Abstract
Insect-induced defenses occur in nearly all plants and are regulated by conserved signaling pathways. As the first described plant peptide signal, systemin regulates antiherbivore defenses in the Solanaceae, but in other plant families, peptides with analogous activity have remained elusive. In the current study, we demonstrate that a member of the maize (Zea mays) plant elicitor peptide (Pep) family, ZmPep3, regulates responses against herbivores. Consistent with being a signal, expression of the ZmPROPEP3 precursor gene is rapidly induced by Spodoptera exigua oral secretions. At concentrations starting at 5 pmol per leaf, ZmPep3 stimulates production of jasmonic acid, ethylene, and increased expression of genes encoding proteins associated with herbivory defense. These include proteinase inhibitors and biosynthetic enzymes for production of volatile terpenes and benzoxazinoids. In accordance with gene expression data, plants treated with ZmPep3 emit volatiles similar to those from plants subjected to herbivory. ZmPep3-treated plants also exhibit induced accumulation of the benzoxazinoid phytoalexin 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside. Direct and indirect defenses induced by ZmPep3 contribute to resistance against S. exigua through significant reduction of larval growth and attraction of Cotesia marginiventris parasitoids. ZmPep3 activity is specific to Poaceous species; however, peptides derived from PROPEP orthologs identified in Solanaceous and Fabaceous plants also induce herbivory-associated volatiles in their respective species. These studies demonstrate that Peps are conserved signals across diverse plant families regulating antiherbivore defenses and are likely to be the missing functional homologs of systemin outside of the Solanaceae.
Keywords: tritrophic interactions, molecular ecology, innate immunity, secondary metabolism
Plants respond to herbivore attack with sophisticated and dynamic immune responses that result in a suite of induced defenses. These responses include structural fortifications such as thorns, trichomes, and cell-wall strengthening, as well as biochemical defenses. For example, antinutritive proteinase inhibitors (PINs) are locally and systemically induced upon insect attack, but many other proteins contribute to antiherbivory responses as well (1). Enzymes such as polyphenol oxidase and threonine deaminase limit protein availability in the midgut, whereas others destabilize insect peritrophic membranes (2–4). Plants also draw upon a complex arsenal of small molecule chemical defenses including terpenoids, alkaloids, phenylpropanoids, glucosinolates, benzoxazinoids, and nonprotein amino acids (5, 6). These metabolites can act as feeding deterrents or negatively impact growth and fitness via direct toxicity or mimicry of insect hormones (5, 7).
Herbivory also stimulates emission of a complex blend of volatiles that function as indirect defenses through oviposition deterrence and attraction of natural enemies such as parasitoids and predators (8–12). These volatiles are also implicated in plant priming effects, alerting closely neighboring plants or tissues to potential attack and enabling a stronger and swifter response (13, 14). As herbivory-induced volatile production is conserved across diverse plant species and perceived across trophic levels, these volatiles exert wide-ranging effects on ecological communities (15, 16). Not surprisingly, a critical focus has been to understand how these responses are regulated (17).
Herbivores are initially perceived by plants through detection of mechanical damage and exposure to elicitors present in oral secretions while feeding, or prior to herbivory, via elicitors contained in oviposition fluid (18–22). Although receptors for herbivore-derived elicitors have not yet been identified, recognition occurs upon exposure to trace levels and is confined to subsets of plant species, indicating that perception is likely to be receptor mediated (23). Herbivory or elicitor treatment triggers a number of signaling processes conserved across species, including calcium flux; MAP kinase activation; and production of secondary signals such as reactive oxygen species (ROS), nitric oxide (NO), and the phytohormones jasmonic acid (JA), ethylene (ET), and salicylic acid (SA) (5, 17, 23–26).
Over 20 y ago, the 18-aa-peptide signal, systemin, was discovered to be a potent regulator of antiherbivore defenses (27). Systemin promotes proteinase inhibitor accumulation, volatile emission, and is critical to resistance against Lepidopteran herbivores in tomato (27–29). Many of the signaling events induced by herbivory were initially described in tomato and as systemin-induced responses, including calcium signaling, activation of MAP kinase activity, and production of the ROS, NO, ET, and oxylipin secondary signals (30–35). Although both signaling and downstream antiherbivore responses similar to those induced by systemin are conserved across the plant kingdom, involvement of peptide regulators has thus far appeared to be specific to the Solanaceae. Peptides similar to systemin, termed hydroxyproline systemins, have been discovered in other Solanaceous species and similarly activate accumulation of proteinase inhibitors and contribute to resistance (36–39). However, orthologous peptides with systemin-like activity have remained elusive in other plant families, raising numerous questions regarding the evolution of herbivory-associated peptide signals. Whether this elusiveness is because such peptides evolved only in the Solanaceae or because the amino acid sequence of functional orthologs in other species diverged to the point of being unrecognizable was unknown.
In the past decade, a number of plant peptides have been identified as defense-related signals in non-Solanaceous species; however, their activities have been consistent with antipathogen defenses (40–42). Among these are the plant elicitor peptides (Peps) isolated from Arabidopsis thaliana. A. thaliana plant elicitor peptide 1(AtPep1) activates expression of pathogen defense genes and confers disease resistance when ectopically expressed (40, 43, 44). Notably, Peps are the only family of plant defense peptide signals for which gene orthologs have been identified in numerous species through amino acid sequence homology (40). A maize ortholog, Zea mays plant elicitor peptide 1 (ZmPep1), was recently demonstrated to be functionally homologous, promoting accumulation of transcripts and metabolites associated with pathogen defense and enhancing resistance to multiple fungi (45). Pep-induced signaling initiates via binding to the plant elicitor peptide receptor (PEPR) LRR receptor kinases and involves secondary signals similar to those induced by herbivory and systemin, including production of the phytohormones JA and ET, calcium, ROS, and NO (40, 43–47). Through examination of maize Pep family activity, we demonstrate that one member, ZmPep3, strongly regulates direct and indirect antiherbivore defenses and that Pep regulation of herbivore-associated responses is a conserved motif across diverse plant species.
Results and Discussion
Maize Pep Regulates Herbivore-Induced Defenses.
The gene encoding ZmPROPEP1, precursor to the maize defense peptide ZmPep1, belongs to a five-gene family (Fig. 1A). Each encoded protein conforms to the established characteristics of PROPEP precursors, lacking a classical secretion signal but containing an amphipathic helix domain and predicted to liberate 23-aa bioactive peptides from the carboxyl terminus (40, 45, 48). Each ZmPep contains a conserved core motif in the carboxyl region similar to the A. thaliana AtPeps. However, whereas the amino end of AtPeps is highly enriched in lys/arg residues, the amino terminal region of the ZmPeps is proline rich (40).
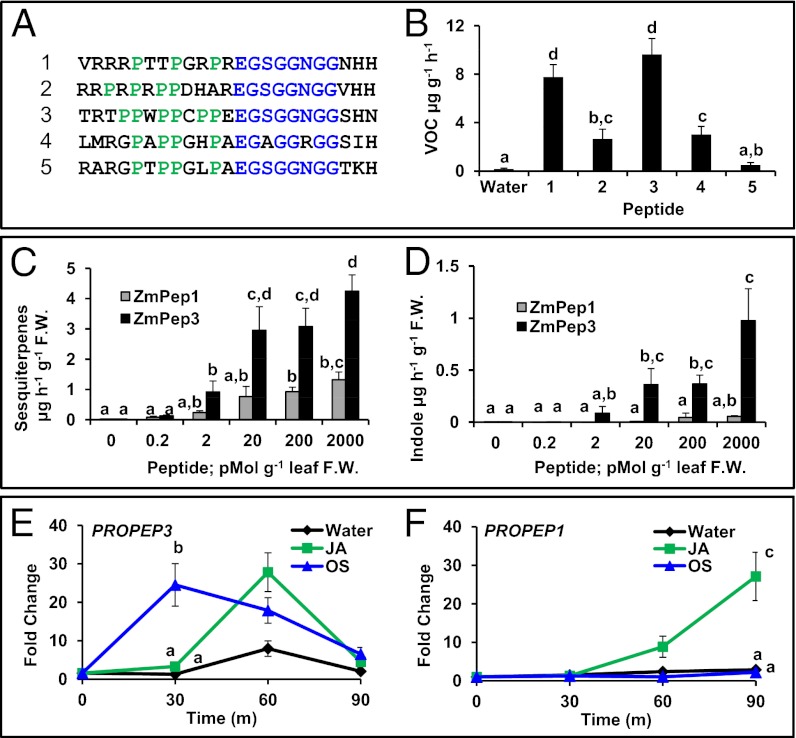
Fig. 1.
Maize plant elicitor peptide 3 (ZmPep3) is an herbivory-inducible signal that regulates emission of plant volatiles. (A) Alignment of maize Pep sequences, with amino-end prolines highlighted in green and core carboxyl motifs designated in blue. (B) Total volatile organic compounds (VOCs) emitted by maize leaves 16 h posttreatment with water or with 2 nmol⋅g−1 FW of each Pep. (C) Total sesquiterpenes emitted from leaves treated with increasing doses of either ZmPep1 (gray bars) or ZmPep3 (black bars). (D) Indole emitted from ZmPep1- or ZmPep3-treated leaves. Expression of the genes encoding ZmPROPEP3 (E) and ZmPROPEP1 (F), respective precursor proteins to ZmPep3 and ZmPep1, in intact leaves treated with water (black), 100 nmol jasmonic acid (JA, green), or 5 μL S. exigua oral secretions (OS, blue) as measured by qRT-PCR. Within each plot, different letters (a–d) represent significant differences between mean values (n = 4, ± SEM; all ANOVAs, P < 0.015; Tukey test corrections for multiple comparisons, P < 0.05).
ZmPep1 promotes accumulation of metabolites associated with pathogen defense, such as benzoxazinoids and pathway precursors including indole and anthranilate (45). Indole and methyl anthranilate are also components of maize herbivore-induced volatiles that attract natural enemies of attacking herbivores (9, 49, 50). To ascertain whether any of the five ZmPeps activate volatile emissions, excised leaves were provided ZmPeps for 16 h. Analysis revealed emission of indole and herbivory-associated terpene volatiles (Fig. 1B). Whereas four of the five peptides stimulated volatile emissions significantly greater than those produced by water-treated leaves, ZmPep3 induced the highest average rate of production. Comparative dose–response experiments confirmed that ZmPep3 is the strongest elicitor of both indole and terpene emission, acting at levels as low as 2–20 pmol⋅g−1 leaf fresh weight (FW) (Fig. 1 C and D). Treatment with ZmPep3 promoted volatile emission in a number of maize varieties and resulted in release of a blend qualitatively matching that induced by Spodoptera exigua herbivory (Figs. S1 and S2). The ZmPeps are naturally occurring variants of a similar amino acid motif, yet ZmPep3 is a potent elicitor of emissions, whereas ZmPep5 is statistically inactive and a functional negative control. This disparity in activity implies that ZmPep3-induced volatile emission results from a specific interaction that is abolished by amino acid differences between ZmPeps.
To examine ZmPROPEP3 transcript accumulation following herbivore elicitation, S. exigua oral secretions (OS) were applied to scratch-wounded intact leaves. Consistent with ZmPep3 as a candidate signal regulating herbivory-associated volatiles, transcripts encoding ZmPROPEP3 rapidly accumulated in response to OS, whereas expression of the pathogen-inducible precursor gene ZmPROPEP1 did not (Fig. 1 E and F). Transcripts for both precursor genes accumulated after scratch-wounding plus application of 100 nmol JA . However, the temporal expression patterns differed, with ZmPROPEP3 transcript levels increasing in a faster but more transient manner. Queries of maize proteome data representing profiles of 34 distinct tissue types and developmental stages revealed that ZmPROPEP1 (Fig. S3) and ZmPROPEP2 precursors are present at detectable levels in the absence of biotic stress. However, ZmPROPEP3 precursor protein was not observed in unchallenged tissues. Taken together, the potent picomolar activity, volatile elicitation, and induced precursor gene expression support a role for ZmPep3 in regulating responses to herbivory (Fig. S4).
ZmPep3 Activates Production of JA, ET, and Expression of Associated Biosynthetic Genes.
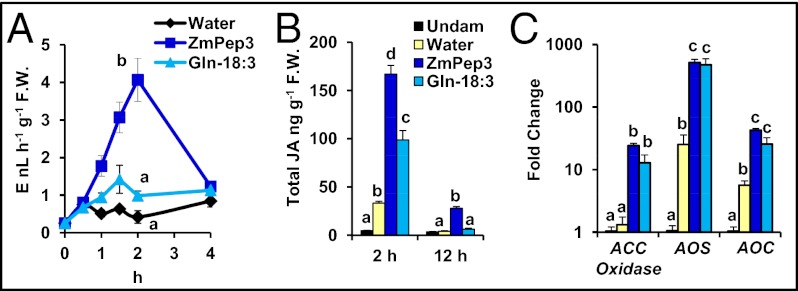
The elicitor-induced phytohormones JA and ET function as signals integral to the initiation of protective responses against pathogens and herbivores (5). N-linolenoyl-L-glutamine (Gln-18:3) is an elicitor present in the oral secretions of many Lepidopteran species, which exhibits strong activity in promoting both phytohormone production and emission of herbivory-associated volatiles (23, 51). Because insect oral secretions induced expression of the ZmPROPEP3 precursor gene, it may be that ZmPep3 mediates signaling in response to oral secretions and elicitors such as Gln-18:3. Relative concentrations of Gln-18:3 and ZmPep3 in leaf tissue subjected to herbivory are not known, and thus we examined equivalent molar quantities to probe the potency of ZmPep3 in regulating hormone biosynthesis and other responses using Gln-18:3 as a positive control. In a comparative treatment of excised leaves supplied with equivalent quantities of either ZmPep3 or Gln-18:3, both resulted in elicitation of JA and ET synthesis (Fig. 2 A and B). However, ZmPep3-induced increases were of greater magnitude and longer duration. Both ZmPep3 and Gln-18:3 treatments caused significantly increased accumulation of transcripts encoding biosynthetic enzymes for ET and JA, 1-aminocyclopropane-1-carboxylate (ACC) oxidase, allene oxide synthase (AOS), and allene oxide cyclase (AOC) (Fig. 2C).
Fig. 2.
ZmPep3 has similar activity to the potent insect-derived elicitor, N-linolenoyl-L-glutamine (Gln-18:3), in activating phytohormone signals. (A) Emission of ethylene (ET) in response to treatment with water (black), ZmPep3 (dark blue), or Gln-18:3 (light blue). (B) Jasmonic acid (JA) in undamaged leaves (black) vs. leaves treated with water (yellow), ZmPep3 or Gln-18:3. (C) Relative expression of genes encoding biosynthetic enzymes for ET (ACC oxidase) and JA (AOS, AOC) 2 h posttreatment as determined by qRT-PCR. Statistics were used to compare plant tissues that were damaged and treated with either water, ZmPep3, or Gln-18:3 each applied at 2 nmol⋅g−1 FW. Within each plot, different letters (a–c) represent significant differences between mean values (n = 4, ± SEM; all ANOVAs, P < 0.005; Tukey test corrections for multiple comparisons, P < 0.05).
The role of JA in defense was first described in the context of peptide-activated responses to herbivory; systemin triggers JA synthesis, and JA treatment results in expression of genes encoding both the prosystemin precursor and systemin-regulated defense genes (30, 35, 52). This amplification loop functions to propagate initial wound signals into systemic plant defense responses (35, 53, 54). Systemin is not mobile, but systemin-mediated amplification of jasmonate production in vascular tissues promotes long-distance defense responses. Plant elicitor peptides are also postulated to act as amplifiers in defense signaling cascades (55). Activation of Peps by exposure to elicitors or defensive phytohormones is likely to propagate defensive signaling temporally and spatially (55). Increased expression of maize ZmPROPEP3 and ZmPROPEP1 in response to JA is consistent with a role in signal amplification. Subsequent production of JA and ET in response to Peps functionally completes this signaling amplification cycle (45, 46; Fig. S5).
ZmPep3 Stimulates Indirect Defense Responses That Are Sufficient to Attract Parasitoid Natural Enemies.
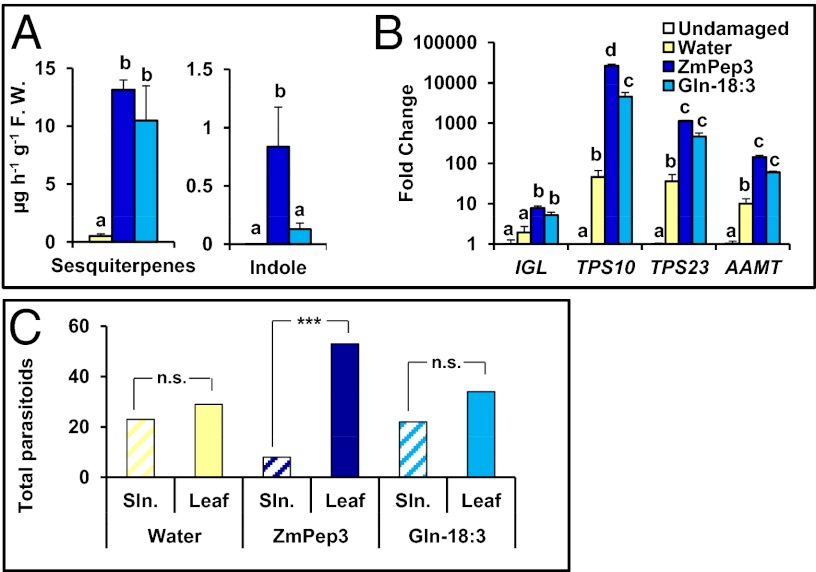
Induced terpene production is vital to antiherbivore defenses in many plant species (12, 56, 57). Gln-18:3 is among the strongest known elicitors of herbivory-induced maize terpene volatiles, and ZmPep3 displays similar activity when supplied to excised leaves at equivalent molar concentrations (Fig. 3A) (23, 51). Both strongly activate expression of genes encoding terpene synthases (TPSs) 10 and 23, enzymes for biosynthesis of E-α-bergamotene, and E-β-caryophyllene production, respectively (58, 59) (Fig. 3B). Although there was no significant quantitative difference in terpenes emitted by leaves treated with ZmPep3 versus Gln-18:3, TPS10 and TPS23 gene expression was more strongly induced by the peptide. These results correspond to significantly greater accumulation of terpenes associated with volatile emission in ZmPep3-treated leaves when internal pools were measured before volatile collection (Fig. S6A).
Fig. 3.
Responses induced by ZmPep3 are effective indirect defenses against S. exigua herbivory. (A) Emission of total sesquiterpenes and indole 16 h posttreatment. Statistics were used to compare plant tissues that were damaged and treated with either water, ZmPep3, or Gln-18:3 each applied at 2 nmol⋅g−1 FW. Within each plot, different letters (a–c) represent significant differences between mean values (n = 4, ± SEM; all ANOVAs, P < 0.005; Tukey test corrections for multiple comparisons, P < 0.05). (B) Relative expression of genes encoding enzymes for the biosynthesis of the emitted volatiles indole (IGL), E-α-bergamotene (TPS10), E-β-caryophyllene (TPS23), and methyl anthranilate (AAMT) 12 h posttreatment with water (yellow), ZmPep3 (dark blue), or Gln-18:3 (light blue) as determined by qRT-PCR. (C) Attraction of the parasitoid C. marginiventris to leaves (crosshatched bars) incubated in water, ZmPep3, or Gln-18:3 solutions (each at 200 pmol⋅g−1 FW) and to the respective solution controls (open bars); representation of combined results from 10 replicate experiments (stars show significant differences between solution controls and incubated leaves; ***P < 0.001; NS, not significant).
Shikimate pathway-derived volatile components, including anthranilate and indole increased in leaves following ZmPep3 and Gln-18:3 treatment; however, the peptide caused significantly greater accumulation (Fig. 3B and Fig. S6B). Indole emission also increased above damage alone in ZmPep3-treated, but not Gln-18:3–treated leaves (Fig. 3A). Interestingly, despite differences at the metabolite level, expression of genes encoding the relevant biosynthetic enzymes, anthranilic acid methyl transferase 1 (AAMT1) and indole-3-glycerol phosphate lyase (IGL) were induced by both peptide and the insect elicitor (Fig. 3B) (49, 50).
ZmPep3 elicits the same spectrum of volatile components as S. exigua herbivory (Fig. S2), indicating that peptide-treated leaves may also be attractive to entomoparasitic wasps. The generalist parasitoid Cotesia marginiventris attacks many Lepidopteran species including S. exigua and is attracted to herbivory-induced maize volatiles (9). Precisely which components are attractive has been difficult to determine; wasps respond to unidentified minor components (60). To determine whether the ZmPep3-induced blend was attractive to naïve C. marginiventris, olfactometer assays were conducted with leaves pretreated with ZmPep3, Gln-18:3, or water for 16 h. To ensure that any preference was due to elicited plant responses and not to an inherent attraction or repellence to any treatment substance, control solutions containing the different elicitors were used. The attractiveness of the different control solutions did not differ significantly (Tukey’s honestly significant difference following ANOVA; water vs. Gln-18:3, P = 0.9999; water vs. ZmPep3, P = 0.4922; and ZmPep3 vs. Gln-18:3, P = 0.5697). When leaves were pretreated with 200 pmol⋅g−1 FW ZmPep3, they were strongly preferred by wasps compared with the paired solution control (Fig. 3C). In contrast, C. marginiventris did not display a statistically significant preference for leaves pretreated with either water or Gln-18:3 (Fig. 3C). Whereas treatment of maize plants with purified fatty acid amide elicitors has been demonstrated to attract Microplitis croceipes, we did not observe significant attraction of C. marginiventris in these assays to Gln-18:3–treated leaves with the 200 pmol⋅g−1 FW dose (61). Although of equal molarity to the applied ZmPep3, this quantity is likely less than would be deposited via oral secretions during feeding. In a second experiment, we compared the capacity of ZmPep1 and ZmPep3 to attract C. marginiventris (Fig. S4B). As before, ZmPep3-treated leaves were highly attractive to the parasitoid, whereas ZmPep1 did not elicit significant attraction compared with the solution control. Together the data indicate that ZmPep3-induced responses generate effective direct and indirect defenses against Lepidopteran herbivores.
ZmPep3 Promotes Direct Defenses Against Herbivores That Inhibit Insect Larval Growth.
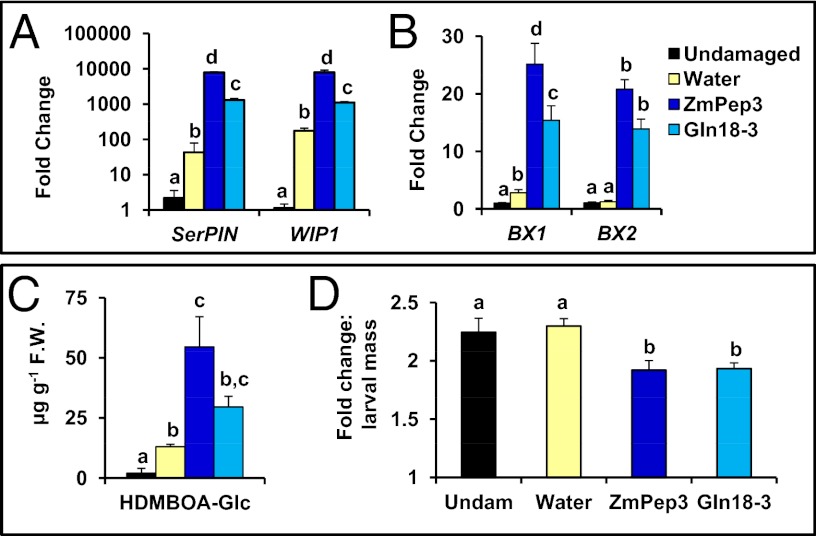
A microarray analysis of transcript levels in excised leaves that were treated for 12 h with either water or ZmPep3 revealed that the most highly up-regulated probe set in ZmPep3-treated leaves corresponded to the Bowman–Birk type proteinase inhibitor SerPIN (Dataset S1). Two additional Bowman–Birk type PIN genes, including wound induced protein 1 (WIP1), were also among the most induced probe sets. Analysis by qRT-PCR validated that both SerPIN and WIP1 were highly expressed in leaves treated with ZmPep3 and to a lesser degree, with Gln-18:3 (Fig. 4A). PINs inhibit insect digestive enzymes and result in reduced larval growth and the discovery that PINs accumulate in response to herbivory contributed to the paradigm of rapidly inducible defenses in plants (1, 62). The assay that identified systemin as a signal regulating tomato defenses was based upon induced PIN accumulation (27). Although this herbivore-induced response is conserved across many plant families, regulation of PIN transcription by endogenous plant peptides had not been observed previously outside of the Solanaceae.
Fig. 4.
Responses induced by ZmPep3 are effective direct defenses against Spodoptora exigua herbivory. (A) Relative expression of genes encoding proteinase inhibitors and (B) genes encoding biosynthetic enzymes for benzoxazinoid defense metabolites associated with direct defense against herbivores 12 h posttreatment with water (yellow), ZmPep3 (dark blue), or Gln-18:3 (light blue). (C) Accumulation of the highly reactive benzoxazinoid precursor 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) 48 h posttreatment in scratch-wounded intact leaves. Statistics were used to compare plant tissues that were damaged and treated with either water, ZmPep3, or Gln-18:3 each applied at 2 nmol⋅g−1 FW. Within each plot, different letters (a–c) represent significant differences between mean values (n = 4, ± SEM; all ANOVAs, P < 0.005; Tukey test corrections for multiple comparisons, P < 0.05). (D) Fold change in S. exigua larval mass after 18 h on leaves that were either untreated (black) or pretreated for 48 h with water, ZmPep3, or Gln-18:3. Within each plot, different letters (a and b) represent significant differences between mean values (n = 24, ± SEM; ANOVA, P < 0.015; Tukey test corrections for multiple comparisons, P < 0.05).
In addition to proteinaceous defenses, maize benzoxazinoids contribute to direct defenses against both herbivores and pathogens (63). Indole is produced by the indole glycerol lyase benzoxazine-deficient 1 (BX1) and modified by a series of cytochrome P450 enzymes (BX2–BX5) (64). The microarray probe sets corresponding to BX1 and BX2 were up-regulated by ZmPep3 treatment, and qRT-PCR confirmed that both genes were expressed in response to ZmPep3 or Gln-18:3 12-h posttreatment (Dataset S1 and Fig. 4B). This BX gene expression corresponds to increased accumulation of a single benzoxazinoid, 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc), a highly reactive analog that is also induced by treatment with ZmPep1, JA, or biotic attack (Fig. 4C) (65, 45). It was observed recently that intact BX1 function is not required for HDMBOA-Glc accumulation, with IGL being the suspected supplier of indole (66). Simultaneous activation of both IGL and BX1 by ZmPep3 may result in increased flux through the benzoxazinoid pathway to produce HDMBOA-Glc.
To assess the role of ZmPep3-induced direct defenses, S. exigua growth on peptide-pretreated leaves was evaluated. Compared with individuals supplied with untreated or water-treated leaves, larvae displayed significantly less biomass gain on ZmPep3-treated leaves (Fig. 4D). This effect was expected given the broad suite of defenses induced by ZmPep3. The antidigestive and growth-inhibiting properties of proteinase inhibitors against larvae have been well characterized (62). HDMBOA_Glc is also is an important component of anti-Lepidopteran defense; associated with resistance to Diatraea grandiosella, Spodoptera littoralis, and Spodoptera frugiperda (63, 65, 67, 68). HDMBOA aglycone released upon tissue damage is more resistant to detoxification than other benzoxazinoids. The specialist herbivore S. frugiperda detoxifies many benzoxazinoid aglycones through reglucosylation, but it is incapable of reconjugating HDMBOA (68).
Plant Elicitor Peptides from Other Species Act as Signals to Regulate Herbivore Defenses.
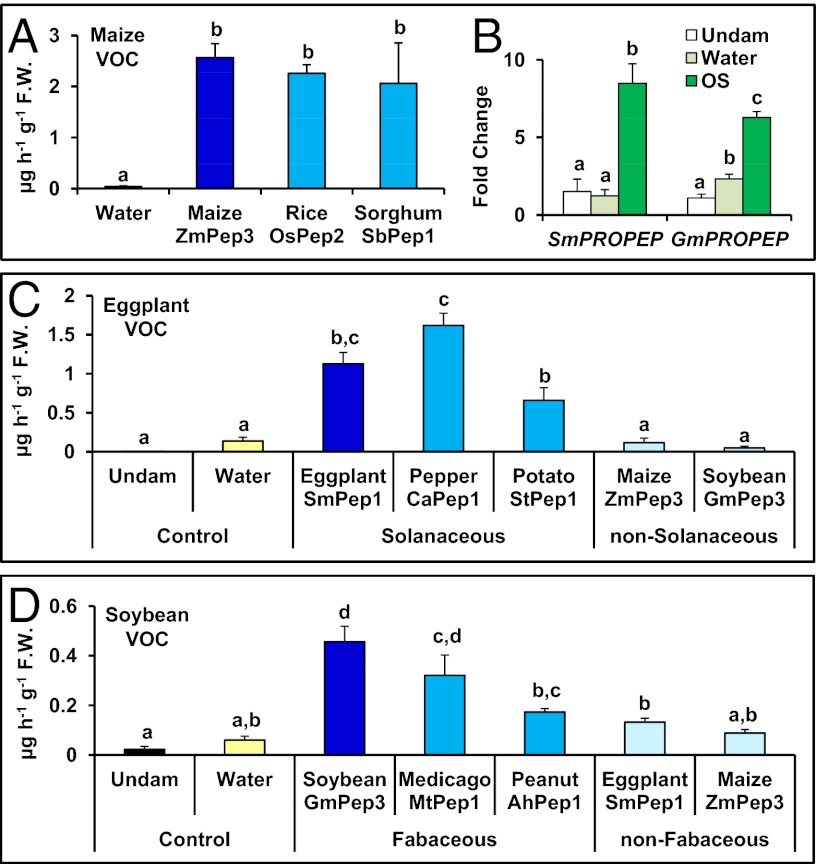
Pep orthologs have been discovered in silico from a diverse array of species and characterized as species-specific signals (40, 43). Nevertheless, predicted Pep sequences from rice and sorghum gene orthologs are similar to ZmPeps in amino acid composition (Dataset S2). To examine potential cross-species functionality, peptide orthologs from both species were assayed for maize volatile elicitation (Fig. S7). Maize leaves produced the full spectrum of herbivory-associated volatiles in response to both Oryzae sativa (OsPep2) and Sorghum bicolor (SbPep1), and to the same magnitude as those induced by ZmPep3 (Fig. 5A). These results indicate that Pep activity is more properly classified as family- rather than species specific.
Fig. 5.
Plant elicitor peptides exhibit conserved volatile-inducing activity in related plant species, but limited functionality between distant plant families. (A) Total emitted volatiles from maize leaves 16 h posttreatment with water or with Peps from maize, rice, and sorghum. (B) Relative expression as determined by qRT-PCR of the genes encoding the peptide precursor proteins from eggplant (SmPROPEP1) or soybean (GmPROPEP3) in eggplant or soybean leaves that were untreated (white) or 30 min posttreatment with either water (light green) or S. exigua OS (dark green). (C) Volatiles emitted from undamaged eggplant leaves or from scratch-wounded leaves 5 h posttreatment with water or Peps from Solanaceous or non-Solanaceous species. (D) Volatiles emitted from undamaged soybean leaves or from scratch-wounded leaves 5 h posttreatment with water or Peps from Fabaceous or non-Fabaceous species. For all experiments, peptides were used at 2 nmol⋅g−1 FW. Within each plot, different letters (a–d) represent significant differences between mean values (n = 4, ± SEM; all ANOVAs, P < 0.001; Tukey test corrections for multiple comparisons, P < 0.05).
Both herbivory-induced volatile emission and PROPEP gene orthologs occur across the plant kingdom, and thus Pep-mediated volatile regulation may be a conserved biological theme. Representative Fabaceous (Glycine max, soybean) and Solanaceous (Solanum melongena, eggplant) species for which both herbivory-associated volatiles and PROPEP gene orthologs have been identified were selected for examination (Fig. S7). In both soybean and eggplant, treatment of leaves with S. exigua OS stimulates expression of G. max PROPEP 3 (GmPROPEP3) or S. melongena PROPEP 1 (SmPROPEP1) precursor genes to levels greater than water treatments (Fig. 5B). As ZmPROPEP3 was also responsive to S. exigua oral secretions, these results indicated that the encoded candidate GmPep3 and SmPep1 peptides might have a role related to herbivore defense. Correspondingly, treatment of eggplant leaves with SmPep1 induced volatile emission, as did treatment of soybean leaves with GmPep3 (Fig. 5 C and D). As observed in maize, other Solanaceous Peps [Capsicum annum Pep 1 (CaPep1) and Solanus tuberosum Pep 1 (StPep1)] had volatile-promoting activity in eggplant, whereas GmPep3 and ZmPep3 did not (Fig. 5C and Fig. S7). Similarly, soybean volatile emission was promoted by other Fabaceous Peps, namely Medicago truncatula Pep 1 (MtPep1) but not by SmPep1 or ZmPep3 (Fig. 5D and Fig. S7). Thus, Pep activity is largely specific to their native plant families, yet the function of Peps as regulators of herbivory-associated volatiles appears conserved across diverse plant species.
The seeming isolation of systemin-like peptides to Solanaceous species implied that peptides were not a conserved signaling mechanism regulating antiherbivore responses, but rather an isolated case of specialized evolution peculiar to the Solanaceae. However, the prevalence of plant elicitor peptides across wide ranging plant families and functional conservation indicates that they are an ancient class of signals regulating numerous suites of defense responses. In addition to Peps mediating innate immunity against pathogens, other Peps are regulators of herbivore defense, and have conserved activity across plant species as functional homologs of systemin.
Materials and Methods
Plant and Insect Materials.
Unless otherwise indicated, experiments were performed using Z. mays var. Golden Queen; additional experiments included Z. mays vars. B73, NC358, and Silver Queen, S. melongena var. Black Beauty and G. max var. Williams 82. All were cultivated in a greenhouse under conditions described in ref. 23. S. exigua were raised on an artificial diet at 29 °C and received as late first instars (Benzon Research).
Precursor Gene Identification and Cloning.
GenBank registered sequences through the National Center for Biotechnology Information (NCBI) were queried with the ZmPROPEP1 sequence (40) using TBLASTN version 2.2.7 algorithm (69). Accession AC209428 encoded a predicted protein sequence similar to ZmPROPEP1, which was termed ZmPROPEP3 and corresponds to the maize locus GRMZM2G339117. Additional genes encoding orthologs and paralogs were identified by the same method; accession numbers for each are listed in Dataset S2. The gene encoding ZmPROPEP3 was cloned from Zea mays var. B73 and var. Golden Queen by previously described methods (45). Primers used are listed in Dataset S2.
Peptide and Elicitor Treatment.
A 23-aa peptide corresponding to the predicted ZmPep3 active sequence, was synthesized, purified, and authenticated at the Protein Core Chemistry Facility (University of Florida, Gainesville). Peptides for comparative experiments were synthesized via a PepScreen library (Sigma-Aldrich). All peptide sequences are listed in Dataset S2. All peptides and Gln-18:3 were diluted in water for plant treatments.
Leaf Bioassays for Analysis of Transcript and Metabolite Abundance.
Assays using either scratch application of leaves on intact plants or leaf excision were performed as previously described (45). Tissue was harvested in liquid nitrogen for RNA and metabolite analysis at time points indicated; for examination of induced volatile compounds, maize leaves were collected 16 h posttreatment, whereas soybean and eggplant leaves were collected 5 h posttreatment.
Measurement of Hormones and Metabolites.
Levels of JA, indole, anthranilate, and terpenoid pools were measured using the previously described vapor phase extraction method coupled with GC/MS analysis (45, 70). Indole levels were quantified by comparison with an external standard curve. ET emitted by leaves was measured by GC using a standard curve as previously described (23). Benzoxazinoids were extracted from freeze-dried tissue collected 48 h posttreatment using 49:1 methanol:acetic acid and analyzed by C18 HPLC using 2-benzoxazolinone (Sigma-Aldrich) as an internal standard (45).
RNA Isolation and qRT-PCR and Microarray Analyses.
RNA isolation, cDNA synthesis, and qRT-PCR analysis were performed as previously described (66). Before reverse transcription, RNA was DNase treated with TURBO DNA-free reagent (Applied Biosystems). All primer sequences and associated GenBank numbers are listed in Dataset S2. Microarray analyses were executed as described by Huffaker et al. (66) using the Affymetrix GeneChip 3′ IVT Express kit and protocol (Affymetrix) and hybridization to the Affymetrix GeneChip Maize Genome Array. Microarray sample preparation, analysis, and data extraction were performed by the University of Florida Interdisciplinary Center for Biotechnology Research (ICBR) Gene Expression Core. Data files were analyzed using ArrayStar 4 software (DNASTAR), with all data preprocessed using robust multiarray analysis (RMA) and quantile normalization. Annotation was assigned using NetAffx Maize Annotation Files in MAGE-ML XML Format, Release 31 and comma separated values format, Release 31 (Affymetrix).
Volatile Collection and Analysis.
Volatiles were collected on 50 mg Super Q (80/100 mesh; Alltech) for 1 h, eluted with methylene chloride containing nonyl acetate as an internal standard, and analyzed by GC as described in ref. 71. Volatile compounds were identified by comparison of retention times with authentic standards and by comparison of mass spectra with Wiley and National Institute of Standards and Technology libraries. The breakdown of total volatile components measured for each species is denoted in Figs. S2 and S7.
Insect Herbivory and Attraction Bioassays.
To observe effects of elicitor-induced defenses on larval growth, intact leaves were scratch wounded and treated. Forty-eight hours posttreatment, preweighed early third instar S. exigua larvae were placed on leaf material, and after 18 h were weighed again. Attraction of C. marginiventris was determined in two experiments. First, a six-arm olfactometer was used to compare the attractiveness of water, Gln-18:3, and ZmPep3-treated leaves as described (72). Ten independent replicates were carried out, each consisting of 24 wasps that were released in groups of six.
Statistical Analyses.
Statistical analyses ANOVAs were performed on quantified levels of phytohormones, benzoxazinoids, volatiles, and qRT-PCR transcripts. Treatment effects were investigated when the main effects of the ANOVAs were significant (P < 0.05). Tukey tests were used to correct for multiple comparisons between control and treatment groups. Before statistical analyses, qRT-PCR transcript data were log transformed, whereas metabolite data were subjected to square root transformation to compensate for elevated variation associated with larger mean values. Analysis was accomplished with JMP 4.0 statistical discovery software (SAS Institute). Parasitoid choice was evaluated using a log-linear model as previously described (72).
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Clarence A. Ryan, in whose laboratory this work began. Research was funded by US Department of Agriculture (USDA)–Agricultural Research Service Project 6615-21000-010-00 and 6615-22000-027-00, by Agriculture and Food Research Initiative competitive Grant 2011-04425 of the USDA National Institute of Food and Agriculture, and by National Science Foundation Division of Integrative Organismal Systems Competitive Award 1139329.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214668110/-/DCSupplemental.
References
- 1.Green TR, Ryan CA. Wound-induced proteinase inhibitor in plant leaves: A possible defense mechanism against insects. Science. 1972;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Gonzales-Vigil E, Wilkerson CG, Howe GA. Stability of plant defense proteins in the gut of insect herbivores. Plant Physiol. 2007;143(4):1954–1967. doi: 10.1104/pp.106.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gooday GW. Aggressive and defensive roles for chitinases. EXS. 1999;87:157–169. doi: 10.1007/978-3-0348-8757-1_11. [DOI] [PubMed] [Google Scholar]
- 4.Vandenborre G, Smagghe G, Van Damme EJ. Plant lectins as defense proteins against phytophagous insects. Phytochemistry. 2011;72(13):1538–1550. doi: 10.1016/j.phytochem.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 6.Adio AM, et al. Biosynthesis and defensive function of Nδ-acetylornithine, a jasmonate-induced Arabidopsis metabolite. Plant Cell. 2011;23(9):3303–3318. doi: 10.1105/tpc.111.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmelz EA, Grebenok RJ, Ohnmeiss TE, Bowers WS. Interactions between Spinacia oleracea and Bradysia impatiens: A role for phytoecdysteroids. Arch Insect Biochem Physiol. 2002;51(4):204–221. doi: 10.1002/arch.10062. [DOI] [PubMed] [Google Scholar]
- 8.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410(6828):577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 9.Turlings TC, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250(4985):1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 10.Kessler A, Baldwin IT. Plant responses to insect herbivory: The emerging molecular analysis. Annu Rev Plant Biol. 2002;53:299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- 11.Rasmann S, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434(7034):732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 12.Unsicker SB, Kunert G, Gershenzon J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr Opin Plant Biol. 2009;12(4):479–485. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH. Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA. 2004;101(6):1781–1785. doi: 10.1073/pnas.0308037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heil M, Silva Bueno JC. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104(13):5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessler A, Halitschke R. Specificity and complexity: The impact of herbivore-induced plant responses on arthropod community structure. Curr Opin Plant Biol. 2007;10(4):409–414. doi: 10.1016/j.pbi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Poelman EH, van Loon JJ, Dicke M. Consequences of variation in plant defense for biodiversity at higher trophic levels. Trends Plant Sci. 2008;13(10):534–541. doi: 10.1016/j.tplants.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- 18.Alborn HT, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 19.Schmelz EA, et al. Fragments of ATP synthase mediate plant perception of insect attack. Proc Natl Acad Sci USA. 2006;103(23):8894–8899. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doss RP, et al. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc Natl Acad Sci USA. 2000;97(11):6218–6223. doi: 10.1073/pnas.110054697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little D, Gouhier-Darimont C, Bruessow F, Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007;143(2):784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mithöfer A, Wanner G, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiol. 2005;137(3):1160–1168. doi: 10.1104/pp.104.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, 3rd, Teal PE. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc Natl Acad Sci USA. 2009;106(2):653–657. doi: 10.1073/pnas.0811861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maffei ME, Mithöfer A, Boland W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry. 2007;68(22-24):2946–2959. doi: 10.1016/j.phytochem.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Gális I, Gaquerel E, Pandey SP, Baldwin IT. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ. 2009;32(6):617–627. doi: 10.1111/j.1365-3040.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- 26.Erb M, Ton J, Degenhardt J, Turlings TC. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol. 2008;146(3):867–874. doi: 10.1104/pp.107.112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 28.McGurl B, Pearce G, Orozco-Cardenas M, Ryan CA. Structure, expression, and antisense inhibition of the systemin precursor gene. Science. 1992;255(5051):1570–1573. doi: 10.1126/science.1549783. [DOI] [PubMed] [Google Scholar]
- 29.Degenhardt DC, Refi-Hind S, Stratmann JW, Lincoln DE. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry. 2010;71(17–18):2024–2037. doi: 10.1016/j.phytochem.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4(2):129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stratmann JW, Ryan CA. Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc Natl Acad Sci USA. 1997;94(20):11085–11089. doi: 10.1073/pnas.94.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orozco-Cardenas M, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96(11):6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergey DR, Ryan CA. Wound- and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol Biol. 1999;40(5):815–823. doi: 10.1023/a:1006247624823. [DOI] [PubMed] [Google Scholar]
- 34.Schaller A, Oecking C. Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell. 1999;11(2):263–272. doi: 10.1105/tpc.11.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan CA. The systemin signaling pathway: Differential activation of plant defensive genes. Biochim Biophys Acta. 2000;1477(1-2):112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- 36.Pearce G, Moura DS, Stratmann J, Ryan CA. Production of multiple plant hormones from a single polyprotein precursor. Nature. 2001;411(6839):817–820. doi: 10.1038/35081107. [DOI] [PubMed] [Google Scholar]
- 37.Pearce G, Ryan CA. Systemic signaling in tomato plants for defense against herbivores. Isolation and characterization of three novel defense-signaling glycopeptide hormones coded in a single precursor gene. J Biol Chem. 2003;278(32):30044–30050. doi: 10.1074/jbc.M304159200. [DOI] [PubMed] [Google Scholar]
- 38.Ren F, Lian HJ, Chen L. TohpreproHypSys- A gene expression and defense protein activity in the tobacco wounding response. J Plant Biol. 2008;51:48–51. [Google Scholar]
- 39.Pearce G. Systemin, hydroxyproline-rich systemin and the induction of protease inhibitors. Curr Protein Pept Sci. 2011;12(5):399–408. doi: 10.2174/138920311796391106. [DOI] [PubMed] [Google Scholar]
- 40.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA. 2006;103(26):10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce G, Yamaguchi Y, Barona G, Ryan CA. A subtilisin-like protein from soybean contains an embedded, cryptic signal that activates defense-related genes. Proc Natl Acad Sci USA. 2010;107(33):14921–14925. doi: 10.1073/pnas.1007568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi Y, Barona G, Ryan CA, Pearce G. GmPep914, an eight-amino acid peptide isolated from soybean leaves, activates defense-related genes. Plant Physiol. 2011;156(2):932–942. doi: 10.1104/pp.111.173096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi Y, Pearce G, Ryan CA. The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA. 2006;103(26):10104–10109. doi: 10.1073/pnas.0603729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22(2):508–522. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huffaker A, Dafoe NJ, Schmelz EA. ZmPep1, an ortholog of Arabidopsis elicitor peptide 1, regulates maize innate immunity and enhances disease resistance. Plant Physiol. 2011;155(3):1325–1338. doi: 10.1104/pp.110.166710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krol E, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem. 2010;285(18):13471–13479. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi Z, et al. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA. 2010;107(49):21193–21198. doi: 10.1073/pnas.1000191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi Y, Huffaker A. Endogenous peptide elicitors in higher plants. Curr Opin Plant Biol. 2011;14(4):351–357. doi: 10.1016/j.pbi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Frey M, et al. An herbivore elicitor activates the gene for indole emission in maize. Proc Natl Acad Sci USA. 2000;97(26):14801–14806. doi: 10.1073/pnas.260499897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Köllner TG, et al. Herbivore-induced SABATH methyltransferases of maize that methylate anthranilic acid using s-adenosyl-L-methionine. Plant Physiol. 2010;153(4):1795–1807. doi: 10.1104/pp.110.158360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paré PW, Alborn HT, Tumlinson JH. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA. 1998;95(23):13971–13975. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell. 1996;8(11):2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stratmann JW. Long distance run in the wound response–jasmonic acid is pulling ahead. Trends Plant Sci. 2003;8(6):247–250. doi: 10.1016/S1360-1385(03)00106-7. [DOI] [PubMed] [Google Scholar]
- 54.Schilmiller AL, Howe GA. Systemic signaling in the wound response. Curr Opin Plant Biol. 2005;8(4):369–377. doi: 10.1016/j.pbi.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Ryan CA, Huffaker A, Yamaguchi Y. New insights into innate immunity in Arabidopsis. Cell Microbiol. 2007;9(8):1902–1908. doi: 10.1111/j.1462-5822.2007.00991.x. [DOI] [PubMed] [Google Scholar]
- 56.Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006;9(3):297–304. doi: 10.1016/j.pbi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 57.Keeling CI, Bohlmann J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006;170(4):657–675. doi: 10.1111/j.1469-8137.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- 58.Schnee C, et al. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA. 2006;103(4):1129–1134. doi: 10.1073/pnas.0508027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Köllner TG, et al. A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20(2):482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouinguené S, Pickett JA, Wadhams LJ, Birkett MA, Turlings TC. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata) J Chem Ecol. 2005;31(5):1023–1038. doi: 10.1007/s10886-005-4245-1. [DOI] [PubMed] [Google Scholar]
- 61.Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH. Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: Isolation and bioactivity. J Chem Ecol. 2000;26:189–202. [Google Scholar]
- 62.Ryan CA. Proteinase inhibitor gene families: Strategies for transformation to improve plant defenses against herbivores. Bioessays. 1989;10(1):20–24. doi: 10.1002/bies.950100106. [DOI] [PubMed] [Google Scholar]
- 63.Niemeyer HM. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: Key defense chemicals of cereals. J Agric Food Chem. 2009;57(5):1677–1696. doi: 10.1021/jf8034034. [DOI] [PubMed] [Google Scholar]
- 64.Frey M, et al. Analysis of a chemical plant defense mechanism in grasses. Science. 1997;277(5326):696–699. doi: 10.1126/science.277.5326.696. [DOI] [PubMed] [Google Scholar]
- 65.Oikawa A, Ishihara A, Hasegawa M, Kodama O, Iwamura H. Induced accumulation of 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) in maize leaves. Phytochemistry. 2001;56(7):669–675. doi: 10.1016/s0031-9422(00)00494-5. [DOI] [PubMed] [Google Scholar]
- 66.Huffaker A, et al. Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 2011;156(4):2082–2097. doi: 10.1104/pp.111.179457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hedin PA, Davis FM, Williams WP. 2-Hydroxy-4,7- dimethoxy-1,4-benzoxazin-3-one, a possible toxic factor in corn to the Southwestern corn borer. J Chem Ecol. 1993;19:531–542. doi: 10.1007/BF00994323. [DOI] [PubMed] [Google Scholar]
- 68.Glauser G, et al. Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 2011;68(5):901–911. doi: 10.1111/j.1365-313X.2011.04740.x. [DOI] [PubMed] [Google Scholar]
- 69.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J. 2004;39(5):790–808. doi: 10.1111/j.1365-313X.2004.02168.x. [DOI] [PubMed] [Google Scholar]
- 71.Schmelz EA, Alborn HT, Tumlinson JH. The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta. 2001;214(2):171–179. doi: 10.1007/s004250100603. [DOI] [PubMed] [Google Scholar]
- 72.Turlings TCJ, Davison AC, Tamo C. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol Entomol. 2004;29:45–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.