Abstract
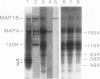
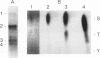
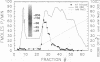
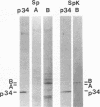
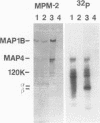
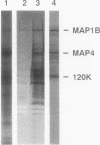
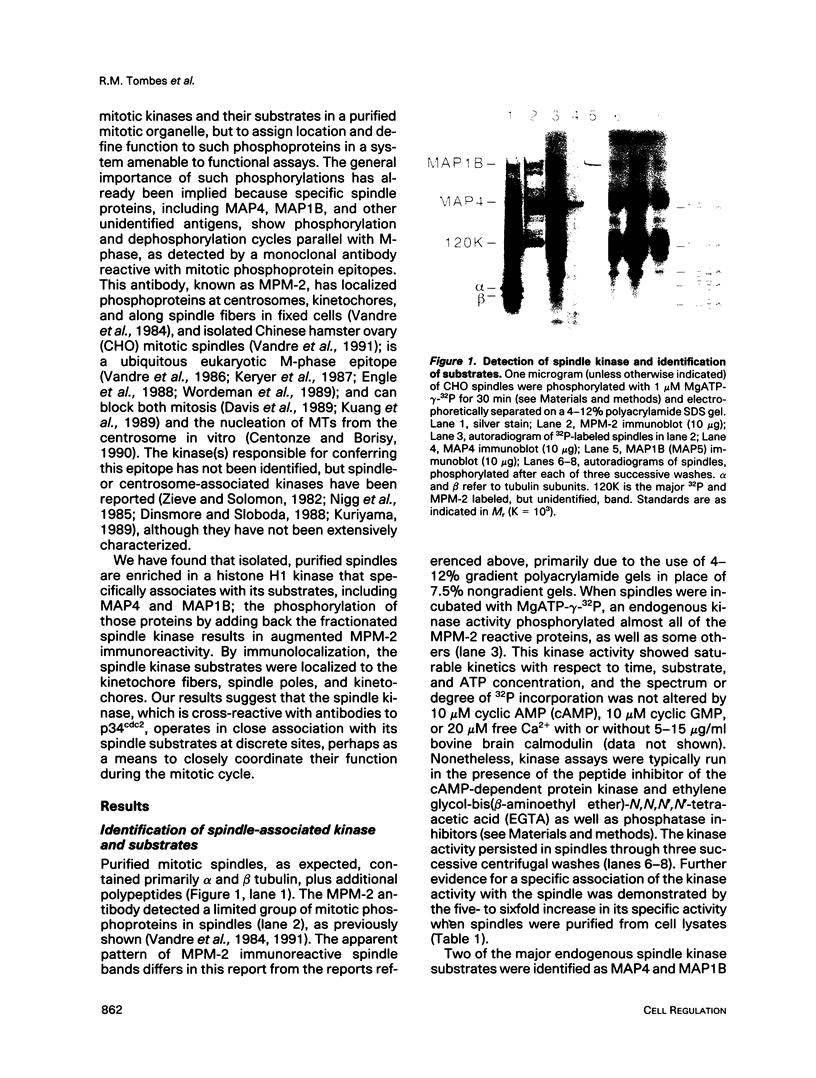
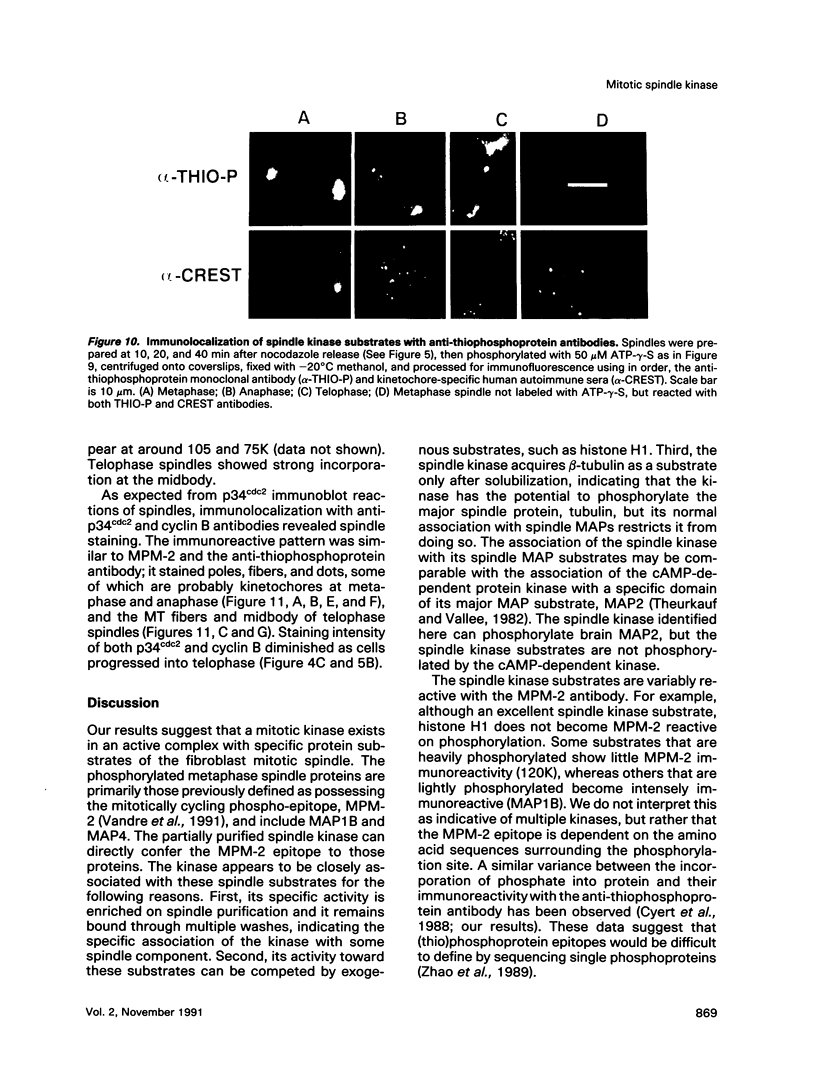
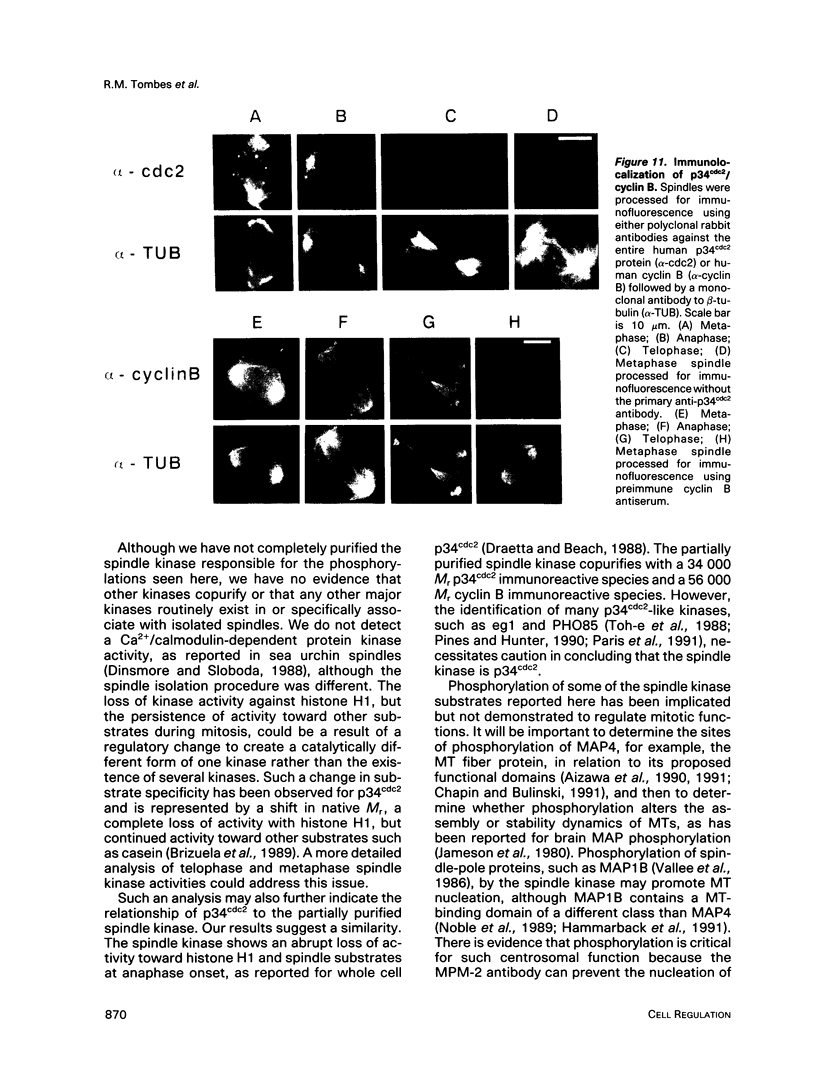
Isolated mammalian (Chinese hamster ovary [CHO]) metaphase spindles were found to be enriched in a histone H1 kinase whose activity was mitotic-cycle dependent. Two substrates for the kinase were identified as MAP1B and MAP4. Partially purified spindle kinase retained activity for the spindle microtubule-associated proteins (MAPs) as well as brain and other tissue culture MAPs; on phosphorylation, spindle MAPs exhibited increased immunoreactivity with MPM-2, a monoclonal antibody specific for a subset of mitotic phosphoproteins. Immunofluorescence using an anti-thiophosphoprotein antibody localized in vitro phosphorylated spindle proteins to microtubule fibers, centrosomes, kinetochores, and midbodies. The fractionated spindle kinase was reactive with anti-human p34cdc2 antibodies and with an anti-human cyclin B but not an anti-human cyclin A antibody. We conclude that spindle MAPs undergo mitotic cycle-dependent phosphorylations in vivo and associate with a kinase that remains active on spindle isolation and may be related to p34cdc2.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Emori Y., Mori A., Murofushi H., Sakai H., Suzuki K. Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U). J Biol Chem. 1991 May 25;266(15):9841–9846. [PubMed] [Google Scholar]
- Aizawa H., Emori Y., Murofushi H., Kawasaki H., Sakai H., Suzuki K. Molecular cloning of a ubiquitously distributed microtubule-associated protein with Mr 190,000. J Biol Chem. 1990 Aug 15;265(23):13849–13855. [PubMed] [Google Scholar]
- Ajiro K., Shibata K., Nishikawa Y. Subtype-specific cyclic AMP-dependent histone H1 phosphorylation at the differentiation of mouse neuroblastoma cells. J Biol Chem. 1990 Apr 15;265(11):6494–6500. [PubMed] [Google Scholar]
- Alfa C. E., Ducommun B., Beach D., Hyams J. S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990 Oct 18;347(6294):680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenguer P., Caizergues-Ferrer M., Labbé J. C., Dorée M., Amalric F. Mitosis-specific phosphorylation of nucleolin by p34cdc2 protein kinase. Mol Cell Biol. 1990 Jul;10(7):3607–3618. doi: 10.1128/mcb.10.7.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Bernat R. L., Borisy G. G., Rothfield N. F., Earnshaw W. C. Injection of anticentromere antibodies in interphase disrupts events required for chromosome movement at mitosis. J Cell Biol. 1990 Oct;111(4):1519–1533. doi: 10.1083/jcb.111.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Klausner R. D., Sandoval I. V. A widely distributed nuclear protein immunologically related to the microtubule-associated protein MAP1 is associated with the mitotic spindle. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1146–1150. doi: 10.1073/pnas.82.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. Activation of human CDC2 protein as a histone H1 kinase is associated with complex formation with the p62 subunit. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4362–4366. doi: 10.1073/pnas.86.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Borisy G. G. Microtubule-associated proteins from cultured HeLa cells. Analysis of molecular properties and effects on microtubule polymerization. J Biol Chem. 1980 Dec 10;255(23):11570–11576. [PubMed] [Google Scholar]
- Centonze V. E., Borisy G. G. Nucleation of microtubules from mitotic centrosomes is modulated by a phosphorylated epitope. J Cell Sci. 1990 Mar;95(Pt 3):405–411. doi: 10.1242/jcs.95.3.405. [DOI] [PubMed] [Google Scholar]
- Chapin S. J., Bulinski J. C. Non-neuronal 210 x 10(3) Mr microtubule-associated protein (MAP4) contains a domain homologous to the microtubule-binding domains of neuronal MAP2 and tau. J Cell Sci. 1991 Jan;98(Pt 1):27–36. doi: 10.1242/jcs.98.1.27. [DOI] [PubMed] [Google Scholar]
- Chou Y. H., Bischoff J. R., Beach D., Goldman R. D. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990 Sep 21;62(6):1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Scherson T., Kirschner M. W. Monoclonal antibodies specific for thiophosphorylated proteins recognize Xenopus MPF. Dev Biol. 1988 Sep;129(1):209–216. doi: 10.1016/0012-1606(88)90175-3. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Wright D. A., Penkala J. E., Rao P. N. Mitosis-specific monoclonal antibodies block cleavage in amphibian embryos. Cell Struct Funct. 1989 Apr;14(2):271–277. doi: 10.1247/csf.14.271. [DOI] [PubMed] [Google Scholar]
- Dinsmore J. H., Sloboda R. D. Calcium and calmodulin-dependent phosphorylation of a 62 kd protein induces microtubule depolymerization in sea urchin mitotic apparatuses. Cell. 1988 Jun 3;53(5):769–780. doi: 10.1016/0092-8674(88)90094-3. [DOI] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Engle D. B., Doonan J. H., Morris N. R. Cell-cycle modulation of MPM-2-specific spindle pole body phosphorylation in Aspergillus nidulans. Cell Motil Cytoskeleton. 1988;10(3):434–437. doi: 10.1002/cm.970100310. [DOI] [PubMed] [Google Scholar]
- Grillner S., Matsushima T. The neural network underlying locomotion in lamprey--synaptic and cellular mechanisms. Neuron. 1991 Jul;7(1):1–15. doi: 10.1016/0896-6273(91)90069-c. [DOI] [PubMed] [Google Scholar]
- Heald R., McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990 May 18;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Mitchison T. J. Two different microtubule-based motor activities with opposite polarities in kinetochores. Nature. 1991 May 16;351(6323):206–211. doi: 10.1038/351206a0. [DOI] [PubMed] [Google Scholar]
- Jameson L., Frey T., Zeeberg B., Dalldorf F., Caplow M. Inhibition of microtubule assembly by phosphorylation of microtubule-associated proteins. Biochemistry. 1980 May 27;19(11):2472–2479. doi: 10.1021/bi00552a027. [DOI] [PubMed] [Google Scholar]
- Keryer G., Davis F. M., Rao P. N., Beisson J. Protein phosphorylation and dynamics of cytoskeletal structures associated with basal bodies in Paramecium. Cell Motil Cytoskeleton. 1987;8(1):44–54. doi: 10.1002/cm.970080107. [DOI] [PubMed] [Google Scholar]
- Kuang J., Zhao J., Wright D. A., Saunders G. F., Rao P. N. Mitosis-specific monoclonal antibody MPM-2 inhibits Xenopus oocyte maturation and depletes maturation-promoting activity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4982–4986. doi: 10.1073/pnas.86.13.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R. 225-Kilodalton phosphoprotein associated with mitotic centrosomes in sea urchin eggs. Cell Motil Cytoskeleton. 1989;12(2):90–103. doi: 10.1002/cm.970120204. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Borisy G. G. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J Cell Biol. 1981 Dec;91(3 Pt 1):822–826. doi: 10.1083/jcb.91.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Keryer G., Borisy G. G. The mitotic spindle of Chinese hamster ovary cells isolated in taxol-containing medium. J Cell Sci. 1984 Mar;66:265–275. doi: 10.1242/jcs.66.1.265. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Watrin A., Labbé J. C., Cavadore J. C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990 Jan 12;60(1):151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- Lowndes J. M., Hokin-Neaverson M., Bertics P. J. Kinetics of phosphorylation of Na+/K(+)-ATPase by protein kinase C. Biochim Biophys Acta. 1990 Apr 9;1052(1):143–151. doi: 10.1016/0167-4889(90)90069-p. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Vallee R. B., Borisy G. G. Identity and polymerization-stimulatory activity of the nontubulin proteins associated with microtubules. Biochemistry. 1977 Jun 14;16(12):2598–2605. doi: 10.1021/bi00631a004. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Schäfer G., Hilz H., Eppenberger H. M. Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell. 1985 Jul;41(3):1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- Noble M., Lewis S. A., Cowan N. J. The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J Cell Biol. 1989 Dec;109(6 Pt 2):3367–3376. doi: 10.1083/jcb.109.6.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Tombes R. M., Meijer L., Krebs E. G. Activation of myelin basic protein kinases during echinoderm oocyte maturation and egg fertilization. Dev Biol. 1988 Nov;130(1):28–36. doi: 10.1016/0012-1606(88)90410-1. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. Identification of major nucleolar proteins as candidate mitotic substrates of cdc2 kinase. Cell. 1990 Mar 9;60(5):791–801. doi: 10.1016/0092-8674(90)90093-t. [DOI] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Rattner J. B., Lew J., Wang J. H. p34cdc2 kinase is localized to distinct domains within the mitotic apparatus. Cell Motil Cytoskeleton. 1990;17(3):227–235. doi: 10.1002/cm.970170309. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Riederer B., Cohen R., Matus A. MAP5: a novel brain microtubule-associated protein under strong developmental regulation. J Neurocytol. 1986 Dec;15(6):763–775. doi: 10.1007/BF01625193. [DOI] [PubMed] [Google Scholar]
- Sellitto C., Kuriyama R. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J Cell Biol. 1988 Feb;106(2):431–439. doi: 10.1083/jcb.106.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Snyder J. A., Hamilton B. T., Mullins J. M. Loss of mitotic centrosomal microtubule initiation capacity at the metaphase-anaphase transition. Eur J Cell Biol. 1982 Jun;27(2):191–199. [PubMed] [Google Scholar]
- Theurkauf W. E., Vallee R. B. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem. 1982 Mar 25;257(6):3284–3290. [PubMed] [Google Scholar]
- Toh-e A., Tanaka K., Uesono Y., Wickner R. B. PHO85, a negative regulator of the PHO system, is a homolog of the protein kinase gene, CDC28, of Saccharomyces cerevisiae. Mol Gen Genet. 1988 Sep;214(1):162–164. doi: 10.1007/BF00340196. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Enzyme termini of a phosphocreatine shuttle. Purification and characterization of two creatine kinase isozymes from sea urchin sperm. J Biol Chem. 1987 Nov 25;262(33):16011–16019. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Bloom G. S., Luca F. C. Differential structure and distribution of the high molecular weight brain microtubule-associated proteins, MAP-1 and MAP-2. Ann N Y Acad Sci. 1986;466:134–144. doi: 10.1111/j.1749-6632.1986.tb38391.x. [DOI] [PubMed] [Google Scholar]
- Vandre D. D., Davis F. M., Rao P. N., Borisy G. G. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandré D. D., Centonze V. E., Peloquin J., Tombes R. M., Borisy G. G. Proteins of the mammalian mitotic spindle: phosphorylation/dephosphorylation of MAP-4 during mitosis. J Cell Sci. 1991 Apr;98(Pt 4):577–588. doi: 10.1242/jcs.98.4.577. [DOI] [PubMed] [Google Scholar]
- Vandré D. D., Davis F. M., Rao P. N., Borisy G. G. Distribution of cytoskeletal proteins sharing a conserved phosphorylated epitope. Eur J Cell Biol. 1986 Jun;41(1):72–81. [PubMed] [Google Scholar]
- Verde F., Labbé J. C., Dorée M., Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990 Jan 18;343(6255):233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Kirschner M. W. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell. 1990 May 18;61(4):561–577. doi: 10.1016/0092-8674(90)90469-u. [DOI] [PubMed] [Google Scholar]
- Wordeman L., Davis F. M., Rao P. N., Cande W. Z. Distribution of phosphorylated spindle-associated proteins in the diatom Stephanopyxis turris. Cell Motil Cytoskeleton. 1989;12(1):33–41. doi: 10.1002/cm.970120105. [DOI] [PubMed] [Google Scholar]
- Zhao J. Y., Kuang J., Adlakha R. C., Rao P. N. Threonine phosphorylation is associated with mitosis in HeLa cells. FEBS Lett. 1989 Jun 5;249(2):389–395. doi: 10.1016/0014-5793(89)80665-9. [DOI] [PubMed] [Google Scholar]
- Zieve G., Solomon F. Proteins specifically associated with the microtubules of the mammalian mitotic spindle. Cell. 1982 Feb;28(2):233–242. doi: 10.1016/0092-8674(82)90341-5. [DOI] [PubMed] [Google Scholar]