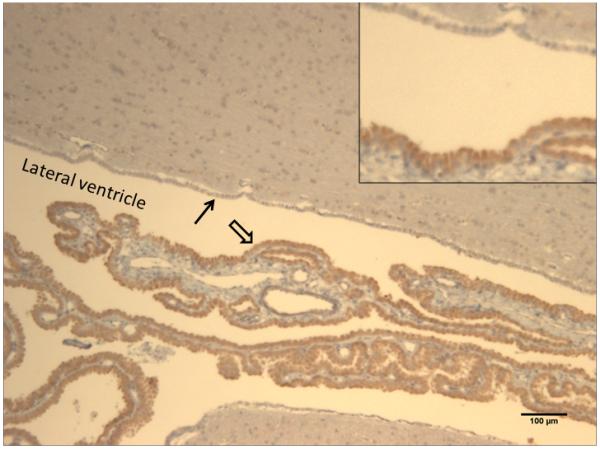
Four weeks following intrathecal injection of scAAV9/CBh-GFP vector, 5-7 micron sections were collected and subjected to IHC against GFP. The image is from NHP #206, block 2A (see supplemental Figure 6), with the lateral ventricle and surrounding parenchyma shown. Dense GFP staining is observed in virtually all of the ependymal cells of the choroid plexus (open arrow), no GFP seen in the ependymal cells lining the ventricles (closed arrow), and light GFP staining is seen in some cells within the surrounding parenchyma. The scale bar is 100 microns, and the inset is a 2x magnification of the area highlighted by the arrows.
DISCUSSION
Based on our results, we conclude that intra-CSF administration of AAV9 or AAV2.5 vectors (via lumbar cistern or cisterna magna) offers a viable, translational option for global CNS gene delivery. Importantly, these studies appear to have a marked improvement in overcoming previously identified translational barriers, such as inhibition by pre-existing NAbs, high peripheral organ biodistribution, reduced efficiency of CNS transduction in NHPs compared to rodents, and high dosage requirement [3]). It should be noted, however, that intrathecal administration, especially for AAV9, did not completely restrict the vector within the CNS. The additional success of AAV2.5 in this approach also demonstrates the ability to rationally engineer improved functionality into the AAV capsid for novel clinical applications. If very high titers of NAbs can inhibit the transduction of AAV9 after intra-CSF delivery, as the results of Samaranch et al suggest [5], the availability of 2 distinct AAV serotypes capable of widespread CNS transduction would allow treatment of patients with high levels of NAbs against one of these serotypes (but not both). Two out of 4 animals showed a lack of NAb cross-presentation between AAV2.5 and AAV9 (Table 2), suggesting the possibility of a second administration of vector in some populations if the serotype capsid was switched on the second administration.
Evasion of anti-AAV neutralizing antibodies is an important consideration for any translational approach, since an estimated 33.5% of the human population are seropositive for AAV9 [18]. In this study and others, we have screened a total of 35 randomly selected naïve NHPs for anti-AAV9 NAbs, and the highest titer detected was 1:128. Although Samaranch et al. reported that anti-AAV9 serum NAb titers ≥1:200 inhibited CNS gene transfer when AAV9 was injected via the cisterna magna[5], in the present studies we found that anti-AAV serum NAb titers up to 1:128 had no inhibitory effect on successful CNS gene transfer. No inhibitory factors were identified in the CSF prior to vector delivery or at 4 weeks post-injection, even when post-injection serum NAb titers rose as high as 1:2048. Previously, we found that a 1:4 NAb titer was sufficient to completely inhibit AAV9 transduction of the CNS and peripheral organs if an intravascular route of administration was used [3]. Although the results of Samaranch et al. indicate that high serum NAb titers can negatively influence intra-CSF gene transfer, our results clearly show that lower NAb titers (that would block intravascular gene delivery) are well tolerated. Moreover, our limited survey of pre-existing NAb levels in NHP would suggest that the presence of naturally-occurring NAbs at levels inhibitory to intra-CSF vector administration would be rare. The methods of titering the NAbs were different in these studies, so we cannot comment on what level of NAb would be inhibitory in relation to our study.
This NHP study yielded some notable differences compared to previous intrathecal AAV9 delivery studies in mice and pigs [13, 15]. In pigs, the vector distribution was mostly limited to the lumbar spinal cord unless a catheter was manipulated to physically distribute the vector to the cervical and thoracic regions. However, in NHPs, a single bolus injection at the lumbar region provided a relatively even level of transduction to the entire spinal cord and brain. Although no clear explanation exists, differences in size, anatomy, and species must be considered. For example, unlike humans and monkeys the pig epidural space contains fatty deposits that may restrict CSF flow [19]. In pigs, the biodistribution to peripheral organs was barely detectable, whereas in NHPs the vector was detected in the liver and spleen at levels that equaled or exceeded levels seen in the CNS. Also, the CSF and CNS volumes across species and age does not always correlate with overall body size, so comparing “equivalent” doses across species becomes a matter of which metric is used for scaling. In our case, we utilized CSF volume as our comparative metric, assuming equivalent CSF volumes of 0.035 mL in mice, 0.09 mL in rats, 20 mL in pigs, 12-15 mL in NHPs, and 140 mL in humans [20-22]. In this case, the high dose in the referenced pig study (~1.5×1011 vg per mL of CSF) [15] is equivalent to the low dose used in the present study in NHPs (~1.5×1011 vg per mL of CSF). Again, it is unclear whether the increased peripheral biodistribution in NHPs compared to pigs is the result of physiological/anatomical differences, differential receptor biology and binding kinetics of AAV9, or differences in the injection protocol that could have led to vector leakage. Previous studies have indicated that IT delivery in mice may restrict the vector to the lower spinal cord, although these studies were done with a lower AAV titer [23]. However, our present results show a similarity in the CNS and peripheral biodistribution between mice and NHPs receiving intrathecal injections at equivalent doses (scaled by CSF volume), including transduction throughout the brain (Supplemental Figure 3). The roughly equivalent translation of the intrathecal approach between mice and NHPs is in contrast to an IV approach, which works considerably better in mice than NHPs [3].
A confusing observation from our rodent studies was the lack of widespread transduction following intraventricular injection of vector, whereas injections into the lumbar cistern produced widespread CNS transduction (Supplemental Figures 2 and 3). Since CSF flows in the direction from the lateral ventricles to the lumbar cistern, it wasn’t clear why the lateral ventricle injection didn’t work. A possible explanation is that AAV9 has a very high affinity for the ependymal cells of the choroid plexus, supported by the very strong transduction of this structure that we observed in the NHPs (Figure 6). Injection into the lumbar cistern allows the vector to circulate in the CSF before contacting the choroid plexus, whereas injection into the ventricles would concentrate the AAV9 vector around those cells from the moment of injection. Transduction of the choroid plexus itself could be beneficial in certain therapeutic applications, since these cells would be ideal “factories” to secrete factors into the CSF. However, the turnover of these cells would likely undermine long-term persistent transgene expression from the choroid plexus, since AAV genomes don’t normally persist in dividing cell populations.
Several factors should be considered in order to achieve efficient, global CNS gene transfer with AAV9. First, the present studies exclusively employed self-complementary AAV vectors, which have at least a 20-fold advantage over traditional single-stranded vectors in terms of transduction efficiency [3, 24, 25]. Since these studies utilized self-complementary AAV vectors, this would limit the entire gene expression cassette (minus ITRs) to approximately 2.1 kb. Larger gene cassettes (up to approximately 4.6 kb) could be packaged in single-stranded vectors, but a larger dose would likely be required to compensate for the lower efficiency of traditional single-stranded AAV vectors. In the present study, we used a hyperosmolar buffer composed of PBS containing 5% sorbitol, which is a common AAV storage buffer to prevent vector aggregation and possible precipitation at higher concentrations. The volume of the injection was low (1 mL compared to a normal CSF volume of ~12-15 mL in a NHP), so only a small and transient overall change in the overall CSF osmolarity would be expected, but we cannot exclude the possibility that this affected the biodistribution in some way. In our previous study on intravascular AAV9 delivery to the CNS in NHPs, we used a dose of ~9×1012 vg/kg (~3-4×1013 vg per animal) [3]. Our present intra-CSF study used a low dose of 1.8×1012 vg (approximately 6×1011 vg/kg or 1.5×1011 vg per mL CSF) per animal and a 3-fold higher dose of 5.5×1012 vg per animal. Interestingly, we did not see an increase in transduction efficiency between our low and high dose intrathecal groups, which may suggest that our low dose is already a saturating dose for AAV9 under the present study parameters. However, with the low number of high dose animals (n=2) we cannot draw any firm conclusions. It is entirely possible that a lower dose could provide a similar level of transduction, but there remains the need for new vector development to increase the transduction efficiency beyond the “saturated” capabilities of AAV9. Samaranch et al. concluded that intravascular and intracisternal AAV9 transduction was equivalent[5], but they were using a higher intravascular dose of 3×1013 vg/kg and if the intra-CSF dose (1.8×1013 vg/kg) was already past saturated levels then their study may have missed the transduction advantage that intra-CSF delivery provides at lower doses. Based on our results, we conclude that AAV9 intrathecal delivery is superior to intravascular delivery to achieve global CNS transduction, both in terms of transduction efficiency and lack of translational barriers.
In summary, we conclude that AAV9 and AAV2.5 can be delivered in a NHP by intra-CSF injection to globally deliver a transgene to the entire CNS. Intrathecal injections are routine non-surgical procedures that are often done in an outpatient setting with minimal risk. This approach has strong translational implications for lysosomal storage diseases, or any other approach in which a secreted gene product is utilized. The efficiency of spinal cord transduction would also suggest feasible applications for motor neuron diseases such as spinal muscular atrophy, giant axonal neuropathy, and amyotrophic lateral sclerosis.
MATERIALS AND METHODS
Virus preparation
Recombinant AAV vectors were generated using HEK293 cells grown in serum-free suspension conditions in shaker flasks, using proprietary methods developed at the UNC Gene Therapy Center Vector Core facility. In brief, the suspension HEK293 cells were transfected using polyethyleneimine (Polysciences) with the following helper plasmids (pGSK2/9 or pXR2.5, and pXX6-80 [26]) plus the ITR-flanked transgene construct pTRS-KS-CBh-EGFP (referred to throughout this manuscript as GFP) [16], to generate self-complementary AAV vectors. Forty-eight hours post-transfection, cell cultures were centrifuged and supernatant was discarded. The cells were resuspended and lysed through sonication as described [27]. 550 Units of DNase was added to the lysate and incubated at 37°C for 45 minutes, followed by centrifugation at 9400 × g to pellet the cell debris and the clarified lysate was loaded onto a modified discontinuous iodixanol gradient followed by column chromatography. Purified vectors were dialyzed in 1x PBS containing 5% D-sorbitol. Titer was obtained by dot blot [27].
Rodent Studies
Animals were maintained in a 12 hour light-dark cycle and had free access to food and water. All care and procedures were in accordance with the Guide for the Care and Use of Laboratory Animals (DHHS Publication No. [NIH] 85-23), and all procedures received prior approval by the University of North Carolina Institutional Animal Care and Usage Committee.
For AAV infusions into the brain, rats (pathogen-free male Sprague-Dawley rats obtained from Charles Rivers, N=3) first were anesthetized with a 50 mg/kg i.p. dose of pentobarbital and placed into a stereotaxic frame. Using a 32 gauge stainless steel injector and a Sage infusion pump, the rats received 10μL of single stranded AAV 2.5 (6.6 × 1010 vector genomes) over 10 minutes into the right lateral ventricle (0.6mm anterior to Bregma, 1.2 mm lateral, 4.0 mm vertical, according to the atlas of Paxinos and Watson [28]). The injector was left in place for 3 minutes post-infusion to allow diffusion from the injectors. In all cases, the incision was sutured, and the animals were allowed to recover for 14 days. Two weeks after ventricular AAV administration, the rats received an overdose of pentobarbital (100 mg/kg pentobarbital, ip) and were perfused transcardially with ice-cold 100 mM sodium phosphate-buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde in PB (pH 7.4).
Mouse IT injections were done in 8-12 week-old female BALB/c mice weighing approximately 20 grams, purchased from Jackson Laboratory (Bar Harbor, ME). Five μL of the vector solution (8.75×109 or 1×1010 total vg per mouse) was injected into the IT space of the lower lumbar cord in a non-sedated mouse, as described in [29]. For IT delivery, scAAV9 vector was prepared in a vehicle solution containing 10-12.5% lidocaine-HCl (MPI Biomedicals, Solon, OH) in 1×PBS + 3.75% sorbitol. Needle penetration into the IT space was indicated by a tail flick, then successful injections were confirmed by transient (10-15 min.) bilateral paralysis of the hindlimbs from the lidocaine [30]. Animals that did not receive a successful injection were removed from the study. Mixing AAV2 with lidocaine did not affect its transduction of HEK293 cells, and injecting AAV9 into mice (IT) without lidocaine did not alter the transduction pattern (data not shown). At termination, the mice were transcardially perfused with PBS containing 1U/mL heparin (Abraxis Pharmaceutic Products, Shaumburn, IL).
Quantitative PCR and biodistribution
Quantitative PCR (qPCR) was used for vector biodistribution studies. Tissue DNA was purified and quantitated as described [3]. Data is reported as the number of double-stranded GFP DNA molecules per 2 copies of the monkey GAPDH locus, or in other words the number of vector DNA copies per diploid NHP genome.
Immunolabeling and imaging of samples (rodent samples)
After 48 hours of fixation in PBS with 4% paraformaldehyde, tissue samples were sectioned at 40 microns using a Leica vibrating microtome at room temperature. To enhance the signal observed with GFP, immunohistochemistry (IHC) was performed. Samples were incubated for 1 hour at room temperature in blocking solution (10% goat serum, 0.1% triton X-100, 1X PBS), then incubated 48-72 hours at 4° C in primary antibody solution (3% goat serum, 0.1% triton X-100, 1X PBS, rabbit anti-GFP [Millipore # AB3080, 1:500]). After washing 3 times in 1X PBS, secondary amplification was performed using a VectaStain ABC Elite Kit (Vector Labs, #PK-6101) with 3,3′-diaminobenzidine tetrachloride (DAB; Polysciences, Inc. #04008) substrate and nickel-cobalt intensification of the reaction product.
For immunofluorescence (Supplemental Figure 1), sections were incubated with a monoclonal antibody to Nestin (1:1000; Chemicon). Following incubation for 48 hrs, the sections were rinsed with PBS and blocked again for 30 minutes at room temperature in PBS containing 10% goat serum. Sections were then incubated for 45 min at 4°C with Alexafluor 594-conjugated goat anti-mouse IgG (1:500, Invitrogen) in PBS containing 3% goat serum. Rinsed sections were mounted and fluorescence was visualized by confocal microscopy.
Microscopy
DAB-processed brain sections were digitized using a Scan-Scope slide scanner (Aperio Technologies, Vista, CA). Virtual slides were viewed using ImageScope software package (v. 10.0; Aperio Technologies), and images were generated using the same software. For immunofluorescence studies, colocalization with native GFP fluorescence was determined from a minimum of five consecutive steps in a Z-series taken at 1 μm intervals through the section of interest using a 40x objective.
NHP Studies
Animals, surgeries, necropsy, and tissue processing (performed by MPI Research)
NHP studies were conducted on a contractual basis by MPI Research (Mattawan, MI), in accordance with NIH guidelines and approved by their Institutional Animal Care and Use Committee. Male cynomolgus macaques (Macaca fascicularis) were used, with weights and ages provided in Table 1, and all study animals received standard housing and care. For injections into the cisterna magna, the animals were sedated with ketamine and the back of the head was shaved for access to the cisterna magna. The animal was placed in lateral recumbence and the head was tilted forward. The cisterna magna was accessed using an over-the-needle catheter and placement was verified by the presence of cerebral spinal fluid (CSF). The animal was slowly infused with 1.5 mL of vector, followed by a flush of 0.2 mL of sterile saline. The needle was removed and the animal was allowed to recover normally. For lumbar intrathecal injections, each animal was sedated, placed on its stomach, a small skin incision made over L3-L4, and a 22 gauge spinal needle introduced into the intrathecal space using clean technique. Placement of the needle was verified by fluoroscopy. The vector (1 mL) was slowly injected by hand, the needle withdrawn, and the incision site closed. Each animal was maintained in a ventral recumbancy until recovered from anesthesia. In all experiments, subjects were sacrificed at 4 weeks post-injection and perfused with PBS. Serum samples were collected prior to injection and at the time of sacrifice. CSF samples were collected just prior to injection, 2 hours post-injection, and at the time of sacrifice.
At sacrifice, samples of the liver, spleen, kidney, heart, lung, and testis were collected. For the intracisternal injections and intrathecal animals 303 and 304, the brain was divided into 6 coronal blocks (see supplemental figure 5) and samples from each block were sectioned at 5-7 microns on a cryostat. Sections from the cervical, thoracic, and lumbar spinal cord and DRG were also collected. These sections were subjected to IHC against GFP (or with no primary antibody as a control) for gene expression analysis. Conditions were optimized so that IHC performed on naïve animal tissue showed no background staining; positive and negative control images are provided in supplemental figure 4. In each sample 7 random fields were selected for histomorphometric analysis of the percentage of the area that was positive for GFP, in order to provide an unbiased quantification of the gene expression biodistribution. For the follow-up intrathecal studies, the same samples were collected except the brain was divided into 5 coronal blocks (see supplemental figure 6) and the optic nerve was also collected. Brain, spinal cord, DRG, optic nerve, liver, and spleen sections were collected and subjected to IHC against GFP and histomorphometric analysis on 2 random fields, as was done for the 4 animals injected into the cisterna magna. These IHC and histomorphometric studies were done by MPI Research (Mattawan, MI). Samples from the identical regions on the contralateral side were sent to UNC for biodistribution analysis.
The above mentioned samples stained for GFP were used for quantification of GFP-positive cells in specific brain areas. An inverted Leica DMIRB microscope with a color Micropublisher camera was used to generate the images at an objective of 20X (Supplemental Figure 7). The number of GFP-positive brown (DAB) cells, as well as the total number of blue (cresyl violet) nuclei, were manually counted and the percentage of GFP positive cells for each brain region was calculated.
In order to perform cellular identification of the GFP-transduced cells, 40 microns brain sections were incubated at 4 degrees in PBS-T (1× PBS + 0.1% Triton X-100) containing 3% serum and a rabbit anti-GFP (1:500 Millipore AB3080), mouse anti-GFAP (1:1000 Sigma 032M4779) or mouse anti-NeuN (1:500 Millipore MAB377). Forty-eight hours post incubation; these samples were washed in PBS-T and incubated with Alexafluor 488-conjugated goat anti-rabbit IgG (1:2000 Invitrogen A11008) and Alexafluor 594-conjugated goat anti-mouse IgG (1:2000 Invitrogen A11032). Forty micron spinal cord sections were incubated with a rabbit anti-GFP (1:500) antibody and a goat anti-ChAT (1:1000 Millipore AB144P) primary. These samples were washed and incubated with Alexafluor 488-conjugated donkey anti-rabbit (1:2000 Invitrogen A21206) and Alexafluor 594-conjugated donkey anti-goat IgG (1:2000 Invitrogen A11058). Both brain and spinal cord samples were washed and mounted onto slides, visualized on a Leica SP2 Confocal microscope with a 20X objective. Each image is merged from at least 5 serial Z-stacks. Unstained brain and spinal cord sections at 40 microns were mounted onto slides for native GFP visualization.
NHP neutralizing antibody titer
NHP neutralizing antibody titer was determined exactly as described [3]. The neutralizing antibody titer reported is the serum or CSF dilution showing a 50% decrease in the transduction of Hela RC32 cells.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by generous grants from Hannah’s Hope Fund (to SJG), Award Number UL1RR025747 from the National Center for Research Resources (to SJG), the Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant U54-AR056953 (to RJS), NIH research grant 5R01AI072176-05 (to RJS), and NIH research grant NS063611 (to TJM), as well as a kind gift from the Jaspar Against Batten Foundation and Partnership for the Cures (for support of SJG’s research). We are extremely thankful to MPI Research for the quality and timeliness of the work and for help with study design; in particular we’d like to acknowledge the roles of Mark Johnson, Missy Peet, and David Serota. We thank the UNC Vector Core, in particular Josh Grieger, for technical assistance with vector production. We thank Lavanya Bachaboina, Huijing Sun, and Erica Jones for help with tissue sectioning and IHC, Brendan Fitzpatrick and Jennifer Coleman in Mark Zylka’s laboratory (UNC) for help with intrathecal mouse injections, and Swati Yadav and Hung-Jui “Sophia” Shih for running qPCR reactions. We’d like to acknowledge Jim Wilson’s group at the University of Pennsylvania for the discovery of AAV9, and thank Xiao Xiao at UNC and the UNC Vector Core for providing the AAV9 helper plasmid.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTAL INFORMATION
Supplementary information is available at Gene Therapy’s website
REFERENCES CITED
- 1.Duque S, et al. Intravenous Administration of Self-complementary AAV9 Enables Transgene Delivery to Adult Motor Neurons. Mol Ther. 2009 doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray SJ, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19(6):1058–69. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray SJ, et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol Ther. 2010;18(3):570–8. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samaranch L, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23(4):382–9. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson BL, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci U S A. 2000;97(7):3428–32. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, et al. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12(20):1503–8. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 8.Ghodsi A, et al. Systemic hyperosmolality improves beta-glucuronidase distribution and pathology in murine MPS VII brain following intraventricular gene transfer. Exp Neurol. 1999;160(1):109–16. doi: 10.1006/exnr.1999.7205. [DOI] [PubMed] [Google Scholar]
- 9.Storek B, et al. Intrathecal long-term gene expression by self-complementary adeno-associated virus type 1 suitable for chronic pain studies in rats. Mol Pain. 2006;2:4. doi: 10.1186/1744-8069-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Towne C, et al. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain. 2009;5(1):52. doi: 10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vulchanova L, et al. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol Pain. 2010;6:31. doi: 10.1186/1744-8069-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao JH, et al. Intrathecal delivery of a mutant micro-opioid receptor activated by naloxone as a possible antinociceptive paradigm. J Pharmacol Exp Ther. 2010;334(3):739–45. doi: 10.1124/jpet.109.165399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder BR, et al. Comparison of Adeno-Associated Viral Vector Serotypes for Spinal Cord and Motor Neuron Gene Delivery. Hum Gene Ther. 2011 doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- 14.Storek B, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A. 2008;105(3):1055–60. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federici T, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2011 doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 16.Gray SJ, et al. Optimizing Promoters for Recombinant Adeno-Associated Virus-Mediated Gene Expression in the Peripheral and Central Nervous System Using Self-Complementary Vectors. Hum Gene Ther. 2011 doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowles DE, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20(2):443–55. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutin S, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 19.Swindle MM. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. 2nd ed CRC Press; Boca Raton: 2007. p. 471. [Google Scholar]
- 20.Morgan CJ, et al. Bilirubin as a cerebrospinal fluid marker of sentinel subarachnoid hemorrhage: a preliminary report in pigs. J Neurosurg. 2004;101(6):1026–9. doi: 10.3171/jns.2004.101.6.1026. [DOI] [PubMed] [Google Scholar]
- 21.Pardridge WM. Peptide Drug Delivery to the Brain. Raven Press; New York: 1991. Transnasal and intraventricular delivery of drugs. [Google Scholar]
- 22.Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS. 2011;8(1):7. doi: 10.1186/2045-8118-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder BR, et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Gene Ther. 2011;22(9):1129–35. doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- 24.McCarty DM, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–8. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 25.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–54. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72(3):2224–32. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1(3):1412–28. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- 29.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67(2-3):313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 30.Sowa NA, et al. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2010;30(31):10282–93. doi: 10.1523/JNEUROSCI.2162-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.