Abstract
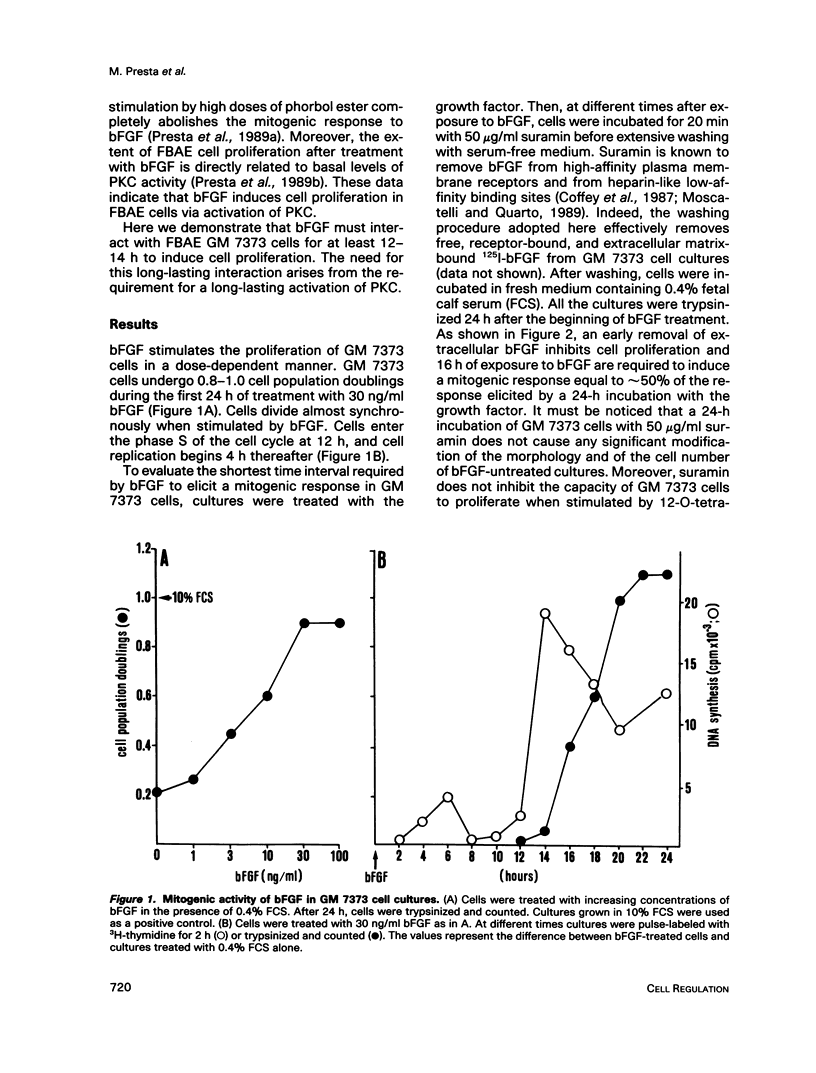
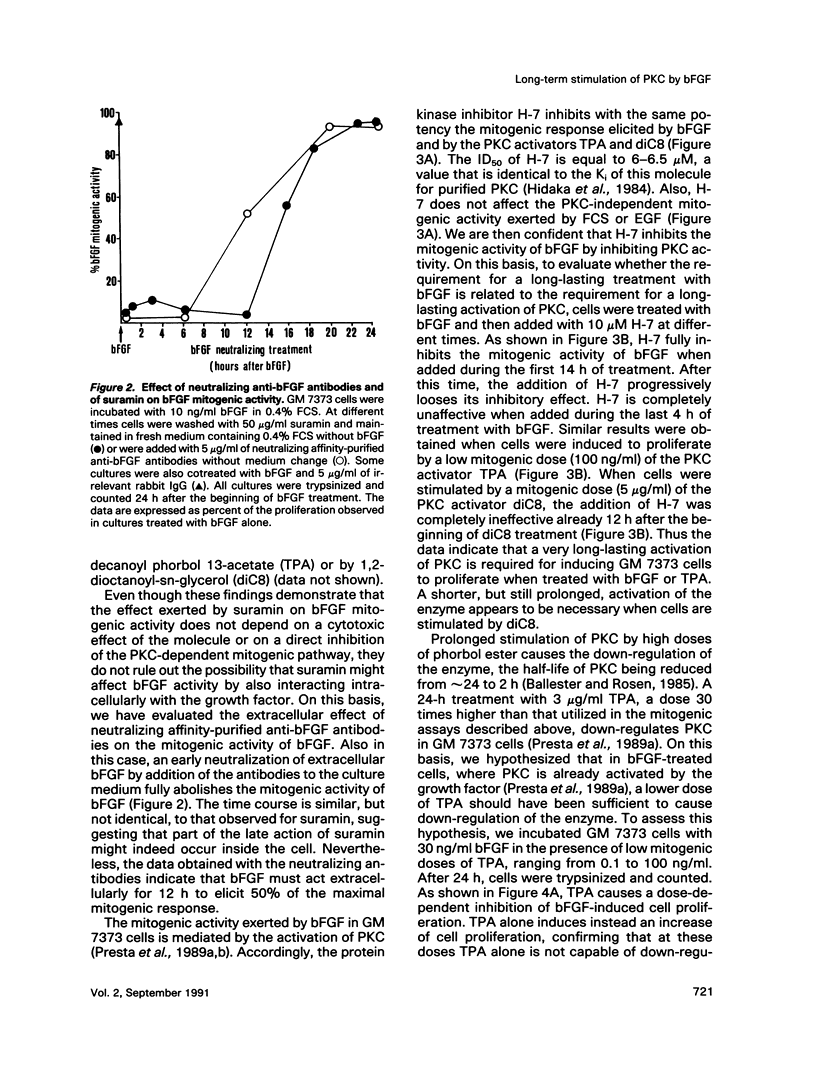
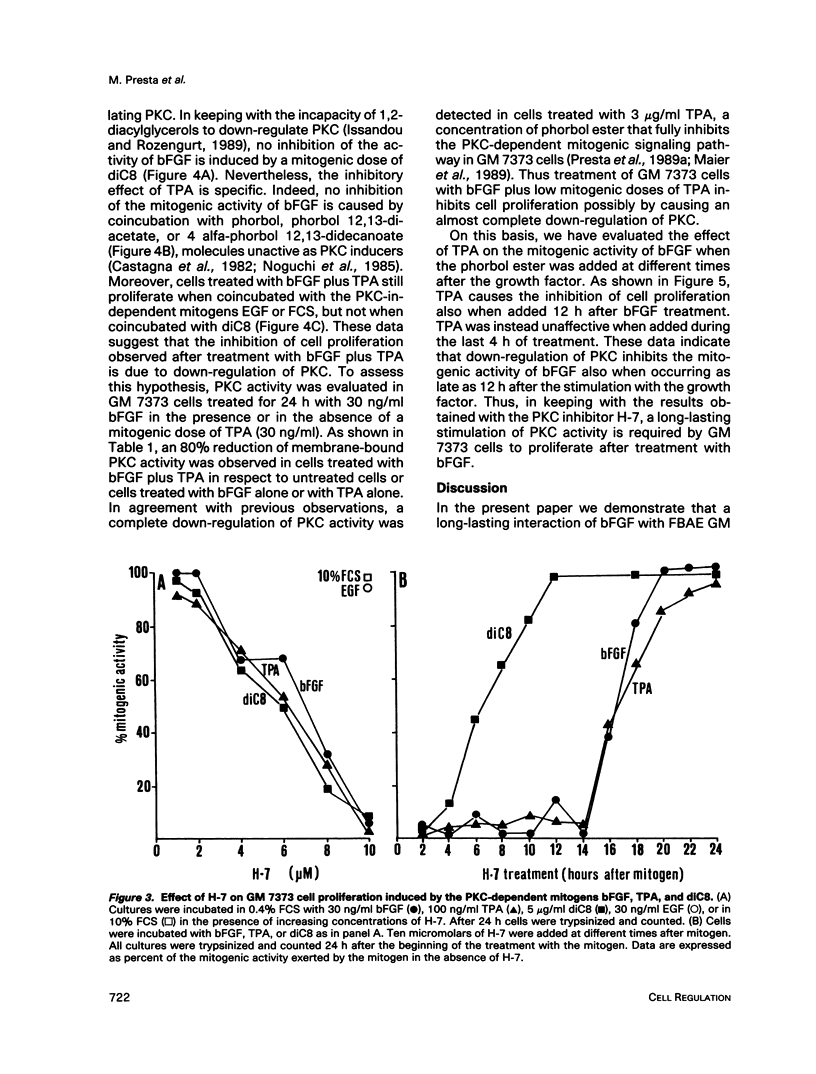
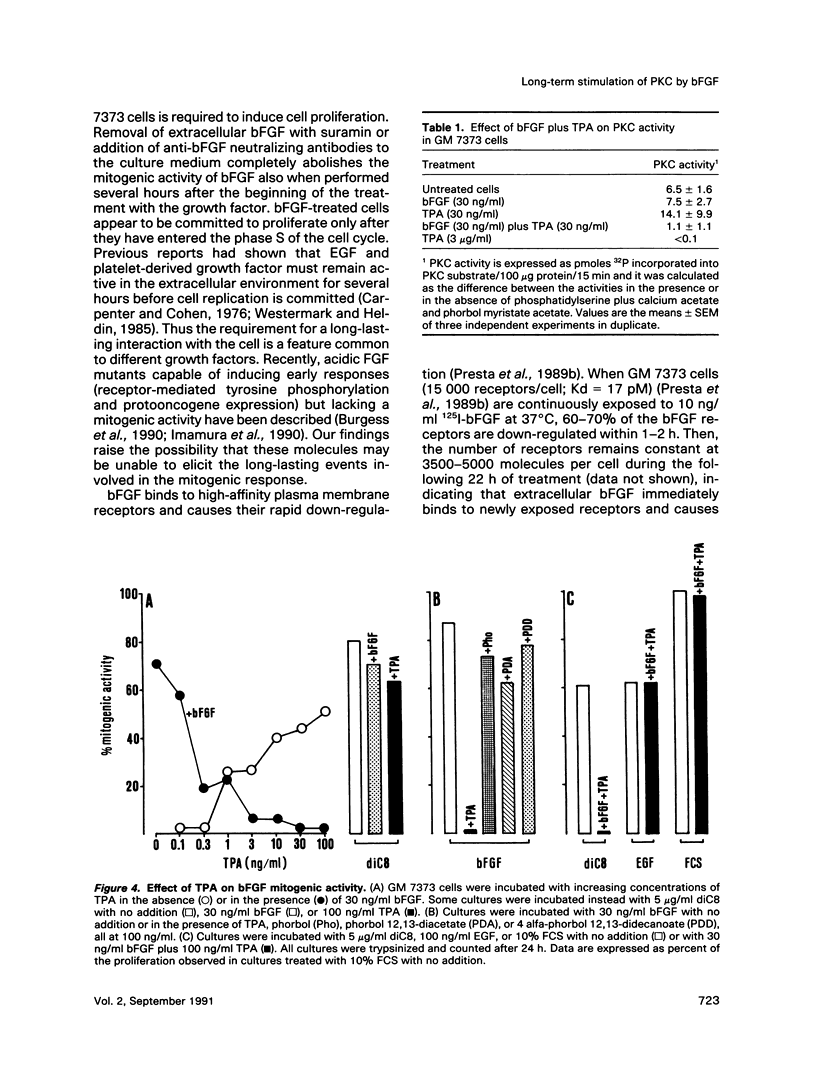
Basic fibroblast growth factor (bFGF) induces a protein kinase C (PKC)-dependent mitogenic response in transformed fetal bovine aortic endothelial GM 7373 cells. A long-lasting interaction of bFGF with the cell is required to induce cell proliferation. bFGF-treated cells are in fact committed to proliferate only after they have entered the phase S of the cell cycle, 12-14 h after the beginning of bFGF treatment. Before that time, the mitogenic response to bFGF is abolished by 1) removal of extracellular bFGF by suramin, 2) addition of neutralizing anti-bFGF antibodies to the culture medium, 3) inhibition of PKC activity by the protein kinase inhibitor H-7, and 4) down-regulation of PKC by cotreatment with phorbol ester. Thus the requirement for a prolonged interaction of bFGF with the cell reflects the requirement for a prolonged activation of PKC. Similar conclusions can be drawn for the PKC activators 12-O-tetradecanoyl phorbol 13-acetate and 1,2-dioctanoyl-sn-glycerol. The two molecules require 16 and 6 h, respectively, of activation of PKC to induce 50% of maximal cell proliferation. The requirement for a long-lasting activation of PKC appears to be a mechanism for the control of cell proliferation capable of discriminating among transient nonmitogenic stimuli and long-lasting mitogenic stimuli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Bazzi M. D., Nelsestuen G. L. Differences in the effects of phorbol esters and diacylglycerols on protein kinase C. Biochemistry. 1989 Nov 28;28(24):9317–9323. doi: 10.1021/bi00450a011. [DOI] [PubMed] [Google Scholar]
- Berry N., Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990 Apr 30;189(2):205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Buchou T., Mester J. Fibroblast growth factor-dependent mitogenic signal transduction pathway in chemically transformed mouse fibroblasts is similar to but distinct from that initiated by phorbol esters. J Cell Physiol. 1990 Mar;142(3):559–565. doi: 10.1002/jcp.1041420315. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Shaheen A. M., Ravera M., Jaye M., Donohue P. J., Winkles J. A. Possible dissociation of the heparin-binding and mitogenic activities of heparin-binding (acidic fibroblast) growth factor-1 from its receptor-binding activities by site-directed mutagenesis of a single lysine residue. J Cell Biol. 1990 Nov;111(5 Pt 1):2129–2138. doi: 10.1083/jcb.111.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Leof E. B., Shipley G. D., Moses H. L. Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol. 1987 Jul;132(1):143–148. doi: 10.1002/jcp.1041320120. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. Structural requirements for diacylglycerols to mimic tumor-promoting phobol diester action on the epidermal growth factor receptor. J Biol Chem. 1985 May 10;260(9):5315–5322. [PubMed] [Google Scholar]
- Grinspan J. B., Mueller S. N., Levine E. M. Bovine endothelial cells transformed in vitro by benzo(a)pyrene. J Cell Physiol. 1983 Mar;114(3):328–338. doi: 10.1002/jcp.1041140312. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Imamura T., Engleka K., Zhan X., Tokita Y., Forough R., Roeder D., Jackson A., Maier J. A., Hla T., Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990 Sep 28;249(4976):1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- Issandou M., Rozengurt E. Diacylglycerols, unlike phorbol esters, do not induce homologous desensitization or down-regulation of protein kinase C in Swiss 3T3 cells. Biochem Biophys Res Commun. 1989 Aug 30;163(1):201–208. doi: 10.1016/0006-291x(89)92121-9. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. N., Paley A. E. 1,2-Diacylglycerols and phorbol esters stimulate phosphatidylcholine metabolism in GH3 pituitary cells. Evidence for separate mechanisms of action. J Biol Chem. 1987 Jul 5;262(19):9204–9210. [PubMed] [Google Scholar]
- Kreutter D., Caldwell A. B., Morin M. J. Dissociation of protein kinase C activation from phorbol ester-induced maturation of HL-60 leukemia cells. J Biol Chem. 1985 May 25;260(10):5979–5984. [PubMed] [Google Scholar]
- Magnaldo I., L'Allemain G., Chambard J. C., Moenner M., Barritault D., Pouysségur J. The mitogenic signaling pathway of fibroblast growth factor is not mediated through polyphosphoinositide hydrolysis and protein kinase C activation in hamster fibroblasts. J Biol Chem. 1986 Dec 25;261(36):16916–16922. [PubMed] [Google Scholar]
- Maier J. A., Presta M., Ragnotti G. Induction of plasminogen activator activity by phorbol ester in transformed fetal bovine aortic endothelial cells. Possible independence from protein kinase C. Biochem Biophys Res Commun. 1989 Apr 28;160(2):682–691. doi: 10.1016/0006-291x(89)92487-x. [DOI] [PubMed] [Google Scholar]
- Mansukhani A., Moscatelli D., Talarico D., Levytska V., Basilico C. A murine fibroblast growth factor (FGF) receptor expressed in CHO cells is activated by basic FGF and Kaposi FGF. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4378–4382. doi: 10.1073/pnas.87.11.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenner M., Magnaldo I., L'Allemain G., Barritault D., Pouysségur J. Early and late mitogenic events induced by FGF on bovine epithelial lens cells are not triggered by hydrolysis of polyphosphoinositides. Biochem Biophys Res Commun. 1987 Jul 15;146(1):32–40. doi: 10.1016/0006-291x(87)90686-3. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Quarto N. Transformation of NIH 3T3 cells with basic fibroblast growth factor or the hst/K-fgf oncogene causes downregulation of the fibroblast growth factor receptor: reversal of morphological transformation and restoration of receptor number by suramin. J Cell Biol. 1989 Nov;109(5):2519–2527. doi: 10.1083/jcb.109.5.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nånberg E., Morris C., Higgins T., Vara F., Rozengurt E. Fibroblast growth factor stimulates protein kinase C in quiescent 3T3 cells without Ca2+ mobilization or inositol phosphate accumulation. J Cell Physiol. 1990 May;143(2):232–242. doi: 10.1002/jcp.1041430206. [DOI] [PubMed] [Google Scholar]
- Presta M., Maier J. A., Ragnotti G. The mitogenic signaling pathway but not the plasminogen activator-inducing pathway of basic fibroblast growth factor is mediated through protein kinase C in fetal bovine aortic endothelial cells. J Cell Biol. 1989 Oct;109(4 Pt 1):1877–1884. doi: 10.1083/jcb.109.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell J. S., Pettit G. R., Tashjian A. H., Jr Three activators of protein kinase C, bryostatins, dioleins, and phorbol esters, show differing specificities of action on GH4 pituitary cells. J Biol Chem. 1986 Dec 25;261(36):17073–17080. [PubMed] [Google Scholar]
- Rush J. S., Waechter C. J. Inhibitors of protein kinase C block activation of B lymphocytes by bacterial lipopolysaccharide. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1315–1320. doi: 10.1016/0006-291x(87)91581-6. [DOI] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T., Hamamori Y., Yamashita T., Fukumoto Y., Takai Y. Involvement of three intracellular messenger systems, protein kinase C, calcium ion and cyclic AMP, in the regulation of c-fos gene expression in Swiss 3T3 cells. FEBS Lett. 1986 Nov 10;208(1):39–42. doi: 10.1016/0014-5793(86)81527-7. [DOI] [PubMed] [Google Scholar]
- Westermark B., Heldin C. H. Similar action of platelet-derived growth factor and epidermal growth factor in the prereplicative phase of human fibroblasts suggests a common intracellular pathway. J Cell Physiol. 1985 Jul;124(1):43–48. doi: 10.1002/jcp.1041240108. [DOI] [PubMed] [Google Scholar]


