Abstract
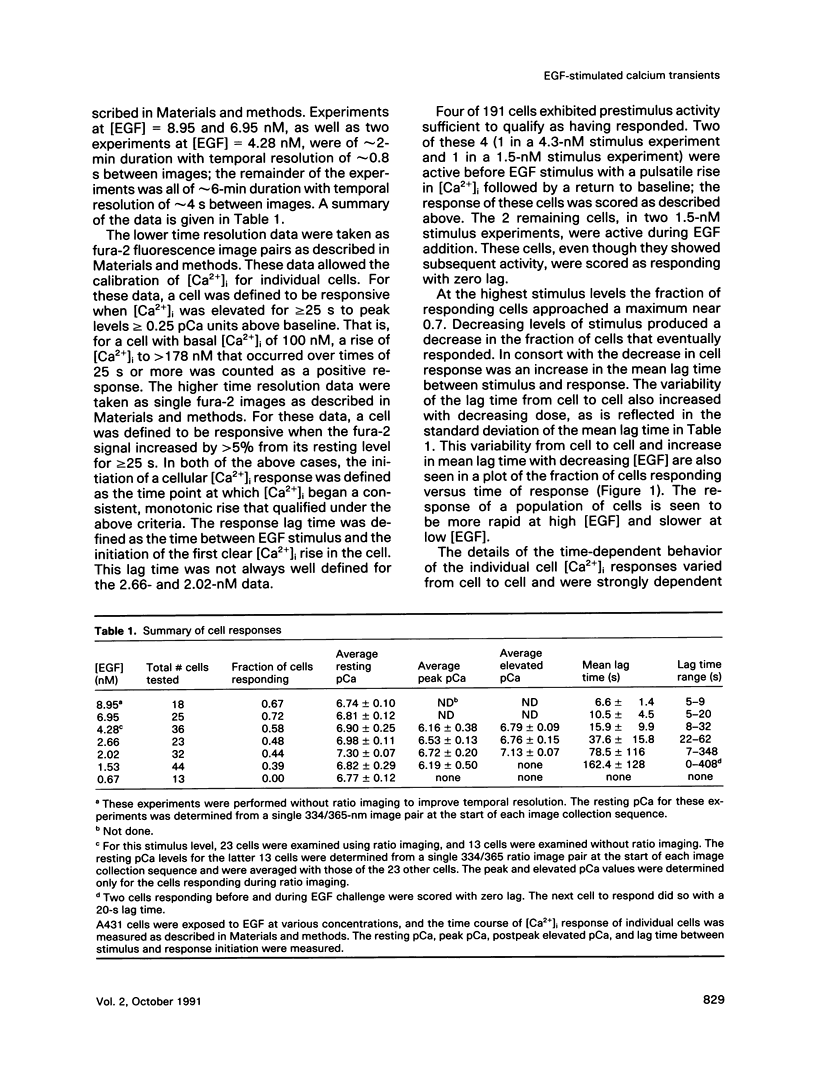
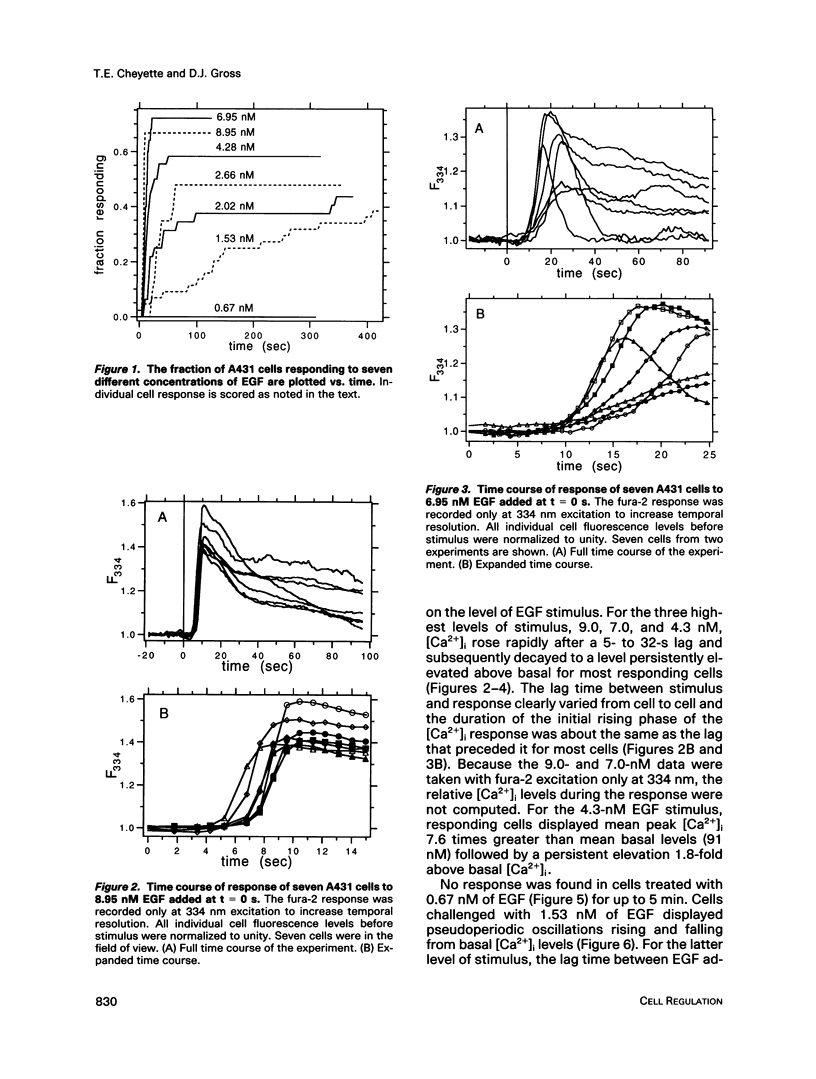
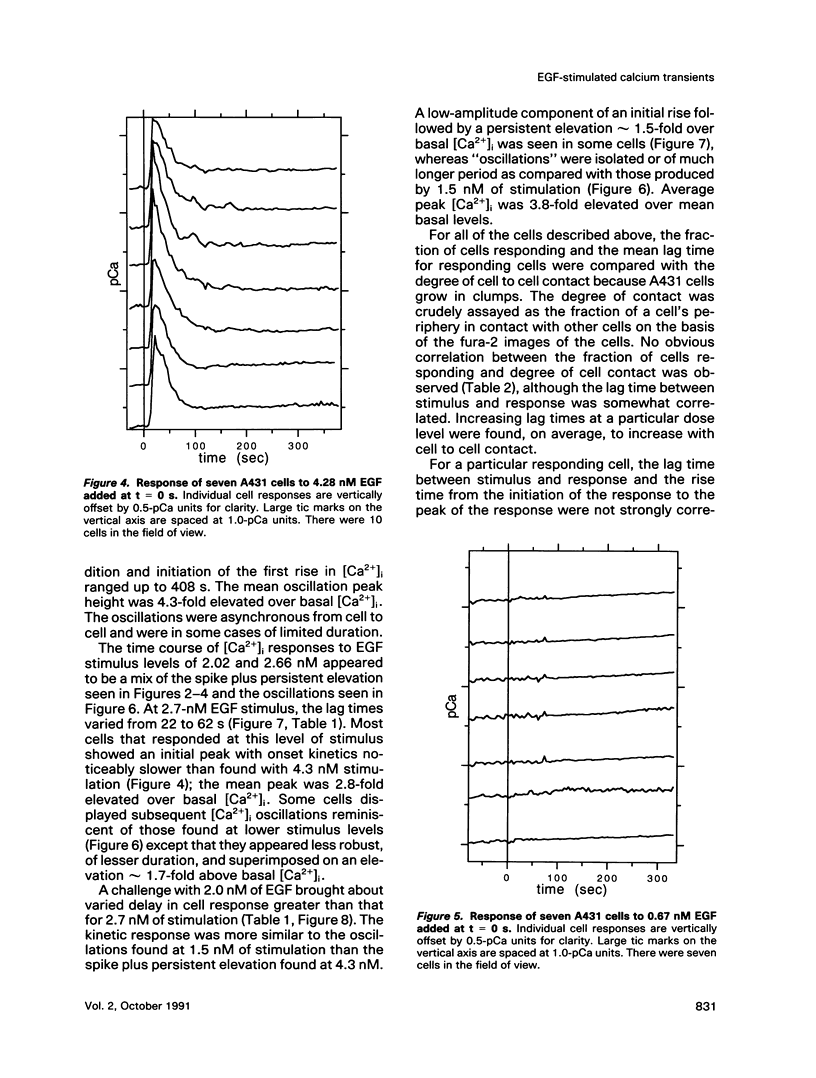
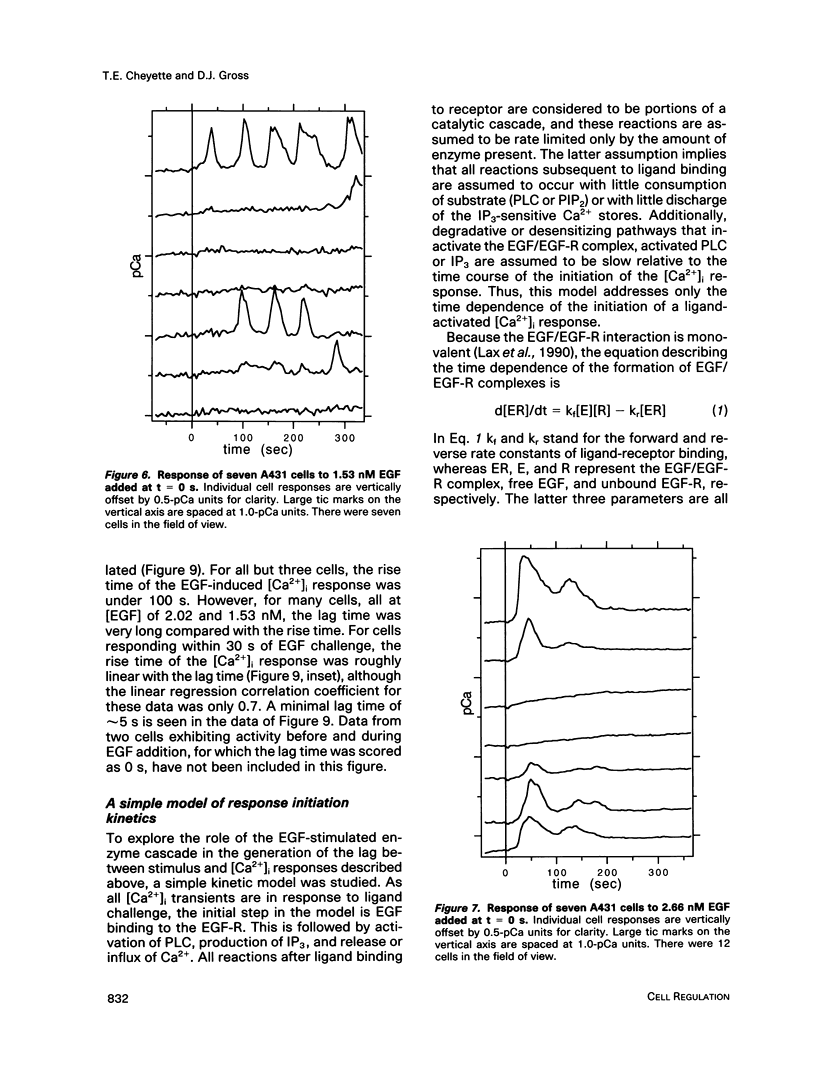
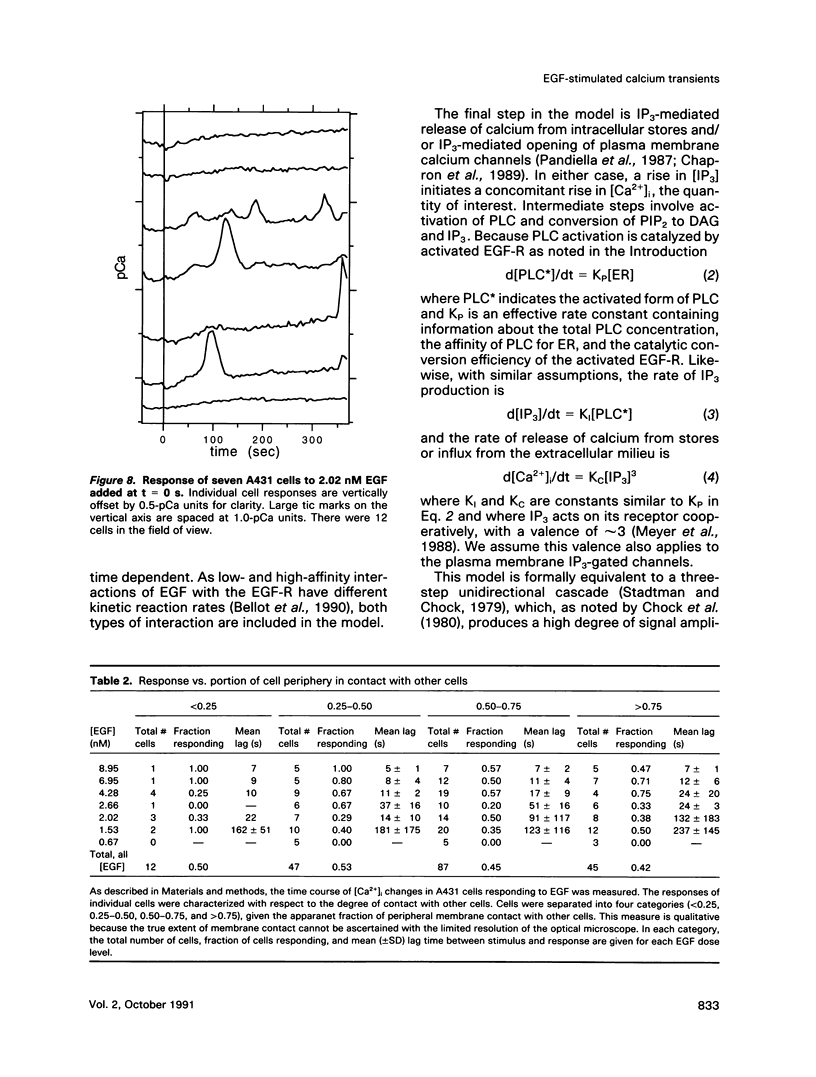
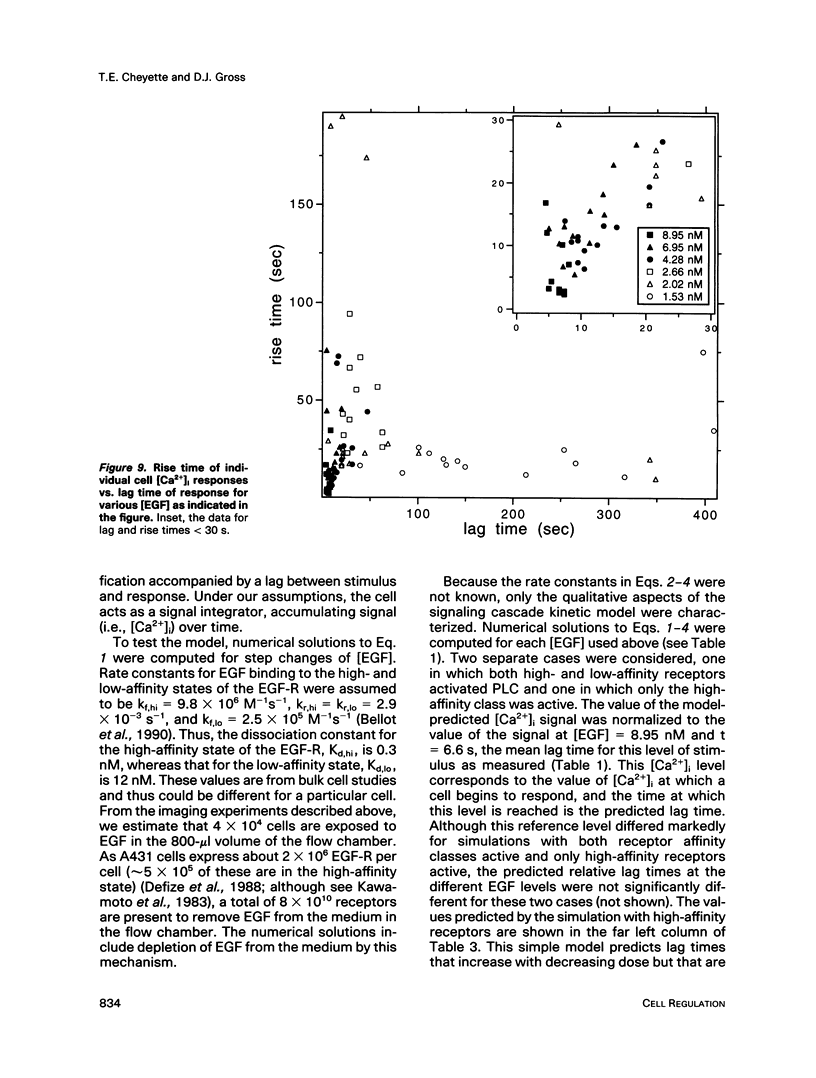
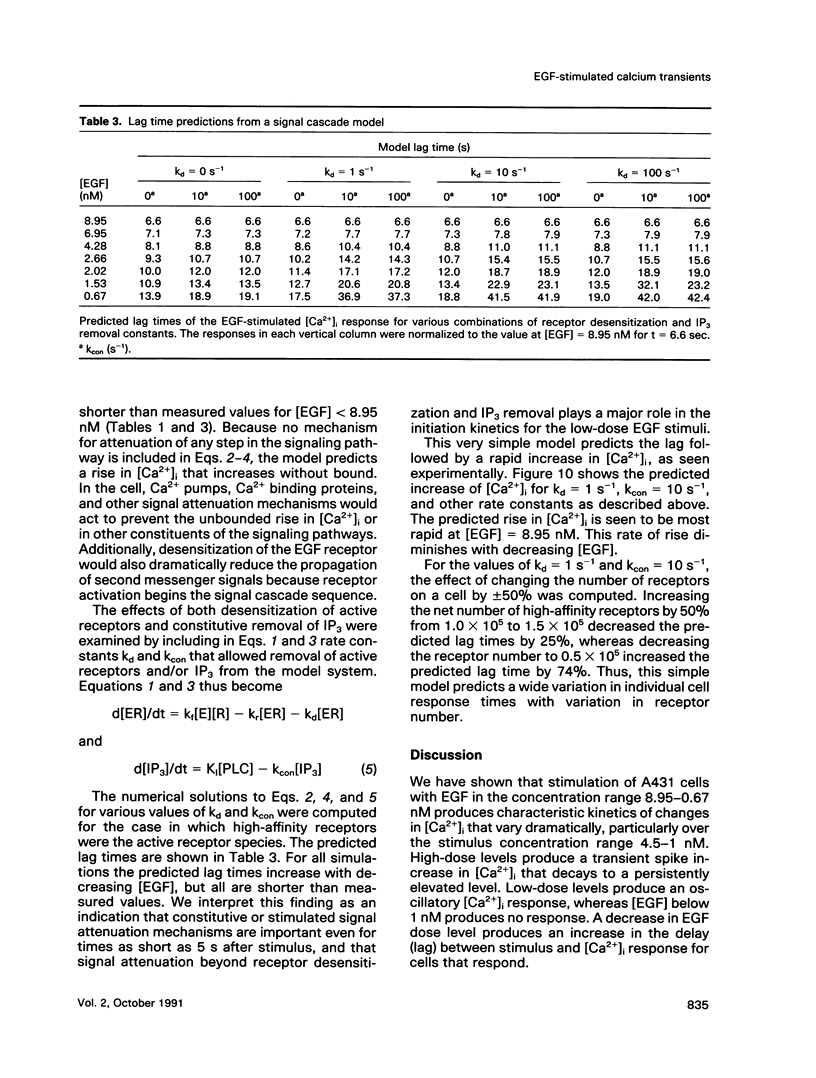
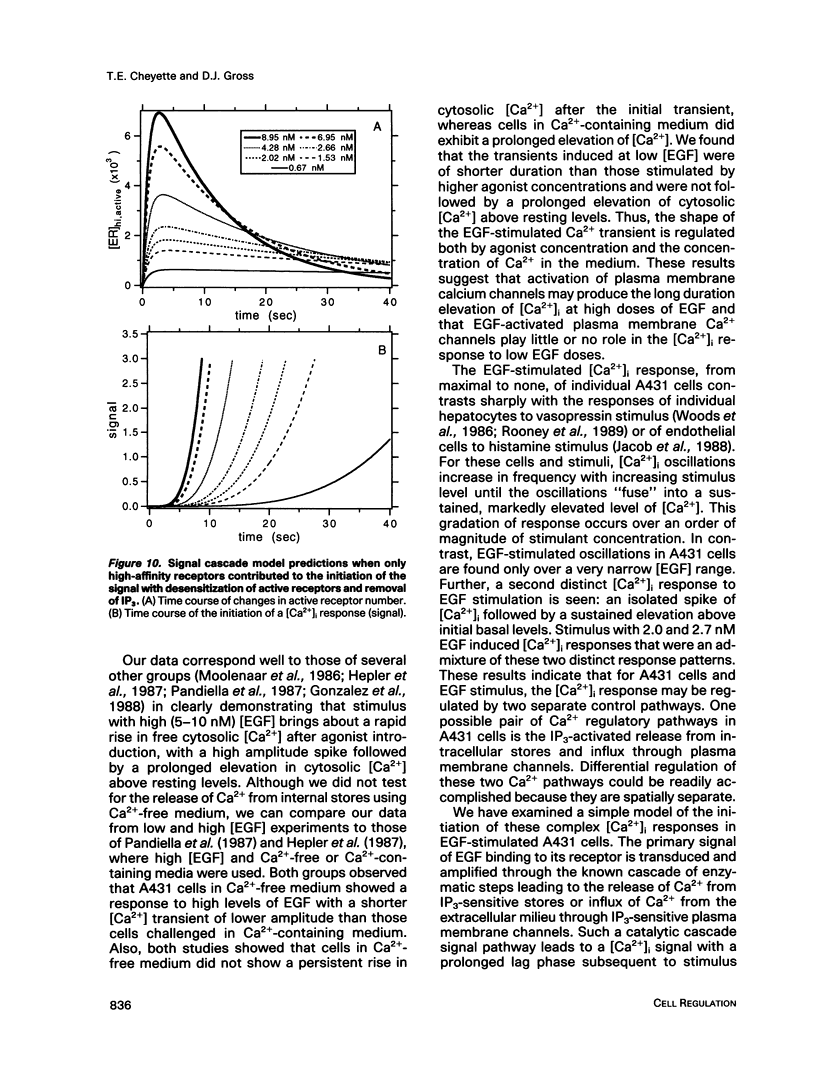
The A431 epidermoid carcinoma cell line responds to epidermal growth factor (EGF) stimulation with a number of rapid changes, including alterations in free cytosolic calcium ion concentration ([Ca2+]i). At the single cell level, these changes in [Ca2+]i are known to proceed after a clear lag phase subsequent to EGF stimulus (Gonzalez et al., 1988). The present study explores the dependence on EGF concentration of this early [Ca2+]i signal. High levels of EGF (9.0-4.3 nM) produce a [Ca2+]i spike followed by an elevation of [Ca2+]i above basal levels. The time of initiation of the spike varies from 5 to 9 s at the high dose and from 8 to 32 s at the low dose in cells that respond. A lower level of EGF (1.5 nM) produces [Ca2+]i oscillations with no prolonged elevation over basal [Ca2+]i. The initiation of response at this [EGF] ranges from 20 to 410 s. Intermediate stimulus levels generate [Ca2+]i responses that are kinetic admixtures of these limiting responses. A simple model based on the enzymatically amplified signal cascade from ligand binding through Ca2+ release or influx is examined. The model predicts a prolonged lag phase followed by a rapid increase in the [CA2+]i signal that compares favorably with the data reported here.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellot F., Moolenaar W., Kris R., Mirakhur B., Verlaan I., Ullrich A., Schlessinger J., Felder S. High-affinity epidermal growth factor binding is specifically reduced by a monoclonal antibody, and appears necessary for early responses. J Cell Biol. 1990 Feb;110(2):491–502. doi: 10.1083/jcb.110.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bjorge J. D., Chan T. O., Antczak M., Kung H. J., Fujita D. J. Activated type I phosphatidylinositol kinase is associated with the epidermal growth factor (EGF) receptor following EGF stimulation. Proc Natl Acad Sci U S A. 1990 May;87(10):3816–3820. doi: 10.1073/pnas.87.10.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapron Y., Cochet C., Crouzy S., Jullien T., Keramidas M., Verdetti J. Tyrosine protein kinase activity of the EGF receptor is required to induce activation of receptor-operated calcium channels. Biochem Biophys Res Commun. 1989 Jan 31;158(2):527–533. doi: 10.1016/s0006-291x(89)80081-6. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Chock P. B., Rhee S. G., Stadtman E. R. Interconvertible enzyme cascades in cellular regulation. Annu Rev Biochem. 1980;49:813–843. doi: 10.1146/annurev.bi.49.070180.004121. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Defize L. H., Arndt-Jovin D. J., Jovin T. M., Boonstra J., Meisenhelder J., Hunter T., de Hey H. T., de Laat S. W. A431 cell variants lacking the blood group A antigen display increased high affinity epidermal growth factor-receptor number, protein-tyrosine kinase activity, and receptor turnover. J Cell Biol. 1988 Sep;107(3):939–949. doi: 10.1083/jcb.107.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defize L. H., Boonstra J., Meisenhelder J., Kruijer W., Tertoolen L. G., Tilly B. C., Hunter T., van Bergen en Henegouwen P. M., Moolenaar W. H., de Laat S. W. Signal transduction by epidermal growth factor occurs through the subclass of high affinity receptors. J Cell Biol. 1989 Nov;109(5):2495–2507. doi: 10.1083/jcb.109.5.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Dupont G., Berridge M. J., Goldbeter A. Latency correlates with period in a model for signal-induced Ca2+ oscillations based on Ca2(+)-induced Ca2+ release. Cell Regul. 1990 Oct;1(11):853–861. doi: 10.1091/mbc.1.11.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearn J. C., King A. C. EGF receptor affinity is regulated by intracellular calcium and protein kinase C. Cell. 1985 Apr;40(4):991–1000. doi: 10.1016/0092-8674(85)90359-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Gross D. J., Heppel L. A., Webb W. W. Studies on the increase in cytosolic free calcium induced by epidermal growth factor, serum, and nucleotides in individual A431 cells. J Cell Physiol. 1988 May;135(2):269–276. doi: 10.1002/jcp.1041350214. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Sim S. S., Kim U. H., Nishibe S., Wahl M. I., Carpenter G., Rhee S. G. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem. 1990 Mar 5;265(7):3940–3943. [PubMed] [Google Scholar]
- Kuppuswamy D., Pike L. J. Ligand-induced desensitization of 125I-epidermal growth factor internalization. J Biol Chem. 1989 Feb 25;264(6):3357–3363. [PubMed] [Google Scholar]
- Lax I., Bellot F., Honegger A. M., Schmidt A., Ullrich A., Givol D., Schlessinger J. Domain deletion in the extracellular portion of the EGF-receptor reduces ligand binding and impairs cell surface expression. Cell Regul. 1990 Jan;1(2):173–188. doi: 10.1091/mbc.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderman J. J., Harris L. J., Slakey L. L., Gross D. J. Charge-coupled device imaging of rapid calcium transients in cultured arterial smooth muscle cells. Cell Calcium. 1990 Feb-Mar;11(2-3):131–144. doi: 10.1016/0143-4160(90)90066-4. [DOI] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Latencies of membrane currents evoked in Xenopus oocytes by receptor activation, inositol trisphosphate and calcium. J Physiol. 1989 Aug;415:189–210. doi: 10.1113/jphysiol.1989.sp017718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Moolenaar W. H., Bierman A. J., Tilly B. C., Verlaan I., Defize L. H., Honegger A. M., Ullrich A., Schlessinger J. A point mutation at the ATP-binding site of the EGF-receptor abolishes signal transduction. EMBO J. 1988 Mar;7(3):707–710. doi: 10.1002/j.1460-2075.1988.tb02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P., Kuryshev YuA Epidermal growth factor activates calcium-permeable channels in A 431 cells. Biochim Biophys Acta. 1989 May 10;1011(2-3):171–175. doi: 10.1016/0167-4889(89)90206-1. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pandiella A., Malgaroli A., Meldolesi J., Vicentini L. M. EGF raises cytosolic Ca2+ in A431 and Swiss 3T3 cells by a dual mechanism. Redistribution from intracellular stores and stimulated influx. Exp Cell Res. 1987 May;170(1):175–185. doi: 10.1016/0014-4827(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Eakes A. T. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987 Feb 5;262(4):1644–1651. [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Thompson D. M., Cochet C., Chambaz E. M., Gill G. N. Separation and characterization of a phosphatidylinositol kinase activity that co-purifies with the epidermal growth factor receptor. J Biol Chem. 1985 Jul 25;260(15):8824–8830. [PubMed] [Google Scholar]
- Todderud G., Wahl M. I., Rhee S. G., Carpenter G. Stimulation of phospholipase C-gamma 1 membrane association by epidermal growth factor. Science. 1990 Jul 20;249(4966):296–298. doi: 10.1126/science.2374928. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Kim J. W., Kim H., Rhee S. G., Carpenter G. Identification of two epidermal growth factor-sensitive tyrosine phosphorylation sites of phospholipase C-gamma in intact HSC-1 cells. J Biol Chem. 1990 Mar 5;265(7):3944–3948. [PubMed] [Google Scholar]
- Wahl M. I., Sweatt J. D., Carpenter G. Epidermal growth factor (EGF) stimulates inositol trisphosphate formation in cells which overexpress the EGF receptor. Biochem Biophys Res Commun. 1987 Feb 13;142(3):688–695. doi: 10.1016/0006-291x(87)91469-0. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Pike L. J. Phosphatidylinositol kinase is activated in membranes derived from cells treated with epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7513–7517. doi: 10.1073/pnas.84.21.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988 Aug;107(2):801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


