Abstract
A reverse-genetics approach has been used to probe the mechanism underlying immune escape for influenza A virus-specific CD8+ T cells responding to the immunodominant DbNP366 epitope. Engineered viruses with a substitution at a critical residue (position 6, P6M) all evaded recognition by WT DbNP366-specific CD8+ T cells, but only the NPM6I and NPM6T mutants altered the topography of a key residue (His155) in the MHC class I binding site. Following infection with the engineered NPM6I and NPM6T influenza viruses, both mutations were associated with a substantial “hole” in the naïve T-cell receptor repertoire, characterized by very limited T-cell receptor diversity and minimal primary responses to the NPM6I and NPM6T epitopes. Surprisingly, following respiratory challenge with a serologically distinct influenza virus carrying the same mutation, preemptive immunization against these escape variants led to the generation of secondary CD8+ T-cell responses that were comparable in magnitude to those found for the WT NP epitope. Consequently, it might be possible to generate broadly protective T-cell immunity against commonly occurring virus escape mutants. If this is generally true for RNA viruses (like HIV, hepatitis C virus, and influenza) that show high mutation rates, priming against predicted mutants before an initial encounter could function to prevent the emergence of escape variants in infected hosts. That process could be a step toward preserving immune control of particularly persistent RNA viruses and may be worth considering for future vaccine strategies.
Virus-specific CD8+ T cells recognize peptide and class I MHC (pMHCI) epitopes derived predominantly from more conserved, internal proteins, offering a promising target for vaccine development. However, some RNA viruses, particularly the influenza A viruses, hepatitis C virus (HCV) and HIV, are a major challenge for preventive immunization as a low-fidelity RNA polymerase allows the rapid emergence of escape mutants. The question asked here is whether it is possible to design an immunization strategy that might minimize the likelihood that such virus variants will escape from immune control and survive.
The influenza A viruses elicit robust and broad CD8+ T-cell immunity (1, 2) that can provide protection against serologically distinct strains, including newly emerged pandemic variants (3, 4). The experimental evidence is that influenza-specific CD8+ T cells, operating in either a primary response or following recall from memory, promote virus elimination and host recovery via the production of proinflammatory cytokines and the killing of virus-infected cells. At the stage of initial priming, the responding CD8+ cytotoxic T lymphocytes (CTLs) are selected as a consequence of the interaction between their clonotypic T-cell receptor (TCR) and pMHCI epitopes expressed on the surface of infected cells. The key to immunogenicity for CD8+ CTLs rests in both the nature of the TCR repertoire and the sequence, or structural complementarity, of peptides targeted in the MHCI groove (5, 6).
Influenza virus-specific CD8+ CTLs can exert selective pressure that leads to the emergence of escape mutations in immunogenic peptides (7). This is a more familiar problem for chronic virus infections (HIV and HCV), with at least some of the variants that persist in the circulation being readily transmissible (8–10). Apart from subverting CD8+ T-cell–mediated control within the infected individual, this constitutes a major barrier to effective vaccine design. With influenza, although mutations have been found for >70% of immunogenic T-cell peptides (7), this finding has received little attention because of the acute nature of the disease. Even so, such escape mutants are readily generated using TCR transgenic mice (11), and “natural” variants occur within the influenza nucleoprotein (NP)380 (HLA-B8), NP383 (HLA-B27), and NP418 (HLA-B35) viral peptides (12–14). Furthermore, given sufficient immune pressure and relative fitness, such mutated viruses can become fixed in the population, leading to the disappearance of WT T-cell specificities (15). For “seasonal” influenza infections, such escape from CD8+ T-cell–mediated immunity can also be relevant to the persistence of variants within the population (longevity and severity of influenza season). Moreover, in the face of a rapidly spreading, novel pandemic strain, established CD8+ T-cell memory constitutes the best protective mechanism. Clearly, any vaccine strategy that focuses on priming the CTLs needs to deal with emerging escape variants.
The immunogenicity of a given epitope can be compromised in a variety of ways. Amino acid variation at an MHCI anchor residue can lead to the failure of pMHCI binding (12). Alternatively, changes at a TCR contact site (14, 16, 17) can prevent or decrease T-cell recognition, although cross-reactive T-cell immunity may still be retained between some influenza variants (18). The present study targets the mechanisms underlying virus escape at TCR contact sites and probes possible compensatory strategies using a readily manipulated C57BL/6J (B6, H2b) influenza mouse model (17). The study focuses principally on escape variants selected in transgenic mice expressing a TCR specific for the immunodominant DbNP366 (ASNENMETM) epitope that reemerged from day 18 after infection and caused lethal disease within a month (11). Sequencing viral RNA recovered from the lungs of these TCR transgenic mice (11) established that NP366 had mutated, especially at position (P) 6, and that the mice were no longer infected with the wt virus. The P6 methionine (M) is the most solvent-exposed residue for the DbNP366 complex (19, 20) and the change from P6M to P6A leads to loss in recognition by CD8+ T cells specific for the wt-DbNP366 epitope (17, 20, 21). The present analysis of the in vivo CTL responses to a panel of P6 mutants indicates that preemptive immunization against the escape variants can generate a good measure of protection.
Results
Primary CD8+ T-Cell Responses to Engineered DbNP366 “Escape” Mutants.
We recreated the variants detected by Price et al. (11) with a reverse-genetics strategy (22) to probe the consequences for TCR recognition and polyclonal CD8+ T-cell responses in wt B6 mice. The viruses with a single amino acid substitution at P6 include the original escape variants NPM6I and NPM6T (11), and new mutants NPM6Y and NPM6W (mutated to “protrude out” from the MHCI peptide binding groove; Fig. S1A). Nontransgenic B6 mice were infected intranasally with the WT HK (H3N2) influenza virus to determine if CD8+ T cells specific for the WT DbNP366 epitope can recognize the various NPM6X peptides. We found that none of the NPM6X peptides induced IFN-γ production by the wt DbNP366-specific CD8+ set (Fig. S1B). Thus, the influenza P6 variants escape recognition by WT DbNP366+ CTLs.
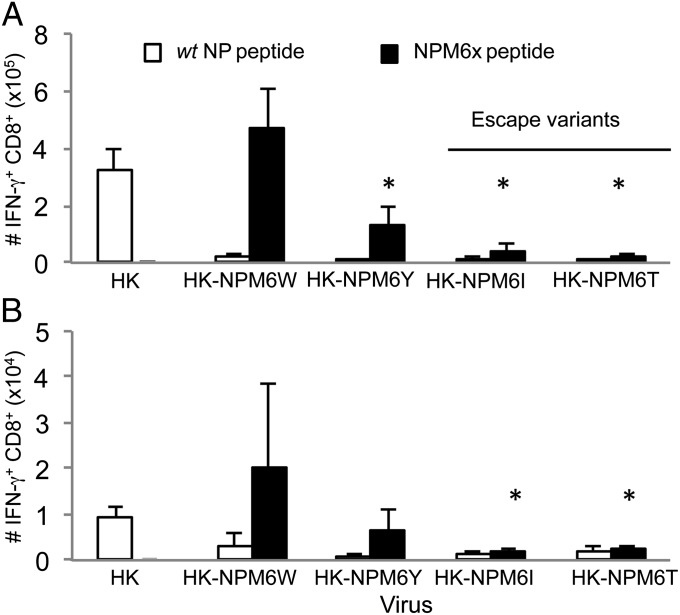
To determine whether these DbNPM6X epitopes generate endogenous CTL responses in a polyclonal (non-TCR–transgenic) system, naïve B6 wt mice were separately infected intranasally with the mutant HK-NPM6X viruses. Analyzing the CD8+ CTLs recovered from both the spleen (Fig. 1A) and the pneumonic lung (Fig. 1B) on day 10 following infection established that the engineered viruses (HK-NPM6T, HK-NPM6I, HK-NPM6W, or HK-NPM6Y) failed to generate effectors specific for the wt DbNP366 epitope. Furthermore, in the case of the previously identified NPM6I and NPM6T escape mutants, the responses to the variant peptides were minimal (Fig. 1 and Fig. S2).
Fig. 1.
The NPM6W and NPM6Y mutants (but not the NPM6I or NPM6T escape variants) generate unique CD8+ T-cell responses. Following intranasal infection with either the WT HK or mutant HK-NPM6X virus, (A) splenocytes or (B) BAL cells (from the site of infection) were stimulated with the WT NP or NPM6X peptides corresponding to the infecting virus. The numbers of epitope specific CD8+ T cells were calculated from the percentage of cells staining and the total cell counts. Data represent mean ± SD (n = 5). Representative FACS plots and the actual numbers of epitope-specific CD8+ T-cell populations are shown in Fig. S2. Experiments were repeated at least twice. *P ≤ 0.01 relative to WT DbNP366+CD8+ response.
More prominent, novel CD8+ T-cell responder sets were, however, generated to a greater (NPM6W) or lesser (NPM6Y) extent following infection with the homologous viruses containing substitutions to these bulky residues (Fig. 1). In addition, the relative lack of de novo CD8+ T-cell responsiveness for the NPM6I or NPM6T epitopes did not obviously reflect decreased binding to H-2Db (Fig. S1A). These findings agree with comparable pMHCI affinities for the M6X mutants detected by the RMAS assay (23).
Generating influenza P6-NP366 mutants that escape wt DbNP366 CTL recognition thus leads either to: (i) the recruitment of novel CTL sets directed at the mutated peptides (bulky substitutions, NPM6W and NPM6Y) or (ii) a lack of primary CTLs (escape mutations, NPM6I and NPM6T). Thus, comparative analyses between those two sets of variants were further performed to provide insights into the mechanism of viral escape and possible compensatory strategies to boost CTL responses.
Structural Basis of the NPM6I and NPM6T Escape Mutants.
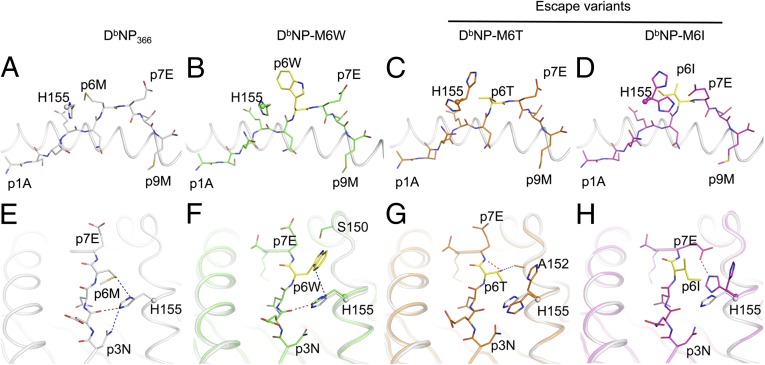
To determine whether topographical constraints within the NPM6I and NPM6T escape variants may provide an explanation for the minimal endogenous primary responses toward the mutated epitopes, the crystal structures were solved for H2Db bound to the variant NPM6I and NPM6T peptides (Figs. 2 and 3, and Table S1). The structures of these escape mutants were compared with those of the responsive DbNPM6W mutant and wt-DbNP366 (19). The overall structures determined for both H2Db molecules and the bound, mutant peptides were comparable to those found for the wt-DbNP366. Perturbations around the p6 position underpinned the observed functional impacts of the mutants. In the WT-DbNP366, the P6M side-chain is exposed to the solvent and stabilized by stacking interactions with H155 of the H2Db molecule (Fig. 2A). The tryptophan in the DbNPM6W structure was perfectly accommodated in the same position as the P6M in the WT-DbNP366 (Fig. 2B), locking its side-chain between H155 and S150 (Fig. 2F). Consequently, compared with P6M, the P6W Cα lies ∼1 Å closer to the Db α2-helix. Interestingly, the P6T and P6I mutations did not affect the overall structure of either H2Db or the peptide. However, both the NPM6T (Fig. 2C) and NPM6I (Fig. 2D) escape variants affected the flexibility of H155. Namely, two different conformations of the H155 side-chain are observed for these escape variants, suggesting an increase in flexibility for this MHCI residue.
Fig. 2.
Unique MHCI H155 movement in DbNPM6I and DbNPM6T crystal structures potentially blocks TCR binding. Stick representation of the M6X peptides bound to the H2Db antigen-binding cleft represented in cartoon. The panel shows H2Db bound to (A) the WT NP peptide in white, (B) NPM6W in green, (C) NPM6T in orange, (D) NPM6I in pink. The sphere represents the Cα of the Histidine (H) 155 of the Db molecule, from which the α2-helix has been removed for clarity. (E–H) The interaction between the Db-H155 and each NPM6X peptide. Hydrogen bonds are represented by red dashed lines, van der Waals contacts in blue.
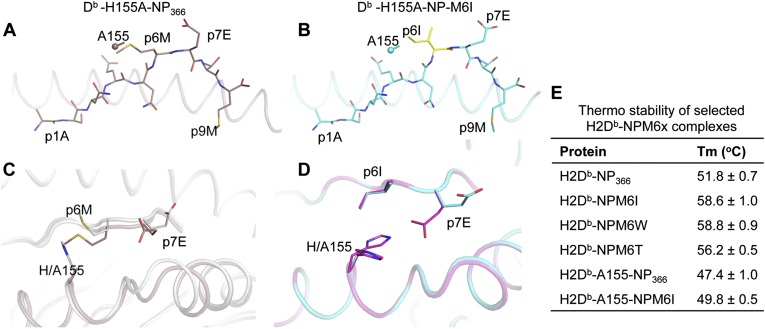
Fig. 3.
The DbH155A mutant crystal structures. (A and B) A stick representation of the (A) NP (brown) and (B) NPM6I (cyan) peptides bound to the Ala-155 mutant of the Db molecule (represented in cartoon), respectively. The sphere represents the Cα atom of the Db Ala-155 from which the α2-helix has been removed for clarity. (C) A superposition of the Db WT (white) and Db-H155A mutant (brown) in complex with the NP peptide. (D) A top view of the Db H155A mutant (cyan) structure superposed with Db WT (pink) bound to the NPM6I peptide. (E) Thermostability (°C) of the different Db-M6X complexes.
In the DbNPM6T structure, the P6T hydroxyl group hydrogen-bonded to the P7E backbone in addition to contacting A152 (Fig. 2G). The P6T could not accommodate H155 in the same conformation as found for the wt NP epitope (Fig. 2E): this side-chain was thus pushed away from the peptide (Fig. 2G). In addition, the P6I mutation mainly affected the H155 conformation, but in a different fashion to that for P6T. The P6I mutation increased the flexibility of H155 and we observed two different conformations of H155, buried and exposed. The solvent-exposed conformation of H155 observed in the NPM6I structure is similar to that for the NPM6T escape peptide. Interestingly, the P6I sat on the top of the H155 (second conformation observed), which became buried inside the Ag-binding cleft (Fig. 2H). This conformation of H155 was further stabilized by its interaction with P7E, the side-chain of which became partly buried relative to the wt complex (Fig. 2H). The structural changes for H155 and P7E induced by P6I generated a more featureless pMHCI surface. Because residue 155 of MHC is important for TCR-mediated interactions, these alterations can potentially influence TCR binding (24, 25).
To evaluate the importance of the H155 structural changes for the NPM6I and NPM6T escape variants, we mutated H155 to A and analyzed the impact on pMHCI stability and conformation. Decreased stability was found for both the DbH155ANP366 and Db-H155ANPM6I epitopes (Fig. 3E), indicating an important role of H155 in stabilizing these pMHCI complexes. The Db-NPM6I and Db-H155A-NPM6I structures (Fig. 3B) were generally comparable (rmsd of 0.46 Å and 0.25 Å for the Cα atoms of the peptides between the two). With the exception of the P7E side-chain, which adopted a similar conformation to that observed for the WT Db-NP366 in the Db-H155A-NP366 complex (Fig. 2A), the peptide backbone and side-chain conformations were identical. That p7E did not adopt the same conformation in the NPM6I peptide for the wt versus the H155A mutant MHCI indicates that the conformational change for H155 in the NPM6I complex was responsible for the movement of P7E. Complexes of the WT MHC and mutant A155 with NP366 were also structurally similar (Fig. 3C). However, the absence of H155 increased the flexibility of the P6M side-chain to such an extent that it was poorly resolved in the electron density. In addition, the peptide sat deeper inside the cleft and pushed away the α2-helix by 1.2 Å at the hinge region of the helix. The P7E also switched its side-chain toward the α2-helix (Fig. 3C). Overall, mutating H155 in the H2Db molecule changed the presentation of the peptide and the MHCI structure, leading to decreased stability for the pMHCI complexes (Fig. 3E).
Decreased Naïve Precursor Frequency for NPM6I and NPM6T.
Our results indicate that the minimal primary CTL responses to the NPM6I and NPM6T escape variants could be a consequence of perturbed structural interactions within the DbNPM6I and DbNPM6T complexes. As the magnitude of acute CTL responses can be influenced by the number of specific naïve precursors (26, 27), this finding would suggest that the naïve CTL pool capable of recognizing these “perturbed” escape variants is also reduced. Analysis using a tetramer enrichment protocol (5, 28) in B6 mice showed that the naïve CTL precursors for the DbNPM6I (10 ± 5) and DbNPM6T (4.5 ± 2) escape mutants were significantly diminished compared with those for the wt Db-NP366 (23 ± 8, P > 0.01). In contrast, the naïve precursor frequencies were comparable for the wt DbNP366 (23 ± 8) and responsive DbNPM6W (23 ± 10) and DbNPM6Y (35 ± 11) variants (Fig. 4).
Fig. 4.
Naïve CD8+ T-cell precursor frequency is lower for the NPM6I and NPM6T escape variants. Naïve DbNP366+ and DbNPM6X+CD8+ precursors within macroscopic lymph nodes and spleens were identified with the DbNP366-PE and DbNPM6X-PE tetramers (28). Data represent the mean (per group) for three to seven B6 [H-2b] mice (black circles), BalbC [H-2d] mice (open squares), and TCR-transgenic OT-I [H-2b] mice (open circles). *P ≤ 0.01; #P < 0.05; **P < 0.05 relative to DbNP366+CD8+ precursors. Experiments were repeated two to four times.
To determine whether the low precursor frequencies for the DbNPM6I and DbNPM6T epitopes are indeed a true reflection of naïve CD8+ CTL precursor numbers, the analysis was repeated in naïve H2-different mice (Balb/C mice H-2d), and OT-I mice [H-2b, with a transgenic TCR specific for OVA257; used previously to determine the background tetramer staining level (28)]. Significantly fewer DbNP366 and DbNPM6I precursors were recovered from the Balb/C and OT-I mice relative to those found for the wt B6 mice. The greater CTL numbers specific for the DbNPM6I and DbNPM6T epitopes in the wt B6 mice are thus likely to be a true reflection of their immune response potential following infection with the homologous influenza viruses (Fig. 4).
Limited TCRβs Elicited by the NPM6I Escape Variant.
To understand the nature of TCRs specific for DbNPM6I (minimal response) versus DbNPM6W (strong response), profiles of TCRVβ use were assessed following infection with the homologous NPM6X variants. The wt DbNP366+CD8+ population is characterized by prominent, public TCRVβ8.3 use across all influenza virus-infected B6 mice (29, 30). After challenge with the HK-NPM6X viruses, the TCRVβ profiles for DbNPM6I- and DbNPM6W-specific CTLs showed varied profiles of TCRVβ bias. The DbNPM6I tetramer+ CTLs in two of four mice used Vβ8.3 (>60%), with Vβ2, Vβ4, Vβ5.1/5.2 being found for the other responding T cells (Fig. S3A). Such limited, and restricted TCRVβ use for the DbNPM6I CD8+ CTLs most likely reflects the low precursor frequency of naive CTLs capable of recognizing this perturbed escape variant (Fig. 4). Conversely, the DbNPM6W+CD8+ T-cell responses were characterized by a broader pattern of TCRVβ use (Fig. S3B).
As the CDR3β region can form important contacts with pMHCI, with the extent of variation being a good measure of clonal diversity for any responding CTL population, we analyzed profiles of TCR CDR3β sequence diversity for the DbNPM6I+CD8+ versus DbNPM6W+CD8+ T cells (Tables S2 and S3). As a consequence of the broad and variable TCRVβ profile found above for the DbNPM6W+CD8+ set, the CDR3β analysis was limited to Vβ elements found at a prevalence of >15% in this response. In contrast, because of its prominence (>60% in two of four mice) and to assess the potential use of wt DbNP366+-specific CDR3β sequences, the Vβ8.3 region was selected for CDR3β analysis within the DbNPM6I+CD8+ population, and as an alternative TCR CDR3βs of Vβ1 and 4 were also studied to determine overall clonality. Overall, the comparison of CDR3β repertoire use for CTLs responding to DbNP366, DbNPM6I, and DbNPM6W showed alternate Jβ use and varied profiles of amino acid loop length (Table S3). The numbers of clonotypes per mouse were similar for DbNP366 (7.95 ± 2.5) and DbNPM6W (5.75 ± 2.2), but the clonotype count for DbNPM6I+ (1.75 ± 0.95) was significantly lower (Tables S2 and S3). Additionally, the DbNPM6I+ Vβ8.3+ CD8+ response was very restricted, compared with the wt DbNP366+ Vβ8.3+ (0.52 ± 0.2) and DbNPM6W+ (0.63 ± 0.25) CDR3β repertoires. Thus, CDR3β diversity was reduced for the NPM6I response, reflecting the hierarchy of naïve CTL precursor frequencies (Fig. 4) and thus the capacity of this immune repertoire to establish endogenous response.
Preemptive Priming with the Escape Mutants.
To determine whether initial exposure to the NPM6X viruses (Fig. 1), primes for secondary CTL responses, WT B6 mice were primed intraperitoneally, with the PR8-NPM6X (H1N1) viruses, then infected intranasally with the homologous HK-NPM6X (H3N2) virus (Fig. 5). Surprisingly, despite the minimal primary CTL responses to the NPM6I and NPM6T variants, secondary DbNPM6I+ and DbNPM6T+ CTLs could be elicited at levels comparable to those found for the wt DbNP366+CD8+ set (Fig. 5). The recall response to the bulky DbNPM6W epitope is equivalent in magnitude to that characteristic of the wt DbNP366 CTL response (Fig. 5). Strikingly, although the recall response following homologous secondary challenge for DbNPM6T was significantly smaller than those found for the wt DbNP366 and the other NPM6X variants (Fig. 5B), the DbNPM6T+CTL set was substantial at the site of infection (Fig. 5C). Thus, although naïve CTL precursor frequencies were lowest for the DbNPM6T epitope, this did not reflect a total CD8+ T-cell repertoire “hole” because there was a substantial secondary response. Thus, despite the limited, naïve CD8+ T-cell numbers and diminished, primary CTL responses to the DbNPM6I and DbNPM6T epitopes, the generated memory populations can be effectively recalled following secondary homologous challenge. The comparable magnitude of secondary mutant-specific CD8+ pools results most likely from greater expansion of secondary effectors and lower decay of primary effectors to the memory sets, because the numbers of memory populations of NPM6I+CD8+ (3.7 × 104 cells) and NPM6T+CD8+ (3.5 × 104 cells) were only twofold lower compared with DbNP366+CD8+ memory pools (6.8 × 104 cells; d37; pooled data from two experiments).
Fig. 5.
Markedly increased recall responses following homologous 2° challenge with the HK-NPM6I and HK-NPM6T escape viruses. (A) Naïve B6 mice were primed intraperitoneally (i.p.) with wt PR8 or mutant PR8-NPM6X viruses and challenged intranasally (i.n.) 6 wk later with 1 × 104 pfu of either the corresponding wt HK or mutant HK-NPM6X virus. (B and C) The magnitude of the CD8+ T-cell response measured on day 8 after secondary infection was determined by an IFN-γ intracellular cytokine staining assay in (B) spleen and (C) BAL. Experiments were repeated at least twice. Data represent mean ± SD (n = 5); *P ≤ 0.01.
To determine whether any possible cross-reactivity between DbNP366+CD8+ and DbNPM6I+ CD8+ T cells could depend on the threshold of antigen stimulation, functional pMHCI avidity was analyzed for the NP366 and NPM6I peptides (Fig. S4). When splenocytes were stimulated with homologous peptides (CD8+ T cells primed by wt-HK stimulated with the NP366 peptide, or CD8+ T cells primed by HK-NPM6I with the NPM6I peptide), the measure of “functional avidity” (threshold for IFN-γ production) was higher for those responding to the homologous versus the heterologous peptide (homologous EC50 0.47 nM and 0.93 nM respectively, versus 1.69 nM and 1.5 nM). Therefore, the highest avidity responses were to the cognate pMHCI complexes. Overall, these data, together with distinct TCR repertoires for DbNPM6I+CD8+ and DbNP366+CD8+ responses, suggest that that priming against variants of a given pMHCI epitope tends to elicit predominantly CD8+ T-cell responses to that mutant rather than cross-reactive CD8+ T cells. Thus, it might be feasible to prime against commonly occurring mutants and generate broadly protective CD8+ T-cell immunity against TCR escape variants.
Discussion
The M at P6 in the NP366 peptide functions as an important TCR contact site (17, 19). A panel of P6 mutant peptides and variant viruses encoding these specific substitutions were used to investigate both the mechanisms underlying immune evasion and the possibility that such changes lead to novel CTL responses (17). These mutant NPM6X peptides are weak agonists compared with the wt DbNP366-specific CD8+ CTL response, enabling escape from preexisting CD8+ T-cell immunity. However, the capacity to develop endogenous responses to such NPM6X variants cannot be predicted from the peptide sequence alone, as demonstrated following priming with the panel of engineered viruses. Infection with viruses expressing the NPM6W366 and NPM6Y366 mutations generated H2Db-restricted CD8+ T-cell responses that were of similar magnitude to (although not cross-reactive with) the CTL expansion characteristically found for the wt DbNP366 epitope. In contrast, primary infection with the NPM6I and NPM6T strains resulted in minimal, mutant-specific CD8+ T-cell responses.
Analyzing the structures of the DbNPM6W, DbNPM6I, and DbNPM6T epitopes showed that both the NPM6T and the NPM6I peptides bind in a way that alters the conformation of H155 in H2Db. Both the central position of the 155 “gate-keeper” residue in the MHCI binding cleft and its involvement in TCR ligation makes it a perfect target for viral escape (25, 31). The present analysis established that H155 residue is important for pMHCI stability. Any conformational change at H155 affects the structure of the pMHCI epitope, and a single amino acid mutation in the immunodominant NP366 peptide (from M to I) changed the conformation of this critical residue resulting in viral escape. Such topographical modifications can contribute to reduced primary immune responses, as the TCR is unable to access a key MHC residue required for recognition. Within the DbNPM6I structure, H155 is buried beneath the viral peptide and is, as a consequence, no longer accessible to the TCR. Our morphological findings, together with the emergence of comparable virus variants in TCR-transgenic mice (11), provide a clear understanding of how structural defects at the level of the pMHCI interaction can promote CTL escape following influenza virus infection. The realization that changes in viral peptides can modify antigenicity in a “cascade” effect that first alters the orientation of the MHCI molecule, then leads to a pMHCI topography that is suboptimal for TCR recognition offers a general explanation for immune escape by a number of viruses. Movement at the 155 residue has also been found for the LCMV escape mutant NP205-V3A (32).
The large secondary response observed following challenge with a serologically different influenza virus incorporating the homologous mutation indicates that, despite what may be a suboptimal pMHCI conformation, the DbNPM6I and DbNPM6T epitopes can be recognized in way that is independent of any “typical” H155 presentation (25). The naïve DbNPM6T-specific precursor frequency detected by pMHCI tetramer binding is significantly smaller than that detected for the other mutant DbNPM6X epitopes. In fact, the frequency was so low that it differed little from the background levels detected in TCR-transgenic OT-1 and Balb/C (H2d) mice (26, 28). The hierarchy for the DbNPM6X epitopes, which correlates with primary CTL response magnitude is: DbNPM6W = DbNPM6Y > DbNPM6I > DbNPM6T. By this analysis, the recovery of >20 antigenic-specific CD8+ CTL precursors is required for the generation of a readily detectable primary response. However, despite the minimal primary DbNPM6I+ and DbNPM6T+ responses, exposure to these escape variants established memory pools that were amplified by secondary challenge to give CTL numbers comparable to these found for the secondary WT response, especially at the site of infection.
Overall, engineered variants based on potentially lethal mutational changes selected in TCR-transgenic mice established at some level of endogenous, polyclonal CD8+ T-cell responses in the WT B6 strain. Although the NPM6I and NPM6T CTLs detected following primary infection were minimal, the homologous recall responses were substantial, similar to what happens with HIV variants (33–35). Although variation at prominent pMHCI residues may manifest as diminished recognition by existing CD8+ T cells, such mutants can recruit their own TCR repertoires, though with different degrees of effectiveness. Even so, it does seem that exposure to mutant viruses of varying immunogenicity can, despite the number of naïve CTL precursors being very low, establish the conditions for an effective secondary response. This finding suggests that it may be useful to prime against commonly occurring mutants and generate broadly protective CD8+ T-cell immunity against TCR escape variants.
Materials and Methods
Mice and Viral Infection.
C57BL/6J (B6, H-2b), Balb/C (H-2d) and OT-I (H-2b TCR transgenic specific for OVA257) mice were bred and housed under specific pathogen-free conditions at the Department of Microbiology and Immunology, University of Melbourne. Recombinant influenza viruses with a single amino acid substitution at P6 within the NP366 peptide, ASNENMETM, were generated using the eight-plasmid reverse genetics system (22). The substitution was incorporated by site directed mutagenesis using PCR primers encoding NPM6X peptide, ASNENxETM (where x = Y, W, I or T amino acids). For acute influenza responses, mice were lightly anesthetized by inhalation of methoxyflurane and infected intranasally with 1 × 104 pfu of HK (H3N2) or one of the modified HK-NPM6X influenza viruses in 30 μL PBS. For recall responses, mice were first primed intraperitoneally with 1.5 × 107 pfu of the serologically distinct PR8 (H1N1) or modified PR8 (PR8-NPM6X) influenza A viruses, in 500 μL PBS, and challenged 6 wk later with the HK viruses. All experiments were approved and conducted under guidelines set by the University of Melbourne Animal Ethics Experimentation Committee.
Tissue Sampling and Cell Preparation.
Spleen and BAL populations were recovered from mice at acute phases of the primary and secondary infections on days 10 and 8, respectively. For assessment of naïve CTL precursor frequencies, spleens and macroscopic lymph nodes (inguinal, brachial, auxiliary, superficial cervical, and mesenteric) were collected from naïve mice and processed to single-cell suspensions.
Tetramer and Phenotypic Staining.
Spleen or BAL cells were stained with pMHCI tetramers conjugated to Strepavidin-APC or -PE (DbNP366 or DbNPM6X366 tetramers) for 1 h at room temperature. Cells were washed twice in FACS buffer [1% (vol/vol) BSA, 0.02% Sodium Azide in PBS], stained with CD8-PerCPCy5.5 and one of 14 different mAb Vβ-FITC (BD Biosciences) for 30 min on ice, washed twice, assessed by flow cytometry using a FACS Calibur (BD Immunocytometry), and analyzed by FlowJo software (Treestar). Naïve DbNPM6X+CD8+ T cells were identified as previously described (5, 26, 28).
Peptide Stimulation and Intracellular Cytokine Staining.
Splenocytes or BAL samples were stimulated with one of NP366, NPM6X or PA224 peptides (AusPep) for 5 h at 37 °C, 5% CO2 in the presence of 1 μg/mL Golgi-Plug (BD Biosciences) and 10 U/mL recombinant human IL-2 (Roche). Cells were washed twice with FACS buffer, stained with CD8-PerCPCy5.5 for 30 min on ice, fixed, permeablized and stained with IFN-γ–FITC mAb (BD Biosciences and Biolegend). In selected experiments, lymphocytes were stimulated with varying concentrations of peptides, threefold dilutions ranging from 300 nM to 0.0008 nM to determine the sensitivity-specific peptides (5).
Isolation of Single CD8+ T cells, RT-PCR, and Sequencing.
Cells were stained with DbNPM6X-PE tetramer in PBS with 0.1% BSA and either Vβ1 Vβ4, 8.1/8.2-, 8.3-, or 9-FITC. Single cells were isolated by sorting with a FACS Aria (BD Immunocytometry) into 80 wells of an empty 96-well twin-tec plate (Eppendorf). mRNA transcripts were reverse-transcribed to cDNA with a Sensiscript kit (Qiagen). The CDR3β region was amplified by a nested PCR (29).
Protein Expression, Crystallization, Structure Determination, and Thermal Stability Assay.
The H2Db and β2-microglobulin molecules were produced and the crystal structures were solved (5) (Table S1) The DbNPM6X complex crystals were obtained by the hanging-drop vapor diffusion technique at 20 °C in 0.1 M Tris⋅HCl pH 8.5, 0.2 M LiSO4, and 25–30% PEG 8000 (wt/vol) (Table S1). The crystals were flash-frozen to a temperature of 100 K before data collection in-house with a Rigaku RU-200 rotating-anode X-ray generator for Db-NPM6W, at the Australian synchrotron on the MX1 beamline with an ADSC-Q210 CCD detector for Db-NPM6I, on the MX2 beamline with an ADSC-Q315 CCD detector for Db-NPM6T, Db-A155-NP and Db-A155-NPM6I structures. The structures were deposited in the Protein Database (Table S1). Each pMHC-I complex was assayed in 10 mM Tris⋅HCl pH 8, 150 mM NaCl, at two concentrations (5 and 10 mM) in duplicate. The thermal melt point (Tm), represents the temperature (°C) for which 50% of the protein was unfolded (5).
Supplementary Material
Acknowledgments
We thank Dr. Weiguang Zeng for providing the NP-M6T peptide and the staff at the MX beamlines of the Australian synchrotron for assistance with data collection. This work was supported by Australian National Health and Medical Research Council (NHMRC) Project Grant AI1008854 (to K.K.), NHMRC Program Grant APP567122 (to P.C.D., S.J.T., and D.C.J.), and National Institutes of Health Grant AI170251 (to P.C.D.). S.A.V. was a recipient of the Australian Postgraduate Award, K.K. is an NHMRC Career Development Fellow Level 2, A.W.P. is a NHMRC Senior Research Fellow, D.C.J. is an NHMRC Senior Principal Research Fellow, S.G. and S.J.T. are Australian Research Council Future Fellows, and J.R. is an NHMRC Australia Fellow.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 4HUU (H2Db-NP-M6I), 4HUV (H2Db-NP-M6W), 4HUW (H2Db-NP-M6T), 4HUX (H2Db-A155-NP), and 4HV8 (H2Db-A155-NP-M6I)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302935110/-/DCSupplemental.
References
- 1.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 2.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175(4):1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreijtz JH, et al. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008;82(11):5161–5166. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenbaum JA, et al. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc Natl Acad Sci USA. 2009;106(48):20365–20370. doi: 10.1073/pnas.0911580106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valkenburg SA, et al. Protective efficacy of cross-reactive CD8+ T cells recognising mutant viral epitopes depends on peptide-MHC-I structural interactions and T cell activation threshold. PLoS Pathog. 2010;6(8):e1001039. doi: 10.1371/journal.ppat.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frankild S, de Boer RJ, Lund O, Nielsen M, Kesmir C. Amino acid similarity accounts for T cell cross-reactivity and for “holes” in the T cell repertoire. PLoS One. 2008;3(3):e1831. doi: 10.1371/journal.pone.0001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assarsson E, et al. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J Virol. 2008;82(24):12241–12251. doi: 10.1128/JVI.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore CB, et al. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296(5572):1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 9.Price DA, et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity. 2004;21(6):793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez CS, et al. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J Virol. 2005;79(9):5721–5731. doi: 10.1128/JVI.79.9.5721-5731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price GE, Ou R, Jiang H, Huang L, Moskophidis D. Viral escape by selection of cytotoxic T cell-resistant variants in influenza A virus pneumonia. J Exp Med. 2000;191(11):1853–1867. doi: 10.1084/jem.191.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voeten JT, et al. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J Virol. 2000;74(15):6800–6807. doi: 10.1128/jvi.74.15.6800-6807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon AC, et al. Recognition of homo- and heterosubtypic variants of influenza A viruses by human CD8+ T lymphocytes. J Immunol. 2004;172(4):2453–2460. doi: 10.4049/jimmunol.172.4.2453. [DOI] [PubMed] [Google Scholar]
- 14.Gras S, et al. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci USA. 2010;107(28):12599–12604. doi: 10.1073/pnas.1007270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gog JR, Rimmelzwaan GF, Osterhaus AD, Grenfell BT. Population dynamics of rapid fixation in cytotoxic T lymphocyte escape mutants of influenza A. Proc Natl Acad Sci USA. 2003;100(19):11143–11147. doi: 10.1073/pnas.1830296100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl A, et al. T-cell tolerance for variability in an HLA class I-presented influenza A virus epitope. J Virol. 2009;83(18):9206–9214. doi: 10.1128/JVI.00932-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedzierska K, et al. Complete modification of TCR specificity and repertoire selection does not perturb a CD8+ T cell immunodominance hierarchy. Proc Natl Acad Sci USA. 2008;105(49):19408–19413. doi: 10.1073/pnas.0810274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boon AC, et al. Sequence variation in a newly identified HLA-B35-restricted epitope in the influenza A virus nucleoprotein associated with escape from cytotoxic T lymphocytes. J Virol. 2002;76(5):2567–2572. doi: 10.1128/jvi.76.5.2567-2572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young AC, Zhang W, Sacchettini JC, Nathenson SG. The three-dimensional structure of H-2Db at 2.4 A resolution: Implications for antigen-determinant selection. Cell. 1994;76(1):39–50. doi: 10.1016/0092-8674(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 20.Turner SJ, et al. Lack of prominent peptide-major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nat Immunol. 2005;6(4):382–389. doi: 10.1038/ni1175. [DOI] [PubMed] [Google Scholar]
- 21.Venturi V, Davenport MP, Swan NG, Doherty PC, Kedzierska K. Consequences of suboptimal priming are apparent for low-avidity T-cell responses. Immunol Cell Biol. 2012;90(2):216–223. doi: 10.1038/icb.2011.36. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann E, et al. Rescue of influenza B virus from eight plasmids. Proc Natl Acad Sci USA. 2002;99(17):11411–11416. doi: 10.1073/pnas.172393399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigal LJ, Wylie DE. Role of non-anchor residues of Db-restricted peptides in class I binding and TCR triggering. Mol Immunol. 1996;33(17–18):1323–1333. doi: 10.1016/s0161-5890(96)00099-5. [DOI] [PubMed] [Google Scholar]
- 24.Tynan FE, et al. T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat Immunol. 2005;6(11):1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 25.Burrows SR, et al. Hard wiring of T cell receptor specificity for the major histocompatibility complex is underpinned by TCR adaptability. Proc Natl Acad Sci USA. 2010;107(23):10608–10613. doi: 10.1073/pnas.1004926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obar JJ, Lefrançois L. Early signals during CD8 T cell priming regulate the generation of central memory cells. J Immunol. 2010;185(1):263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Gruta NL, et al. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest. 2010;120(6):1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedzierska K, Turner SJ, Doherty PC. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc Natl Acad Sci USA. 2004;101(14):4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kedzierska K, La Gruta NL, Turner SJ, Doherty PC. Establishment and recall of CD8+ T-cell memory in a model of localized transient infection. Immunol Rev. 2006;211:133–145. doi: 10.1111/j.0105-2896.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 31.Godfrey DI, Rossjohn J, McCluskey J. The fidelity, occasional promiscuity, and versatility of T cell receptor recognition. Immunity. 2008;28(3):304–314. doi: 10.1016/j.immuni.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen AT, et al. Loss of anti-viral immunity by infection with a virus encoding a cross-reactive pathogenic epitope. PLoS Pathog. 2012;8(4):e1002633. doi: 10.1371/journal.ppat.1002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oxenius A, et al. Loss of viral control in early HIV-1 infection is temporally associated with sequential escape from CD8+ T cell responses and decrease in HIV-1-specific CD4+ and CD8+ T cell frequencies. J Infect Dis. 2004;190(4):713–721. doi: 10.1086/422760. [DOI] [PubMed] [Google Scholar]
- 34.Allen TM, et al. De novo generation of escape variant-specific CD8+ T-cell responses following cytotoxic T-lymphocyte escape in chronic human immunodeficiency virus type 1 infection. J Virol. 2005;79(20):12952–12960. doi: 10.1128/JVI.79.20.12952-12960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iglesias MC, et al. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood. 2011;118(8):2138–2149. doi: 10.1182/blood-2011-01-328781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.