Abstract
Respiratory syncytial virus (RSV) infects most children in the first year of life and is a major single cause of hospitalization in infants and young children. There is no effective vaccine, and antibody generated by primary neonatal infection is poorly protective against reinfection even with antigenically homologous viral strains. Studying the immunological basis of these observations in neonatal mice, we found that antibody responses to infection were low and unaffected by CD4 depletion, in contrast with adult mice, which had stronger CD4-dependent antibody responses. Natural killer cell depletion or codepletion of CD4+ and CD8+ cells during neonatal RSV infection caused a striking increase in anti-RSV antibody titer. These cells are major sources of the cytokine IFN-γ, and blocking IFN-γ also enhanced RSV-specific antibody responses in neonates. In addition, infection with a recombinant RSV engineered to produce IFN-γ reduced antibody titer, confirming that IFN-γ plays a pivotal role in inhibition of antibody responses after neonatal infection. These unexpected findings show that the induction of a strong cellular immune response may limit antibody responses in early life and that vaccines that induce IFN-γ–secreting cells might, in some situations, be less protective than those that do not.
Keywords: viral infection, bronchiolitis, lung infection
Antibody responses to vaccines are poor in infancy (1), and this can lead to increased infection severity and decreased vaccination efficacy. It is believed that the paucity of the infantile antibody response is caused by immaturity of the cells involved (2, 3). Neonatal B cells are immature, expressing lower levels of MHC-II (4), and when B cells are transferred from adult mice into the footpads of neonates, the antibody response is restored to adult levels (5). In addition to poor B- and T-cell interactions, the development of follicular dendritic cells is delayed in neonatal mice (6) and infants (7). Improved understanding about B-cell responses is required to improve early life vaccine strategies, particularly in the control of respiratory infections, which are a major cause of morbidity and mortality in infancy.
Respiratory syncytial virus (RSV) is the principal cause of viral lung infection in infants (8). The hospitalization rate resulting from RSV bronchiolitis in the United States is 17 per 1,000 children younger than 6 mo of age (9), with an estimated 33.8 million cases of RSV-associated lower respiratory tract infection and 66,000 to 199,000 deaths globally per annum (10). Reinfection is common and does not necessarily depend on antigenic changes (11), and 47% of children infected in the first year of life are reinfected in their second year of life (12). An effective RSV vaccine would be a major breakthrough in child health, and, as most children with RSV bronchiolitis are younger than 6 mo of age, it would need to be effective in infancy. A core component of protection against RSV infection is antibody: high-titer, maternally derived, RSV-neutralizing antibody is protective against hospitalization (13), and protection against reinfection in humans is associated with high titers of antibody (14). Antibody responses seen in infants after RSV infection (15, 16) and vaccination (17, 18) are weak, an effect usually attributed to reduced T-cell help. Few CD4 T-cell lymphocytes are found in the lungs of RSV-infected infants (19), and the V gene repertoire for anti-RSV antibody is restricted in neonatal humans (20). We have developed a mouse model of neonatal RSV infection (21) in which we observed that infection of neonatal mice led to a T-cell response that caused disease on subsequent reinfection (22). Furthermore, we subsequently showed that this pathogenic cellular response is dependent upon the mouse MHC haplotype (23).
Antibody titers are lower following experimental neonatal mouse RSV infection compared with adult infection (24). Studying the basis of these observations, we wished to assess whether the cellular response to neonatal RSV affected the antibody titer. We found that CD4+ cell depletion did not reduce neonatal antibody titers, but depleting natural killer (NK) cells or CD4+ and CD8+ cells together increased antibody titer. During neonatal RSV infection, NK and T cells produced the cytokine IFN-γ, which has been shown to inhibit the antibody response (25). When IFN-γ was depleted during neonatal RSV infection, the antibody titer significantly increased. This demonstration of the inhibition of antibody production by the cellular response during neonatal infection has important implications for the control of RSV in early life.
Results
Reduced Antibody Response in Neonates Compared with Adults After RSV Infection.
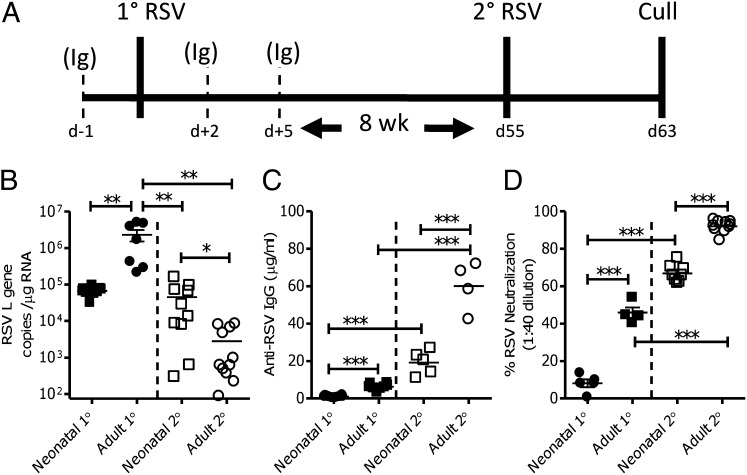
BALB/c mice were intranasally infected with 4 × 104 pfu/g body weight RSV at 4 d (neonatal; 105 pfu) or 28 d (adult; 106 pfu) of life (schematic of experimental protocol in Fig. 1A). Mice infected as neonates had significantly lower viral loads than adults on day 4 post primary infection (P < 0.01; Fig. 1B), as previously observed (21, 26). Serum was collected 8 wk after primary infection to allow maturation of responses. Sera from mice that had primary RSV infection as adults had significantly more RSV-specific ELISA binding (P < 0.001; Fig. 1C) and neutralizing (P < 0.001; Fig. 1D) antibody than mice that had a primary infection as neonates. RSV-specific IgG subtypes were quantified by ELISA in sera. The neonatal RSV-IgG response was significantly skewed toward IgG1 [indicating a T helper 2 (Th2) bias; P < 0.001], whereas adult primary infection led to a balanced IgG1/IgG2a response (Fig. S1A).
Fig. 1.
Antibody responses to RSV infection in early life are less than adult responses. BALB/c mice were infected with 4 × 104 pfu RSV per gram body weight intranasally as neonates (age 4 d, ▪) or as immature adults (age 4–6 wk, ●; primary 1° RSV). At 8 wk after the primary infection, mice were given a secondary infection of 106 pfu RSV (secondary 2° RSV). Schematic experimental protocol (A) including cellular depletion protocol (Ig indicates an antibody depletion time point). Lung viral load was measured by quantifying RSV L gene expression on day 4 of primary (1° RSV, closed symbols) or secondary (2° RSV, open symbols) infection (B). RSV-specific IgG (C) and in vitro RSV neutralization (D) measured in sera collected after primary and secondary infections. Points represent individual mice, lines represent mean of n ≥ 4 mice per group ± SEM (A and C show pooled data from two experiments, B is representative of two experiments; *P < 0.05, **P < 0.01, and ***P < 0.001, t test).
Mice were reinfected with 106 pfu of the same RSV strain 8 wk after their initial infection (Fig. 1A). Previous exposure to RSV, regardless of age, significantly reduced viral load during secondary RSV infection, compared with primary RSV infection (P < 0.01; Fig. 1B). However, mice that were primed as adults had a significantly reduced viral load than mice primed as neonates (P < 0.05). Although reinfection significantly boosted antibody titers in neonatally and adult primed mice (P < 0.001; Fig. 1C), adult primed mice still had significantly greater antibody titers (P < 0.001; Fig. 1C) and more potent neutralizing responses (P < 0.001; Fig. 1D) than neonatally primed mice after reinfection. Adult reinfection of neonatally primed mice reversed the initial IgG1 bias, leading to a predominantly IgG2a response (Fig. S1B). Neonatal mice generated weaker antibody responses to RSV infection, which may reduce protection against subsequent viral reexposure and contribute to the delayed sequelae of neonatal RSV infection (22).
T Cells and NK Cells Inhibit Neonatal Antibody Response.
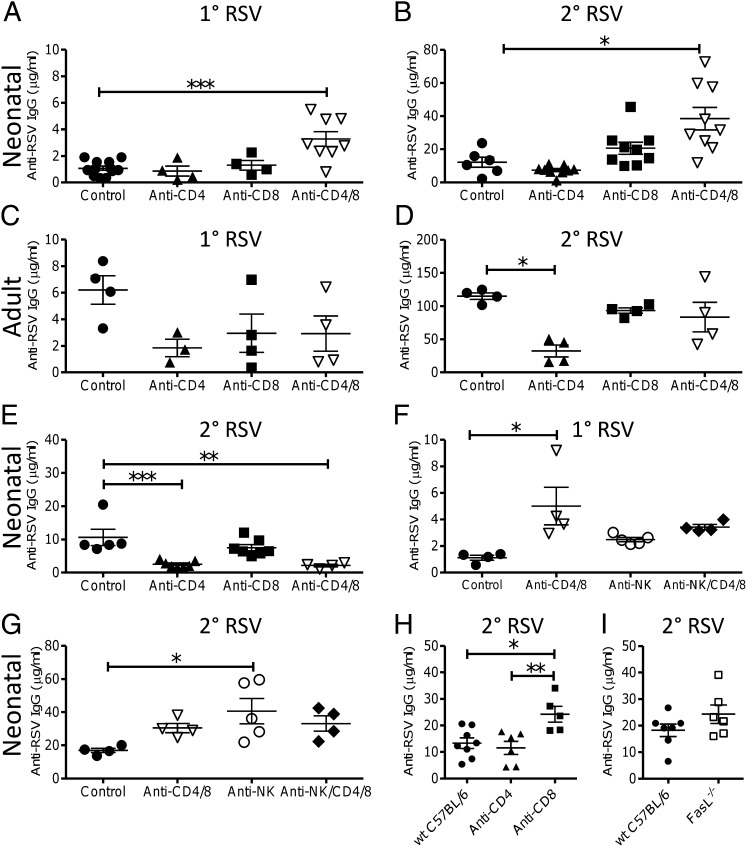
We have previously observed that depletion of T cells during neonatal RSV infection can have a protective effect during reinfection (22). To assess their effect on antibody responses, T cells were depleted during primary neonatal RSV infection and antibody responses were measured before and after reinfection (schematic in Fig. 1A). Cell depletion significantly reduced the targeted cell type (Fig S2). CD4+ cell depletion had no effect on the weak anti-RSV antibody response in neonates after primary (Fig. 2A) or secondary infection (Fig. 2B). However, depletion of both CD4+ and CD8+ cells together significantly boosted IgG after primary (P < 0.001) and secondary (P < 0.05) RSV infection. Depletion of CD4+ cells during primary adult RSV infection did not significantly change the antibody titer before reinfection (Fig. 2C), but caused a significant reduction in anti-RSV IgG titer after reinfection (P < 0.05; Fig. 2D), as previously observed (27). CD4 and CD8 codepletion during primary adult RSV infection had no effect on IgG titer (Fig. 2D). To define the timing of the effect, T cells were depleted during secondary RSV infection of neonatally primed mice. When T cells were depleted during secondary RSV infection of neonatally primed mice, CD8+ cell depletion had no effect on antibody titer, but CD4+ cell depletion or depletion of CD4+ and CD8+ cells together significantly reduced the titer (P < 0.01; Fig. 2E). In addition, cell depletion during primary neonatal infection increased levels of IgG1 compared with control neonatal infection (Fig. S1 C and D).
Fig. 2.
Cell depletion increases neonatal antibody response to RSV infection. CD4, CD8, or NK cells were depleted during primary RSV infection of neonatal (A, B, F, and G) or adult (C and D) BALB/c mice or during secondary RSV infection of BALB/c mice initially infected as neonates (E). RSV-specific IgG was measured on day −1 (A, C, and F) or day 7 (B, D, E, and G) of RSV secondary infection. WT C57BL/6 mice were infected as neonates with RSV, with or without cell depletion (H) or compared with FasL−/− mice (I), and serum anti-RSV IgG measured on day 7 of RSV secondary infection. Points represent individual mice, lines represent mean of n ≥ 3 mice per group ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001, ANOVA and post-test). Data presented are pooled from two experiments except in C and D, which are from a single experiment.
To test whether NK cells also affected the neonatal antibody response, we depleted NK cells with anti-asialo GM1 during primary neonatal RSV infection. NK cell depletion had no effect on antibody titer before reinfection (Fig. 2F). However, NK cell-depleted neonatal mice had significantly higher anti-RSV IgG responses than untreated mice after reinfection (P < 0.05; Fig. 2G). Suppression of antibody responses by NK cells has been previously observed (28) and was linked to inhibition of antigen presenting cells (APC) (29). This suppression may be caused by NK cell killing the antigen presenting cells by using the Fas ligand (FasL) pathway. To test this, antibody responses to RSV infection were measured in FasL−/− deficient mice. The FasL−/− mice are on a C57BL/6 background, so we confirmed that cell depletion affected antibody levels in C57BL/6 mice (Fig. 2H). Neonatal CD4+ cell depletion had no effect, but CD8+ cell depletion significantly increased RSV-specific antibody titer (P < 0.05). When levels were compared, there was no significant difference in antibody titer between WT and FasL−/− deficient mice (Fig. 2I).
IFN-γ Inhibits Neonatal Antibody Response.
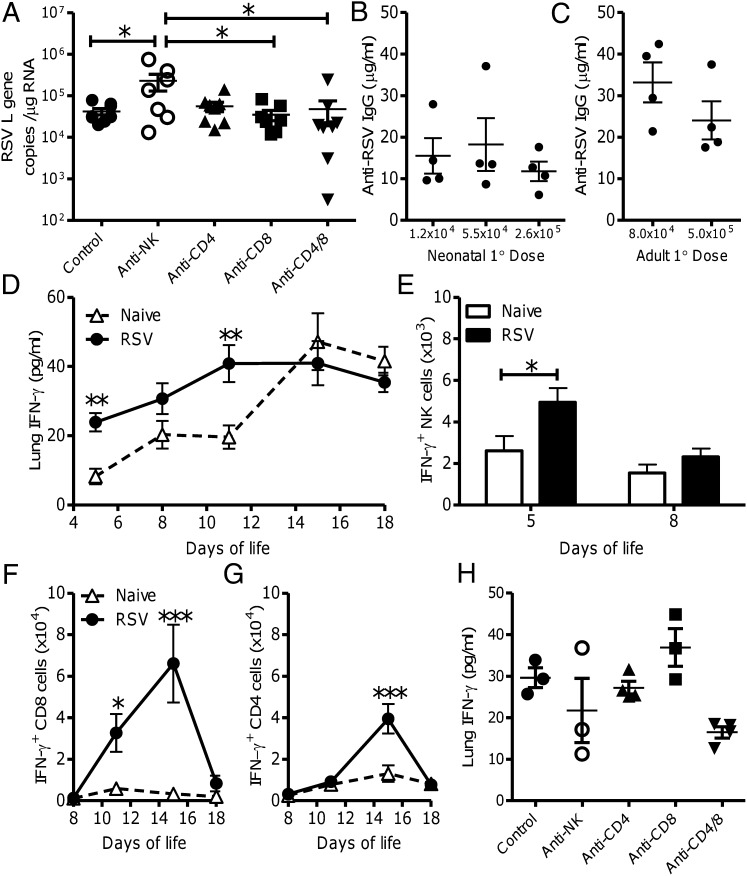
The lower viral load during primary neonatal infection may contribute to the reduced neonatal antibody responses. RSV viral titer was measured on day 4 after primary neonatal infection with and without cell depletion (Fig. 3A). As previously demonstrated (30), NK depletion significantly increased viral load (P < 0.05). However, T-cell depletion had no effect on viral load, suggesting that increased viral load does not account for the increased antibody titer after T-cell depletion. To further address the role of viral load on antibody response, neonatal mice were infected with different doses of virus (1.2 × 104 pfu, 5.5 × 104 pfu, and 2.6 × 105 pfu). There was no apparent difference in antibody responses between groups after secondary infection (Fig. 3B). Likewise, there was no difference in antibody titer when adult mice (Fig. 3C) were infected with different primary doses of virus (8.0 × 104 pfu or 5.0 × 105 pfu). These data suggest that the increased antibody titer seen after cell depletion is not caused by differences in viral load.
Fig. 3.
IFN-γ is produced during neonatal RSV infection. Neonatal BALB/c mice were infected with 105 pfu RSV; CD4, CD8, or NK cells were depleted during RSV infection, and lung viral load was measured by quantifying RSV L gene expression on day 4 post primary infection (A). Neonatal (B) and adult (C) mice were infected with different primary doses of RSV and RSV-specific IgG titer measured on day 7 after secondary RSV infection. Four-day-old mice were infected with RSV (●) or left naive (∆), IFN-γ was measured in lung homogenate by ELISA (D), and IFN-γ expression was measured in PMA/ionomycin-stimulated lung NK (DX5+) (E), CD8 (F), and CD4 (G) cells by flow cytometry. IFN-γ levels were measured in lung homogenate at day 4 post primary neonatal RSV infection, with and without cell depletion (H). Points represent individual mice, lines represent mean of n ≥ 3 mice per group (A–C, and H), and bars/points represent mean of n ≥ 4 mice per group ± SEM (D–G). Data shown are representative of one experimental repeat (*P < 0.05, **P < 0.01, and ***P < 0.001, ANOVA and post-test).
IFN-γ has been shown to have an inhibitory effect on antibody responses (25). In previous studies, we observed that neonatal mice infected with recombinant RSV expressing IFN-γ have reduced antibody responses (31). Although there is a well described Th2 bias in neonatal life (3), we and others have shown that there are RSV-specific IFN-γ–secreting CD8 cells following neonatal RSV infection (22, 32). Mice were infected at day 4 of life and cytokine levels were measured in lung homogenate supernatants at various time points after infection. RSV-infected mice had significantly more IFN-γ in the lungs at days 1 and 7 post infection than naive littermate controls (P < 0.01; Fig. 3D). IFN-γ levels increased in the lungs with age in naive and infected mice, which may reflect increased immunological maturity or the increased size of the lungs. To define which cells produced IFN-γ, cells were isolated during primary infection and stimulated with PMA/ionomycin. Infected mice had significantly more IFN-γ–producing NK cells on day 1 post infection (day 5 of life) compared with age-matched controls (P < 0.05; Fig. 3E). A significant increase in IFN-γ–producing CD8 cells was observed on days 7 and 11 post infection (P < 0.05; Fig. 3F) and CD4 cells on day 11 post infection (P < 0.001; Fig. 3G). To determine whether cell depletion affected IFN-γ production, levels were measured in lung homogenates on day 4 after neonatal infection with and without cell depletion (Fig. 3H). Reduced levels were seen in the anti-CD4 and CD8 double depletion group and the anti-NK group.
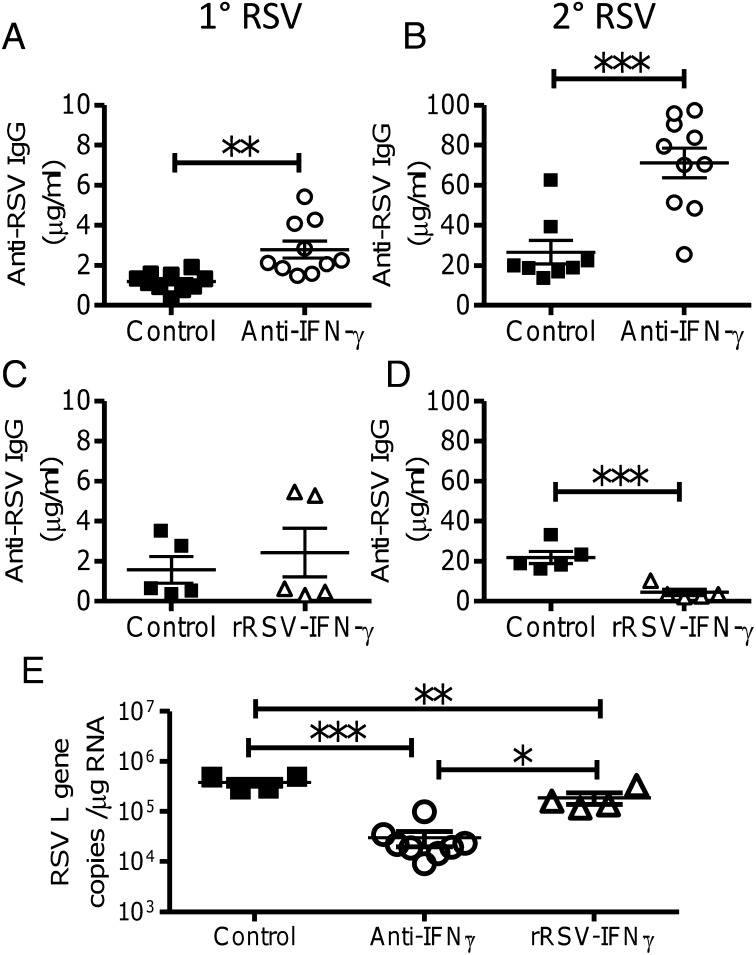
We next investigated whether IFN-γ might have an inhibitory effect on antibody responses. Neonatal mice were infected with RSV, and IFN-γ was blocked by using three doses of anti–IFN-γ (clone XMG1.2) i.p. on days −1, +2, and +5. Blocking IFN-γ during primary infection significantly boosted antibody before (P < 0.05; Fig. 4A) and after (P < 0.001; Fig. 4B) reinfection. IFN-γ blockade lead to a more balanced IgG1:IgG2a ratio than control treatment (P < 0.05; Fig. S1E). To confirm that the IFN-γ was affecting the antibody response, we used a recombinant RSV that expressed IFN-γ (33). As we have observed previously (31), there was no effect on antibody titer before rechallenge (Fig. 4C), but, after rechallenge with WT virus, the antibody response in the recombinant RSV (rRSV)–IFN-γ–primed animals was significantly lower than in WT RSV-primed mice (P < 0.001; Fig. 4D). Infection with rRSV–IFN-γ significantly reduced the IgG1 response (P < 0.05; Fig. S1F). To support the argument that increases in antibody responses are independent of viral load, mice treated with anti–IFN-γ had significantly lower viral loads during primary neonatal infection than control-treated mice but greater antibody responses (P < 0.001; Fig. 4E). As previously observed (31), the rRSV–IFN-γ–infected mice had a significantly lower viral titer than control infected neonatal mice (P < 0.05). We therefore conclude that neonatal anti-RSV antibody responses are inhibited by the cellular immune response, and that IFN-γ plays a vital role in this inhibition.
Fig. 4.
IFN-γ inhibits antibody responses to neonatal RSV infection. During primary RSV infection, neonatal BALB/c mice were treated with anti–IFN-γ or left untreated, and, 8 wk later, mice were reinfected with RSV. RSV-specific IgG was measured in sera on day −1 (A) and day 7 (B) of secondary infection. Neonatal mice were infected with recombinant RSV expressing IFN-γ (rRSV–IFN-γ) or WT RSV A2, and, 8 wk later, mice were reinfected with WT RSV A2. RSV-specific IgG was measured in sera on day −1 (C) and day 7 (D) of secondary infection. Viral load (RSV L gene) was measured during primary neonatal infection by RT-PCR in lungs of control mice or mice infected with RSV and treated with anti–IFN-γ or infected with rRSV–IFN-γ (E). Points represent individual mice; lines represent mean of n ≥ 4 mice per group. The data are pooled from two experimental repeats (*P < 0.05, **P < 0.01, and ***P < 0.001, ANOVA and post-test).
Discussion
The antibody response following neonatal RSV infection was lower than the adult response. Cellular depletion (CD4, CD8, or NK) during primary neonatal infection significantly increased the antibody response. These cells all produced IFN-γ during neonatal infection, and, if IFN-γ was blocked, the antibody response was significantly enhanced. The absence of effect of neonatal CD4+ cell depletion suggests that neonatal B cells have a reduced requirement for CD4 help and that, possibly, the early-life antibody response to RSV is T-cell–independent, unlike the adult response. This would fit with the observation that the neonatal anti-RSV response has reduced somatic hypermutation (20) and that there are reduced numbers of T follicular helper cells in neonates (34). Levels of the TNF family receptors critical for the development and maintenance of B cells [B cell activating factor receptor (BAFF-R), B cell maturation antigen (BCMA), and transmembrane activator and CAML interactor (TACI)] have been demonstrated to be lower in cord blood (35) and neonatal mice (36). Although germinal centers are immature in early life (6), adjuvants that induce maturation of follicular dendritic cells can restore antibody levels (37). Although we did not directly address the role of IFN-γ on antibody response in adult mice, we did observe that they have CD4-dependent antibody responses to RSV infection (Fig. 2D). In addition, we have previously shown that there is a significantly greater RSV-specific IFN-γ response in adult mice than neonatal mice (22). This suggests that the absence of strong CD4:B-cell interactions in neonates contributes to the suppression of the antibody response by IFN-γ.
Inhibition of antibody by IFN-γ has been observed in other systems, IFN-γ receptor-deficient mice have enhanced antibody responses to Bordetella pertussis infection (38) and STAT1 receptor-deficient mice have enhanced antibody responses to RSV infection (39). This inhibitory role for IFN-γ in the neonatal immune response is surprising because of the reported Th2 skew of responses, but Th1 responses can be observed in human neonates, for example, to bacillus Calmette–Guérin (40), and, in mice, can be boosted by the addition of Toll like receptor (TLR) ligands (41). There are several mechanisms by which IFN-γ might inhibit the antibody response. It is possible that the cell and cytokine manipulations increased the viral load, thereby increasing the antigen exposure. However, multiple strands of evidence suggest that antibody titer is not solely influenced by viral load: depleting neonatal T cells had no effect on viral load (Fig. 3A), anti–IFN-γ treatment decreased viral load (Fig. 4E), the rRSV–IFN-γ virus was significantly attenuated as previously observed (33, 42), and, when neonatal or adult mice were infected with different amounts of virus, there was no effect on antibody response (Fig. 3 B and C). The decrease in viral load after neonatal anti–IFN-γ treatment was in contrast to adult mice, which had unchanged (43) or increased (44) viral load after anti–IFN-γ treatment. The decreased viral titer after neonatal anti–IFN-γ treatment may suggest that removing the suppression of antibody response by IFN-γ also has an effect on the viral load. From our studies, we cannot rule out the role of other effectors or cells in the inhibition of the neonatal antibody response, but believe that, during neonatal RSV infection, NK and T cells produce IFN-γ, which acts on cells important for the generation of antibody inhibiting their function, for example B cells (25, 45, 46).
The cell depletion experiments reported in the present study do not distinguish between cells producing IFN-γ or cells responding to IFN-γ and the effect on antibody response. Possible targets for IFN-γ include NK cells, T cells, and B cells, and further studies are required to identify which plays a role. Codepletion of NK and T cells together did not further increase the antibody titer vs. single cell type depletion. As NK cells have been described as limiting antibody by inhibiting APC (29), it may be that the T cells generate the IFN-γ in response to the viral infection, activating the NK cells, leading to APC inhibition. However, we observed no difference in FasL−/− deficient mice, suggesting the inhibitory mechanism is independent of this pathway. It may be that other killing mechanisms or cell types are involved (47), for example, Treg cells, which use granzyme B to resolve inflammation after RSV infection (48), or CD8 cells, which have been shown to kill lymphocytic choriomeningitis virus-infected B cells (49). CD8 T cells have been shown to down-regulate the Th2 CD4 T-cell response to RSV (50), and transferring RSV-specific CD8 cells into adult mice has an inhibitory effect on antibody responses (51). However, this does not explain why CD4 and CD8 codepletion enhanced antibody responses. Neonatal B cells have been shown to have reduced expression of IL-4 receptor, which may potentiate the inhibitory effects of IFN-γ (52). Although IFN-γ has been shown to inhibit IL-4 and thereby reduce classical Th2 help for B cells, the effect of IFN-γ on other CD4 helper subsets that may play a role, such as T follicular help, is not well characterized; recently, IL-21 depletion has been shown to reduce anti-RSV antibody (53).
The effect of IFN-γ on the anti-RSV antibody response in early life may go some way to explain why infants can be reinfected with the same virus in the same or consecutive seasons. We have not succeeded in our attempts to determine how general our observations are and so are unable to say whether this is an RSV-specific effect. The inhibition of antibody responses by the cellular response may have an impact on the development of RSV vaccines for use in early life. If vaccines induce a strong cellular response producing IFN-γ, it may reduce the anti-RSV antibody response. By understanding the effect of IFN-γ on antibody responses, we can optimize pediatric vaccine formulations and schedules to minimize the inhibitory effects.
Methods
Mice and Virus.
Time-mated pregnant BALB/c or C57BL/6 mice (Harlan) were purchased at < 14 d gestation, and pups were weaned at 3 wk of age. Mice were infected intranasally with 4 × 104 pfu/g body weight RSV A2 at 4 d (neonatal, ∼105 pfu in 20 μL) or 4 to 6 wk of age (adults, ∼5 × 105 pfu in 100 μL) under isoflurane anesthesia. Mice were reinfected intranasally 8 wk later with 106 pfu RSV in 100 µL. rRSV–IFN-γ (i.e., recombinant RSV expressing IFN-γ) was obtained from P.L.C. and was generated as described previously (33). Breeding pairs of Fas ligand (FasL−/−) deficient mice [generalized lymphoproliferative disease (gld)] were obtained as a gift from Anthony Warrens (Imperial College London, London, United Kingdom), and the litters from these were used in the same protocol as the WT mice. RSV A2 strain was grown in HEp-2 cells, and viral titer was determined by immune-plaque assay.
RSV viral load was assessed by extracting RNA from lung tissue by using STAT-60 or RNeasy mini kit (Qiagen) and then converting it into cDNA. Quantitative RT-PCR for the RSV L gene using primers and probes previously described (21) was performed, and the results were normalized against 18s or GAPDH endogenous RNA levels. L gene copy number was determined by using an RSV L gene standard and presented relative to micrograms lung RNA.
For cell depletion, mice were treated with 500 µL (adults) or 100 µL (neonates) of 1 mg/mL antibody i.p. on day −1, day +2, and day +5 post infection. Cells were depleted using anti-CD4 (hybridomas YTA 191 and YTA 3), anti-CD8 (clone YTS 156), and control treatment used an irrelevant matched isotype control. All antibodies were IgG2b and a gift of Steve Cobbold (Oxford University, Oxford, United Kingdom). IFN-γ was blocked in neonatal mice by using 100 µL of 1 mg/mL antibody from clone XMG1.2 (eBioscience) i.p. on day −1, day +2, and day +5 post infection. NK cells were depleted with 100 μL anti-asialo GM1 (Wako) on day −1 and day +2 post infection. Prior to commencement, all animal work was approved by a local ethical review board, as described by the Animals (Scientific Procedures) Act 1986, and the work was licensed by the UK Home Office.
RSV-Specific Antibody Quantification.
A quantitative assay was used to determine serum antibody levels as adapted from Donnelly et al. (54). Ninety-six–well plates were coated with RSV antigens and blocked with 1% BSA. A dilution series of recombinant murine IgG was used on each plate as a standard to quantify the RSV-specific antibodies. Sera were diluted 1:500, 1:5,000, and 1: 50,000 to ensure the absorbance reading measured fell within the linear range of the standard curve. Bound IgG was detected by incubation for 2 h at 37 °C with HRP-conjugated goat anti-mouse IgG (AbD Serotec). Plates were washed and developed with 50 μL TMB/E substrate and the reaction was terminated by the addition of 50 μL of 2M H2SO4 and read at A450. For IgG subtype measurements, a similar protocol was performed by using biotinylated anti-IgG1 or anti-IgG2a and detected by using HRP-Streptavidin, compared with a standard curve of recombinant murine IgG1 or IgG2a. Viral neutralization by antibody was assessed as described before (55).
IFN-γ Cytokine ELISA.
Cytokine levels were assessed in lung homogenate supernatants by ELISA using a pair of capture and biotinylated detection antibodies (R&D Systems) following the manufacturer’s instructions. Mediator concentrations were quantified by comparison with recombinant cytokine standards.
Cell Preparation and Flow Cytometry.
After infection, animals were culled by using i.p. pentobarbitone. Cells were harvested as described previously (56). Before staining, cells were blocked with CD16/32 (BD). For surface staining, antibodies against the surface markers CD3 (17A2, FITC), CD4 (RM4-5, APC), CD8 (53.6-7, PerCP), and CD49b (DX5, PE) were added in 1:100 dilution. For intracellular staining, cells were stimulated for 4 h at 37 °C in the presence of 10 μg/mL Brefeldin A, 100 μg/mL phorbol 12-myristate 13- acetate (PMA), and 10 μg/mL ionomycin. Cells were permeabilized with 0.5% saponin and stained with directly conjugated anti–IFN-γ (XMG2.1, FITC). Samples were run on an LSR (BD) and analyzed by using Winlist (Verity).
Statistical Analysis.
Results are expressed as mean ± SEM; statistical significance was calculated by ANOVA followed by Tukey tests or t tests using GraphPad Prism software, as indicated in figure legends.
Supplementary Material
Acknowledgments
The authors thank Steve Cobbold for depleting antibodies, Anthony Warrens for the FasL−/− deficient mice, the staff of St. Mary’s and St. George’s University of London Central Biomedical Services for their support with in vivo studies, and Dr. Paul McKay (Imperial College London) for assistance with the development of the quantitative RSV IgG ELISA. In particular, the authors acknowledge the longstanding support of Prof. Ita Askonas and all her invaluable advice. This work was funded by a Research Grant from The Royal Society (to J.S.T.), research funding from St. George’s University of London (to J.S.T.), Wellcome Trust Programme Grant 071381/Z/03/Z (to P.J.O.), a Medical Research Council Career Development Award Grant G0800311 (to C.J.), and an intramural research program, National Institute of Allergy and Infectious Diseases, National Institutes of Health (to P.L.C. and A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214247110/-/DCSupplemental.
References
- 1.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9(3):185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 2.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: Faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30(12):585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 4.Tasker L, Marshall-Clarke S. Immature B cells from neonatal mice show a selective inability to up-regulate MHC class II expression in response to antigen receptor ligation. Int Immunol. 1997;9(4):475–484. doi: 10.1093/intimm/9.4.475. [DOI] [PubMed] [Google Scholar]
- 5.Astori M, Finke D, Karapetian O, Acha-Orbea H. Development of T-B cell collaboration in neonatal mice. Int Immunol. 1999;11(3):445–451. doi: 10.1093/intimm/11.3.445. [DOI] [PubMed] [Google Scholar]
- 6.Pihlgren M, et al. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J Immunol. 2003;170(6):2824–2832. doi: 10.4049/jimmunol.170.6.2824. [DOI] [PubMed] [Google Scholar]
- 7.Timens W, Rozeboom T, Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology. 1987;60(4):603–609. [PMC free article] [PubMed] [Google Scholar]
- 8.Tregoning JS, Schwarze J. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall CB, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair H, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agoti CN, et al. Genetic relatedness of infecting and reinfecting respiratory syncytial virus strains identified in a birth cohort from rural Kenya. J Infect Dis. 2012;206(10):1532–1541. doi: 10.1093/infdis/jis570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 13.Stensballe LG, et al. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J Allergy Clin Immunol. 2009;123(2):398–403. doi: 10.1016/j.jaci.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 15.Murphy BR, et al. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24(5):894–898. doi: 10.1128/jcm.24.5.894-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandenburg AH, et al. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J Med Virol. 1997;52(1):97–104. doi: 10.1002/(sici)1096-9071(199705)52:1<97::aid-jmv16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Wright PF, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182(5):1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 18.Karron RA, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191(7):1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 19.Reed JL, et al. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis. 2009;199(8):1128–1138. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 20.Williams JV, Weitkamp JH, Blum DL, LaFleur BJ, Crowe JE., Jr The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol Immunol. 2009;47(2-3):407–414. doi: 10.1016/j.molimm.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Culley FJ, Pollott J, Openshaw PJ. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196(10):1381–1386. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tregoning JS, et al. The role of T cells in the enhancement of RSV infection severity during adult re-infection of neonatally sensitized mice. J Virol. 2008;82(8):4115–4124. doi: 10.1128/JVI.02313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tregoning JS, et al. Genetic susceptibility to the delayed sequelae of neonatal respiratory syncytial virus infection is MHC dependent. J Immunol. 2010;185(9):5384–5391. doi: 10.4049/jimmunol.1001594. [DOI] [PubMed] [Google Scholar]
- 24.Tasker L, Lindsay RW, Clarke BT, Cochrane DW, Hou S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin Exp Immunol. 2008;153(2):277–288. doi: 10.1111/j.1365-2249.2008.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson HM, Torres BA. Recombinant mouse interferon-gamma regulation of antibody production. Infect Immun. 1983;41(2):546–548. doi: 10.1128/iai.41.2.546-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Empey KM, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS ONE. 2012;7(7):e40499. doi: 10.1371/journal.pone.0040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88(3):1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abruzzo LV, Rowley DA. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- 29.Shah PD, Gilbertson SM, Rowley DA. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985;162(2):625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F, Zhu H, Sun R, Wei H, Tian Z. Natural killer cells are involved in acute lung immune injury caused by respiratory syncytial virus infection. J Virol. 2012;86(4):2251–2258. doi: 10.1128/JVI.06209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harker JA, et al. Delivery of cytokines by recombinant virus in early life alters the immune response to adult lung infection. J Virol. 2010;84(10):5294–5302. doi: 10.1128/JVI.02503-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruckwardt TJ, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7(12):e1002377. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bukreyev A, Whitehead SS, Bukreyeva N, Murphy BR, Collins PL. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc Natl Acad Sci USA. 1999;96(5):2367–2372. doi: 10.1073/pnas.96.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastelic B, et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189(12):5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 35.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110(8):2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 36.Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol. 2008;181(2):976–990. doi: 10.4049/jimmunol.181.2.976. [DOI] [PubMed] [Google Scholar]
- 37.Bjarnarson SP, Adarna BC, Benonisson H, Del Giudice G, Jonsdottir I. The adjuvant LT-K63 can restore delayed maturation of follicular dendritic cells and poor persistence of both protein- and polysaccharide-specific antibody-secreting cells in neonatal mice. J Immunol. 2012;189(3):1265–1273. doi: 10.4049/jimmunol.1200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186(11):1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durbin JE, et al. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168(6):2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 40.Vekemans J, et al. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol. 2001;31(5):1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, et al. Preexposure to CpG protects against the delayed effects of neonatal respiratory syncytial virus infection. J Virol. 2012;86(19):10456–10461. doi: 10.1128/JVI.01082-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harker J, et al. Virally delivered cytokines alter the immune response to future lung infections. J Virol. 2007;81(23):13105–13111. doi: 10.1128/JVI.01544-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plotnicky-Gilquin H, et al. Gamma interferon-dependent protection of the mouse upper respiratory tract following parenteral immunization with a respiratory syncytial virus G protein fragment. J Virol. 2002;76(20):10203–10210. doi: 10.1128/JVI.76.20.10203-10210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Schaik SM, et al. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol. 2000;62(2):257–266. doi: 10.1002/1096-9071(200010)62:2<257::aid-jmv19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds DS, Boom WH, Abbas AK. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987;139(3):767–773. [PubMed] [Google Scholar]
- 46.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14(5):558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 47.Dix RD, Podack ER, Cousins SW. Loss of the perforin cytotoxic pathway predisposes mice to experimental cytomegalovirus retinitis. J Virol. 2003;77(6):3402–3408. doi: 10.1128/JVI.77.6.3402-3408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loebbermann J, et al. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 2012;5(2):161–172. doi: 10.1038/mi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Planz O, Seiler P, Hengartner H, Zinkernagel RM. Specific cytotoxic T cells eliminate B cells producing virus-neutralizing antibodies [corrected] Nature. 1996;382(6593):726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 50.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186(3):421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alwan WH, Record FM, Openshaw PJM. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin Exp Immunol. 1992;88(3):527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian C, Kron GK, Dischert KM, Higginbotham JN, Crowe JE., Jr Low expression of the interleukin (IL)-4 receptor alpha chain and reduced signalling via the IL-4 receptor complex in human neonatal B cells. Immunology. 2006;119(1):54–62. doi: 10.1111/j.1365-2567.2006.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodd JS, et al. Endogenous IL-21 regulates pathogenic mucosal CD4 T-cell responses during enhanced RSV disease in mice. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donnelly L, et al. Intravaginal immunization using the recombinant HIV-1 clade-C trimeric envelope glycoprotein CN54gp140 formulated within lyophilized solid dosage forms. Vaccine. 2011;29(27):4512–4520. doi: 10.1016/j.vaccine.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moghaddam A, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12(8):905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 56.Tregoning JS, et al. The chemokine MIP1α/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung. PLoS ONE. 2010;5(2):e9381. doi: 10.1371/journal.pone.0009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.