Abstract
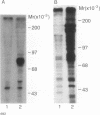
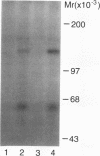
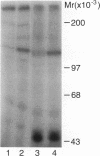
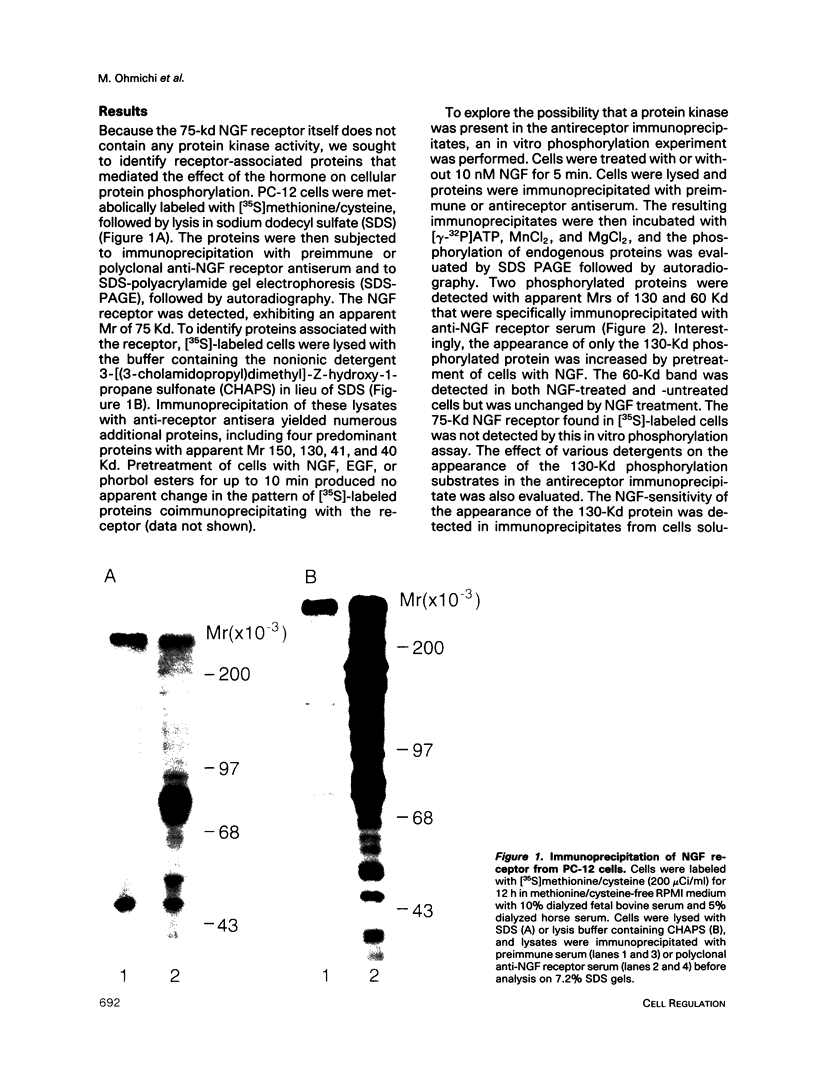
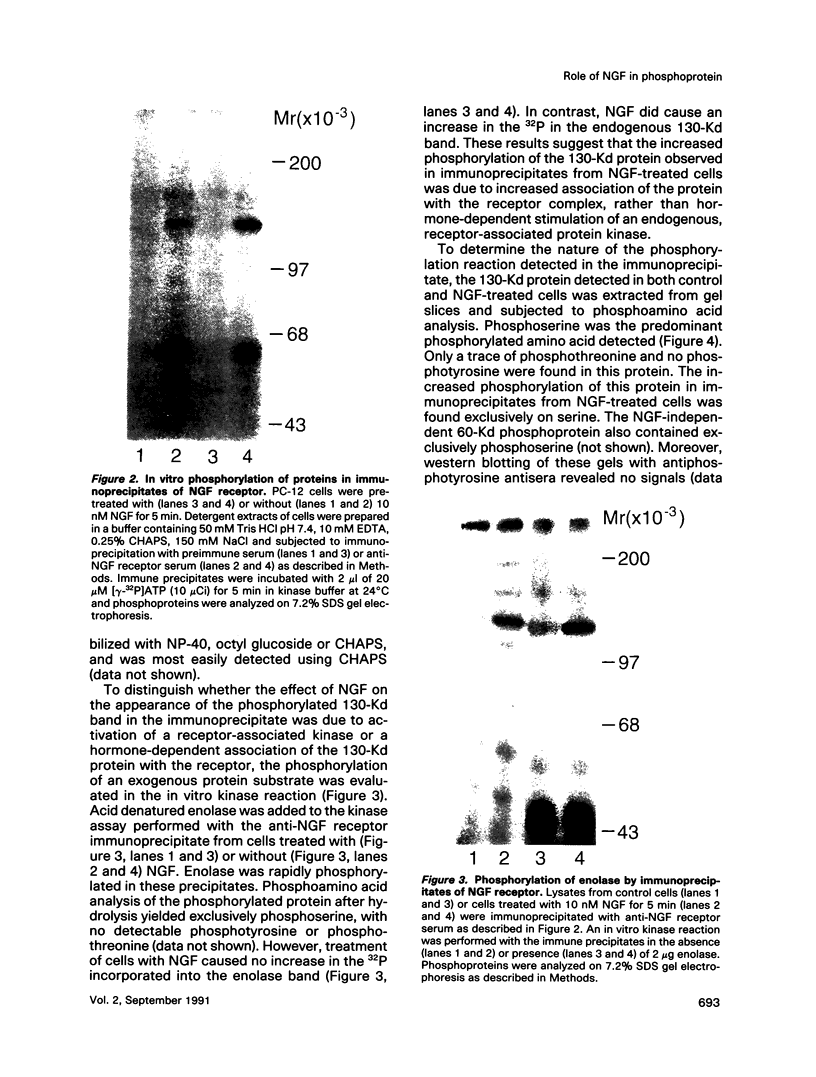
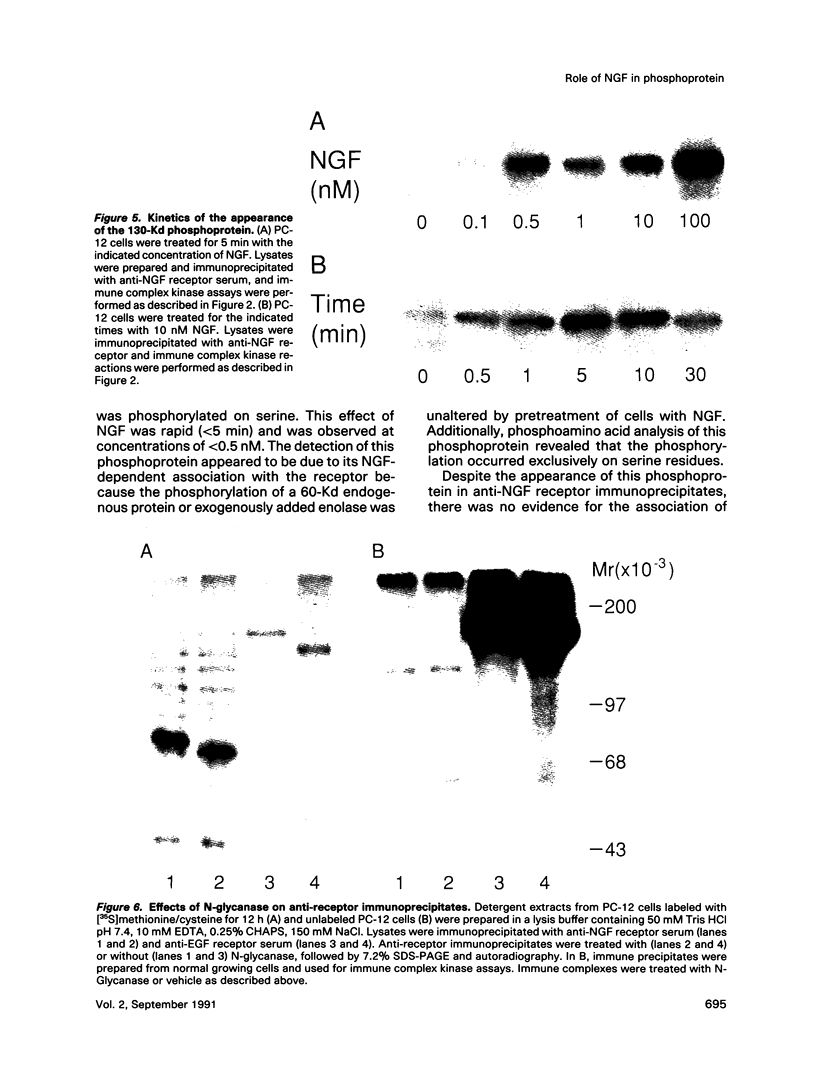
To explore the molecular mechanisms of nerve growth factor (NGF) action, we have attempted to identify proteins that immunoprecipitate with the NGF receptor. An anti-NGF receptor antibody was developed that immunoprecipitated the 75-Kd receptor in PC-12 cells. In [35S]methionine-labeled cells lysed with nonionic detergent, immunoprecipitation with this antireceptor antisera specifically brought down several associated proteins, although prior treatment of cells with NGF produced no apparent change in the distribution of these proteins. However, in vitro phosphorylation assays of the immunoprecipitated complex revealed the presence of a serine kinase that phosphorylated two predominant substrates with Mrs of 60 and 130 Kd. Prior treatment of cells produced no change in the appearance of the 60-Kd phosphoprotein, but NGF did stimulate the appearance of the 130-Kd protein. This effect was observed with as little as 0.1 nM NGF and was maximal at 5 min, but declined thereafter. Prior treatment of cells with NGF did not increase the phosphorylation of enolase added exogenously to the immunoprecipitates, suggesting that this action of NGF may have reflected the hormone-dependent association of the 130-Kd protein with the receptor, rather than activation of a receptor-associated kinase. Thus the association of the NGF 75-Kd receptor with a 130-Kd protein may be involved in signal transduction for the growth factor, although the role of this receptor in the NGF-dependent tyrosine phosphorylation remains unclear.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aletta J. M., Lewis S. A., Cowan N. J., Greene L. A. Nerve growth factor regulates both the phosphorylation and steady-state levels of microtubule-associated protein 1.2 (MAP1.2). J Cell Biol. 1988 May;106(5):1573–1581. doi: 10.1083/jcb.106.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. R. Insulin dependent neurite outgrowth in cultured embryonic mouse brain cells. Brain Res. 1983 Dec;313(2):315–318. doi: 10.1016/0165-3806(83)90231-6. [DOI] [PubMed] [Google Scholar]
- Chan B. L., Chao M. V., Saltiel A. R. Nerve growth factor stimulates the hydrolysis of glycosylphosphatidylinositol in PC-12 cells: a mechanism of protein kinase C regulation. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1756–1760. doi: 10.1073/pnas.86.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Decker S. J. Aspects of the metabolism of the epidermal growth factor receptor in A431 human epidermoid carcinoma cells. Mol Cell Biol. 1984 Apr;4(4):571–575. doi: 10.1128/mcb.4.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S. J. Effects of epidermal growth factor and 12-O-tetradecanoylphorbol-13-acetate on metabolism of the epidermal growth factor receptor in normal human fibroblasts. Mol Cell Biol. 1984 Sep;4(9):1718–1724. doi: 10.1128/mcb.4.9.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Patrick J. Nerve growth factor mediates phosphorylation of specific proteins. Cell. 1980 Nov;22(2 Pt 2):571–581. doi: 10.1016/0092-8674(80)90367-0. [DOI] [PubMed] [Google Scholar]
- Hama T., Huang K. P., Guroff G. Protein kinase C as a component of a nerve growth factor-sensitive phosphorylation system in PC12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2353–2357. doi: 10.1073/pnas.83.8.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Koizumi S., Contreras M. L., Matsuda Y., Hama T., Lazarovici P., Guroff G. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J Neurosci. 1988 Feb;8(2):715–721. doi: 10.1523/JNEUROSCI.08-02-00715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landreth G. E., Rieser G. D. Nerve growth factor- and epidermal growth factor-stimulated phosphorylation of a PC12 cytoskeletally associated protein in situ. J Cell Biol. 1985 Mar;100(3):677–683. doi: 10.1083/jcb.100.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y., Seeley P. J., Müller T. H., Helmer-Matyjek E., Sabban E., Goldstein M., Greene L. A. Regulation of tyrosine hydroxylase phosphorylation in PC12 pheochromocytoma cells by elevated K+ and nerve growth factor. Evidence for different mechanisms of action. Mol Pharmacol. 1985 Aug;28(2):220–228. [PubMed] [Google Scholar]
- Maher P. A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Guroff G. Purification and mechanism of activation of a nerve growth factor-sensitive S6 kinase from PC12 cells. J Biol Chem. 1987 Feb 25;262(6):2832–2844. [PubMed] [Google Scholar]
- Miyasaka T., Sternberg D. W., Miyasaka J., Sherline P., Saltiel A. R. Nerve growth factor stimulates protein tyrosine phosphorylation in PC-12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2653–2657. doi: 10.1073/pnas.88.7.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N., Guroff G. Nerve growth factor-induced increase in the cell-free phosphorylation of a nuclear protein in PC12 cells. J Biol Chem. 1985 Jun 25;260(12):7791–7799. [PubMed] [Google Scholar]
- Radeke M. J., Misko T. P., Hsu C., Herzenberg L. A., Shooter E. M. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987 Feb 12;325(6105):593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E., Rechler M. M., Ishii D. N. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986 May;6(5):1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland E. A., Müller T. H., Goldstein M., Greene L. A. Cell-free detection and characterization of a novel nerve growth factor-activated protein kinase in PC12 cells. J Biol Chem. 1987 Jun 5;262(16):7504–7513. [PubMed] [Google Scholar]
- Schechter A. L., Bothwell M. A. Nerve growth factor receptors on PC12 cells: evidence for two receptor classes with differing cytoskeletal association. Cell. 1981 Jun;24(3):867–874. doi: 10.1016/0092-8674(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Shooter E. M. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]