Abstract
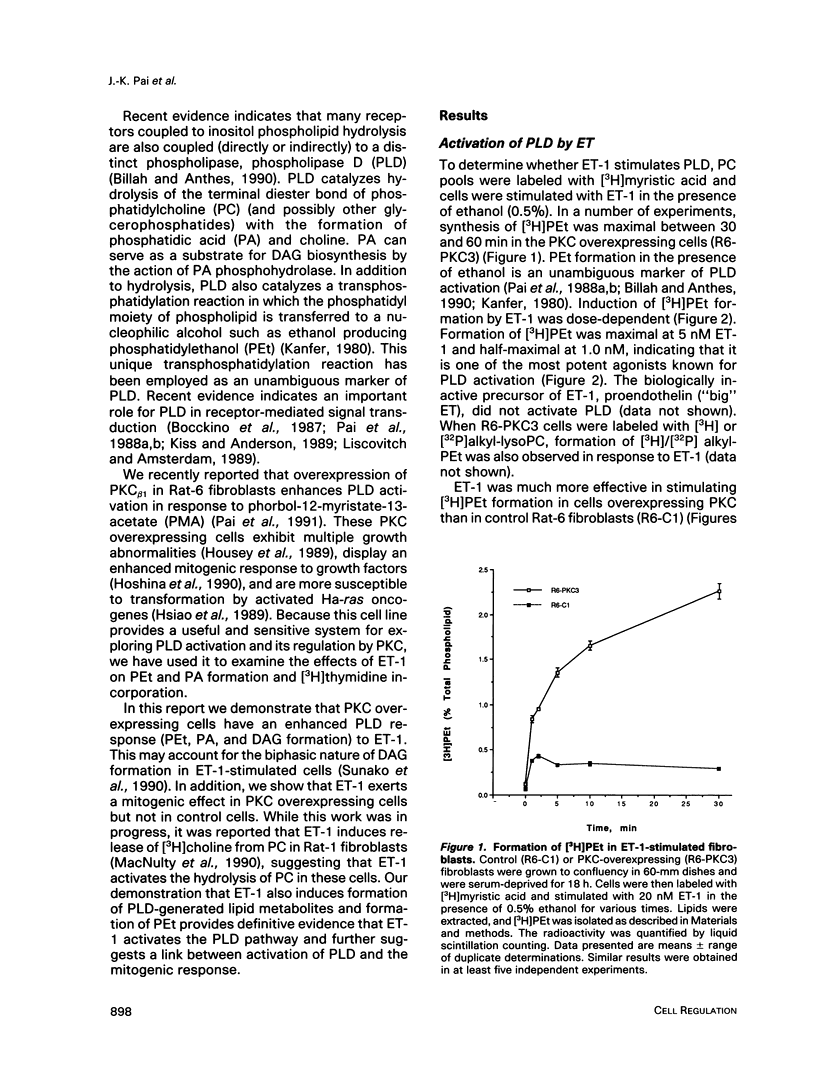
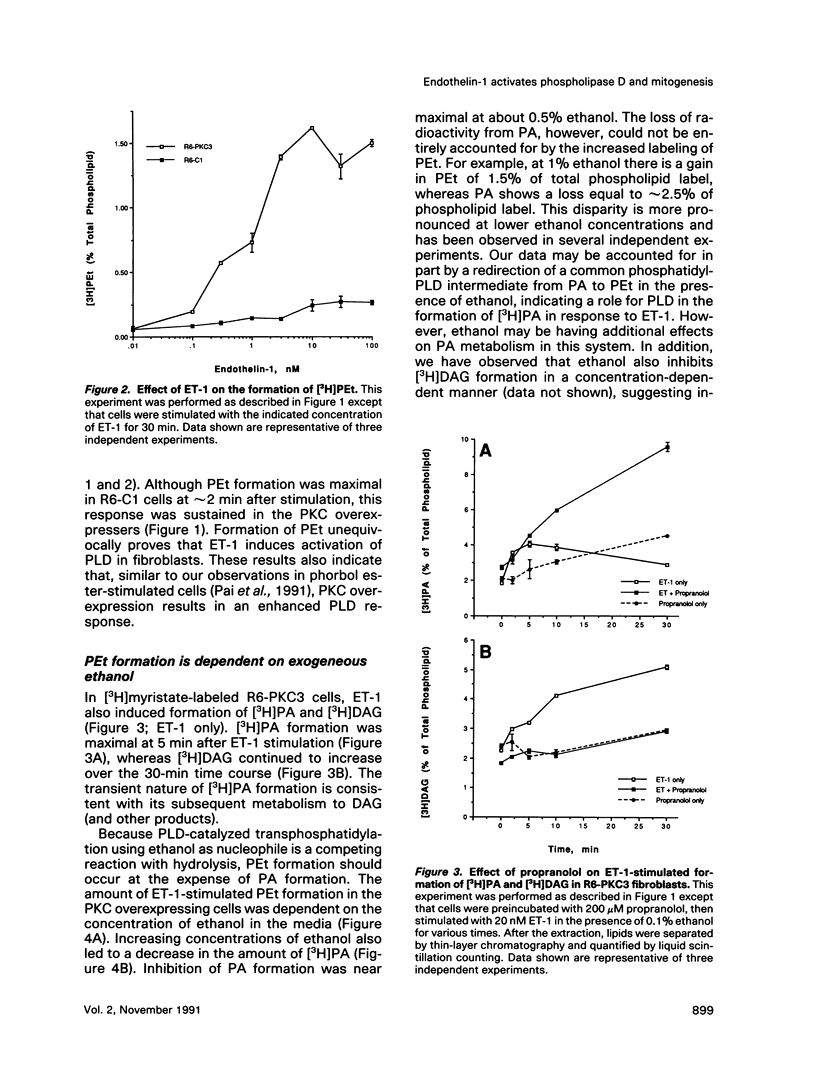
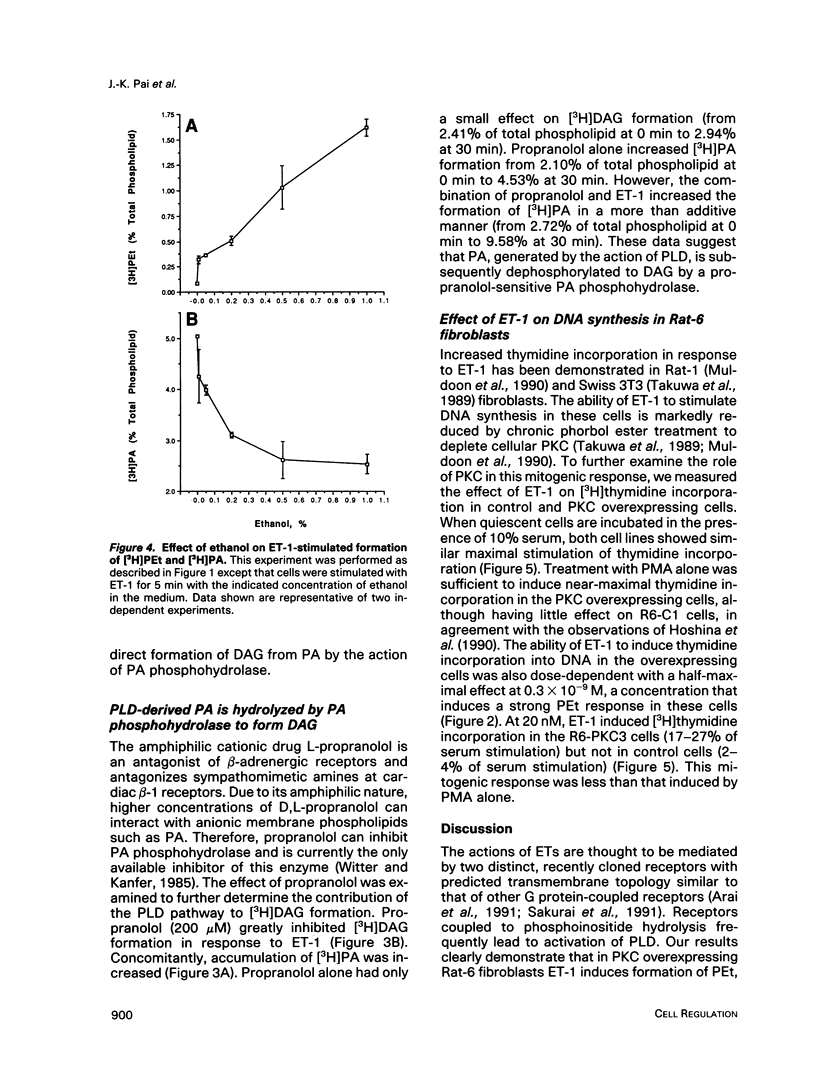
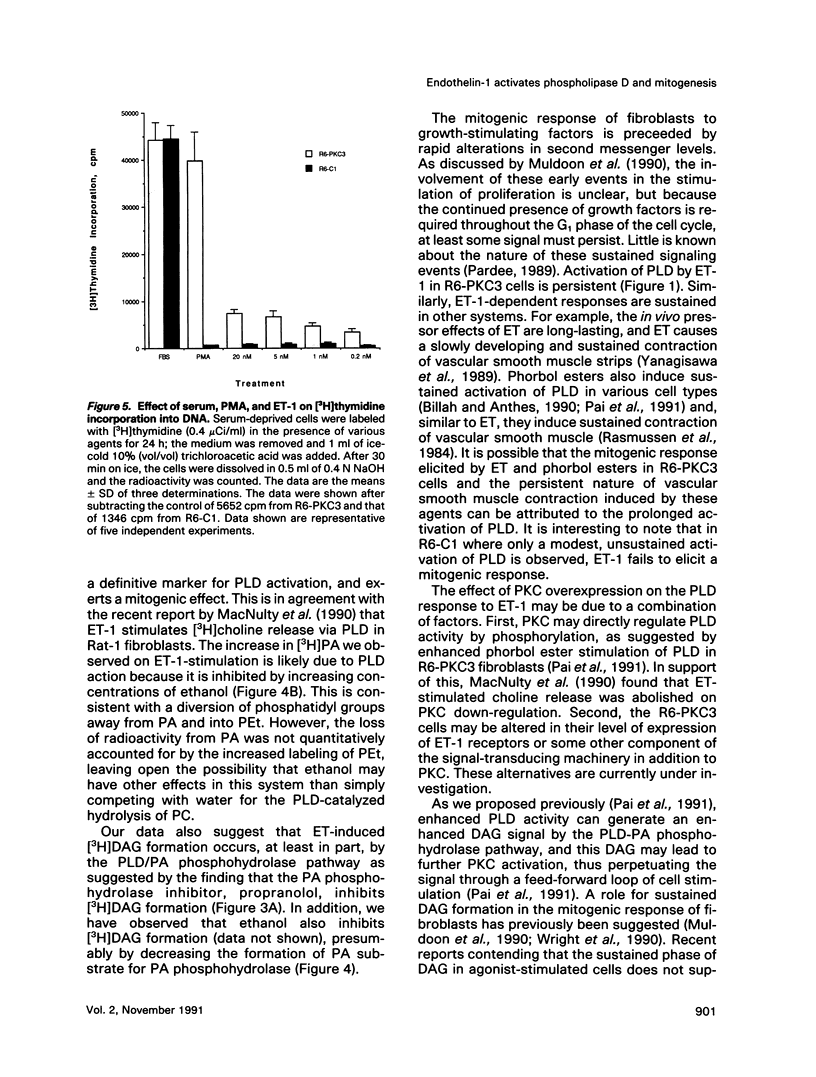
Endothelins (ETs) are a family of extremely potent vasoconstrictor peptides. In addition, ET-1 acts as a potent mitogen and activates phospholipase C in smooth muscle cells and fibroblasts. We examined the effects of ET-1 on phosphatidylcholine (PC) metabolism and thymidine incorporation in control Rat-6 fibroblasts and in cells that overexpress protein kinase C beta 1 (PKC). PC pools were labeled with [3H]myristic acid, and formation of phosphatidylethanol (PEt), an unambiguous marker of phospholipase D (PLD) activation, was monitored. ET-1 stimulated much greater PEt formation in the PKC overexpressing cells. ET-1 action was dose-dependent with a half-maximal effect at 1.0 x 10(-9) M. With increasing ethanol concentrations, [3H]PEt formation increased at the expense of [3H]phosphatidic acid (PA). Propranolol, an inhibitor of PA phosphohydrolase, increased [3H]PA accumulation and decreased [3H]diacylglycerol (DAG) formation. These data are consistent with the formation of [3H]DAG from PC by the sequential action of PLD and PA phosphohydrolase. Phorbol esters are known to stimulate thymidine incorporation and PLD activity to a greater extent in PKC overexpressing cells than in control cells. ET-1 also stimulates thymidine incorporation to a greater extent in the PKC overexpressing cells. The effect of ET-1 on thymidine incorporation into DNA in the overexpressing cells was also dose-dependent with a half-maximal effect at 0.3 x 10(-9) M. Enhanced PLD activity induced by ET-1 in the overexpressing cells may contribute to the mitogenic response, especially in light of a possible role of the PLD product, PA, in regulation of cell growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Hoshina S., Ueffing M., Weinstein I. B. Growth factor-induced DNA synthesis in cells that overproduce protein kinase C. J Cell Physiol. 1990 Nov;145(2):262–267. doi: 10.1002/jcp.1041450210. [DOI] [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Housey G. M., Johnson M. D., Weinstein I. B. Cells that overproduce protein kinase C are more susceptible to transformation by an activated H-ras oncogene. Mol Cell Biol. 1989 Jun;9(6):2641–2647. doi: 10.1128/mcb.9.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer J. N. The base exchange enzymes and phospholipase D of mammalian tissue. Can J Biochem. 1980 Dec;58(12):1370–1380. doi: 10.1139/o80-186. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J Biol Chem. 1989 Jan 25;264(3):1483–1487. [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Wright T. M., Pessin M. S., Raben D. M. Dissociation of protein kinase C activation and sn-1,2-diacylglycerol formation. Comparison of phosphatidylinositol- and phosphatidylcholine-derived diglycerides in alpha-thrombin-stimulated fibroblasts. J Biol Chem. 1991 Feb 15;266(5):3215–3221. [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- MacNulty E. E., Plevin R., Wakelam M. J. Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J. 1990 Dec 15;272(3):761–766. doi: 10.1042/bj2720761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. F., Hsieh K. P., Porter B. W. The sustained second phase of hormone-stimulated diacylglycerol accumulation does not activate protein kinase C in GH3 cells. J Biol Chem. 1990 May 5;265(13):7623–7631. [PubMed] [Google Scholar]
- Muldoon L. L., Pribnow D., Rodland K. D., Magun B. E. Endothelin-1 stimulates DNA synthesis and anchorage-independent growth of Rat-1 fibroblasts through a protein kinase C-dependent mechanism. Cell Regul. 1990 Mar;1(4):379–390. doi: 10.1091/mbc.1.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon L. L., Rodland K. D., Forsythe M. L., Magun B. E. Stimulation of phosphatidylinositol hydrolysis, diacylglycerol release, and gene expression in response to endothelin, a potent new agonist for fibroblasts and smooth muscle cells. J Biol Chem. 1989 May 25;264(15):8529–8536. [PubMed] [Google Scholar]
- Nakaki T., Nakayama M., Yamamoto S., Kato R. Endothelin-mediated stimulation of DNA synthesis in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Feb 15;158(3):880–883. doi: 10.1016/0006-291x(89)92804-0. [DOI] [PubMed] [Google Scholar]
- Pai J. K., Pachter J. A., Weinstein I. B., Bishop W. R. Overexpression of protein kinase C beta 1 enhances phospholipase D activity and diacylglycerol formation in phorbol ester-stimulated rat fibroblasts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):598–602. doi: 10.1073/pnas.88.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Forder J., Kojima I., Scriabine A. TPA-induced contraction of isolated rabbit vascular smooth muscle. Biochem Biophys Res Commun. 1984 Jul 31;122(2):776–784. doi: 10.1016/s0006-291x(84)80101-1. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kimura S., Shinmi O., Sugita Y., Yanagisawa M., Goto K., Masaki T. Purification and characterization of putative endothelin converting enzyme in bovine adrenal medulla: evidence for a cathepsin D-like enzyme. Biochem Biophys Res Commun. 1990 May 16;168(3):1230–1236. doi: 10.1016/0006-291x(90)91160-t. [DOI] [PubMed] [Google Scholar]
- Sunako M., Kawahara Y., Hirata K., Tsuda T., Yokoyama M., Fukuzaki H., Takai Y. Mass analysis of 1,2-diacylglycerol in cultured rabbit vascular smooth muscle cells. Comparison of stimulation by angiotensin II and endothelin. Hypertension. 1990 Jan;15(1):84–88. doi: 10.1161/01.hyp.15.1.84. [DOI] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Bollag W. E., Rasmussen H. The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem. 1987 Jan 5;262(1):182–188. [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- Witter B., Kanfer J. N. Hydrolysis of endogenous phospholipids by rat brain microsomes. J Neurochem. 1985 Jan;44(1):155–162. doi: 10.1111/j.1471-4159.1985.tb07125.x. [DOI] [PubMed] [Google Scholar]
- Wright T. M., Shin H. S., Raben D. M. Sustained increase in 1,2-diacylglycerol precedes DNA synthesis in epidermal-growth-factor-stimulated fibroblasts. Evidence for stimulated phosphatidylcholine hydrolysis. Biochem J. 1990 Apr 15;267(2):501–507. doi: 10.1042/bj2670501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Endothelin, a novel endothelium-derived peptide. Pharmacological activities, regulation and possible roles in cardiovascular control. Biochem Pharmacol. 1989 Jun 15;38(12):1877–1883. doi: 10.1016/0006-2952(89)90484-x. [DOI] [PubMed] [Google Scholar]


