Abstract
Background
There is no angiographically demonstrable obstructive coronary artery disease (CAD) in a significant minority of patients with myocardial infarction (MI), particularly women. We sought to determine mechanism(s) of MI in this setting using multiple imaging techniques.
Methods and Results
Women with MI were enrolled prospectively, prior to angiography if possible. Women with ≥50% angiographic stenosis or use of vasospastic agents were excluded. Intravascular ultrasound (IVUS) was performed during angiography and cardiac magnetic resonance imaging (CMR) within one week. Fifty women (age 57±13 years) had median peak troponin 1.60 ng/ml; 11 had ST elevation. Median diameter stenosis of the worst lesion was 20% by angiography; 15 patients (30%) had normal angiograms. Plaque disruption was observed in 16/42 patients (38%) undergoing IVUS. There were abnormal myocardial CMR findings in 26/44 patients (59%) undergoing CMR: late gadolinium enhancement (LGE) in 17 and T2 signal hyperintensity indicating edema in 9 additional patients. The most common LGE pattern was ischemic (transmural/subendocardial). Non-ischemic LGE patterns (midmyocardial/subepicardial) were also observed. LGE was infrequent with plaque disruption but T2 signal hyperintensity was common with plaque disruption.
Conclusions
Plaque rupture and ulceration are common in women with MI without angiographically demonstrable obstructive CAD. LGE is also common in this cohort of women, with an ischemic pattern of injury most evident. Vasospasm and embolism are possible mechanisms of ischemic LGE without plaque disruption. IVUS and CMR provide complementary mechanistic insights in female MI patients without obstructive CAD and may be useful in identifying potential etiologies and therapies.
Keywords: myocardial infarction, magnetic resonance imaging, coronary disease, imaging
Background
A substantial proportion of patients with myocardial infarction (MI) have no angiographically obstructive (≥50% diameter stenosis) coronary artery disease (CAD), including approximately 7-32% of women and 6-12% of men.1-4 The underlying degree of CAD ranges from absolutely no detectable luminal irregularities through moderate stenosis. Several pathogenetic mechanisms of MI in the absence of obstructive CAD have been postulated, including atherosclerosis with plaque disruption that does not lead to luminal occlusion, endothelial dysfunction with inability to augment coronary flow in response to stress, and vasospasm, among others.5 However, there are extremely limited data. Women are less likely to display obstructive coronary lesions when presenting with all forms of ischemic heart disease, from stable chest pain through MI and fatal ischemic heart disease.6,7 There are also sex differences in morphologic characteristics of the vasculature. Outward remodeling, plaque erosion and microvascular embolization are all more common in women.6 We sought to determine the mechanisms of MI in women without obstructive CAD by examining these patients during their index admission using intravascular ultrasound (IVUS) to determine the presence and extent of plaque and occult plaque disruption. Cardiac magnetic resonance imaging (CMR) was performed within one week thereafter to determine the presence and pattern of myocardial injury.
Methods
The study was approved by the NYU School of Medicine Institutional Review Board. All patients provided informed consent.
Patient Population
Patients were eligible for enrollment if they were women age ≥18 who presented with acute MI and did not have any lesion with ≥50% diameter stenosis or evidence of plaque rupture on coronary angiography, at NYU Langone Medical Center or Bellevue Hospital Center. MI was defined based on ischemic symptoms with elevation and fall of troponin, with or without electrocardiographic (ECG) changes, per the universal definition of MI.8 Cardiac troponin I was measured in the clinical laboratories (VITROS immunodiagnostic assay, Ortho Clinical Diagnostics, Rochester, NY; 99th percentile=0.034ng/ml). Patients with troponin elevation due to heart failure, hypertensive crisis or chronic kidney disease were not included. The target population was women with MI who were clinically referred for angiography. Exclusion criteria were known obstructive CAD based on prior angiography or revascularization, contraindication to study procedures and recent use of vasospastic agents such as cocaine or ergot alkaloids (≤2 weeks). Patients provided informed consent prior to angiography but were excluded from further participation if the angiogram showed ≥50% stenosis of a major epicardial vessel, coronary dissection or excessive tortuosity which in the opinion of the operator increased risk of IVUS.
Angiographically eligible patients underwent IVUS at the time of angiography and CMR within 7 days of angiography. A small subset of patients who could not be approached for consent until after angiography due to logistic issues did not undergo concomitant IVUS (n=8). In these cases only CMR was performed. Demographic, electrocardiographic, left ventricular (LV) function and laboratory data were collected. Severity of stenosis was determined by visual assessment by experienced angiographers. LV wall motion and ejection fraction (LVEF) were obtained by echocardiography, angiography or CMR.
Women suspected to have tako-tsubo cardiomyopathy were included if they met eligibility criteria. Tako-tsubo cardiomyopathy was considered to be present when there was a transient mid LV wall motion abnormality with or without apical involvement extending beyond a single epicardial vascular territory in the absence of evidence of myocarditis or pheochromocytoma.9
Intravascular Ultrasound (IVUS)
Patients who met angiographic eligibility criteria underwent IVUS using a standard clinical scanner (Boston Scientific Corp./SCIMED, Natick, Massachusetts) consisting of a rotating 40-MHz transducer within a 3.2-F imaging sheath. The goal of IVUS was to image at least the proximal 40 mm of the vessel deemed by the angiographer to be the most likely culprit vessel (based on ECG, results of any wall motion studies and the angiogram itself) and at least one other major epicardial vessel, using automated pullback. When a culprit artery could not be identified or if the right coronary artery (RCA) was not suspected as a culprit, the left anterior descending (LAD) and left circumflex (LCX) arteries were imaged. Manual advancement and withdrawal of the IVUS probe were used if additional images were needed to fully characterize any areas of the vessel.
Images were interpreted by an independent core laboratory (Cardiovascular Imaging Research Core Laboratory, University of British Columbia) for determination of plaque rupture, ulceration/erosion, thickness and presence/absence of thrombus, dissection and calcification. Ruptured plaque was defined according to the published standard as a plaque ulceration with a tear detected in the fibrous cap, typically with a dissection into the plaque.10 Ulceration was defined as a recess in the plaque beginning at the luminal-intimal border, typically without enlargement of the external elastic lamina compared to the reference segment, and without dissection into the plaque.10 Contrast injections were used as needed to prove and define the communication point. These definitions have very high reproducibility (99%).11 Maximal thickness >0.5 mm was considered abnormal.12
Qualitative parameters (rupture, ulceration, thrombus, dissection) were assessed by two independent, experienced readers at the core laboratory and verified by a cardiologist. Parameters were also reviewed by a cardiologist board-certified in cardiovascular ultrasound at the enrolling center (HRR). The core laboratory was blinded not only to clinical, ECG, laboratory, angiographic and CMR information at the time of interpretation but also to the nature of the study protocol, i.e., inclusion of women with MI and the entry criterion of no obstructive CAD. The cardiologist at the enrolling center reviewed the IVUS studies offline, blinded to all other information. Disagreements were resolved by consensus of two cardiologists at the core laboratory.
Cardiac Magnetic Resonance Imaging (CMR)
Patients underwent CMR within 7 days of angiography. All patients were imaged using a 1.5-Tesla MRI system (Avanto, Siemens, Erlangen, Germany) with a phased-array body coil and standard ECG monitoring. Images were acquired during repeated end-expiratory breath holds. Initial scout images were acquired to identify the cardiac axes. For evaluation of cardiac function, ECG-gated cine images were acquired in standard long- and short-axis planes, using a segmented steady state free precession sequence. A stack of 10-15 short-axis slices was used for full coverage of the LV. T2-weighted inversion recovery or turbo spin echo images were acquired prior to contrast administration in standard long- and short-axis planes.
T2-weighted imaging was incorporated into the protocol after enrollment of 12 patients. Delayed contrast-enhanced T1-weighted images were obtained approximately 10 minutes after a total dose of 0.15mmol/kg gadolinium-DTPA, using a magnetization-prepared spoiled gradient-echo sequence with the inversion time set to null normal myocardial signal intensity.13 Blood pressure and pulse oximetry were monitored.
Image Analysis
All images were reviewed by two experienced CMR readers (MBS, LA) who were blinded to ECG, laboratory, angiographic and IVUS results at the time of interpretation. Any disagreements were resolved by consensus.
The LV was divided according to the AHA 17-segment model for all analyses. T2-weighted images were evaluated on a segmental basis for presence of increased signal. The presence and pattern of late gadolinium enhancement (LGE) were evaluated for each segment and classified as subendocardial, subepicardial, midwall, or transmural in distribution. Segments with >75% LGE based in the subendocardium were classified as transmural. Determination of LGE is highly reproducible.14
Statistical Analysis
Descriptive analysis techniques were employed to characterize study participants. Center and variability of continuous measures are presented using means and standard deviations when they followed a normal distribution and using medians and interquartile range otherwise. We compared baseline characteristics and clinical outcomes of the group with plaque disruption and with no plaque disruption; LGE vs. none, abnormal T2 vs. normal T2, ST-segment elevation vs. no ST-elevation. Statistical testing was performed using two-sample t-tests and non-parametric version Wilcoxon rank-sum tests for continuous variables, depending on whether or not their distribution was Gaussian; and Fisher’s exact test for categorical variables.
Results
Patients and Testing
A total of 121 women who met clinical inclusion criteria, including no known obstructive CAD, provided informed consent between June 2007-August 2010; 71 were ineligible due to ≥50% stenosis (n=69), coronary dissection (n=1) or excessive tortuosity (n=1). Fifty women were fully eligible with <50% angiographic stenosis of all major vessels. We have previously demonstrated that 32% of women undergoing angiography for MI in our laboratory have <50% stenosis; this consecutive sample included women with previously known obstructive CAD.4 Eight of the 50 patients in the current study did not undergo IVUS for logistical reasons (see Figure 1). Forty-four patients underwent CMR, including 36 of the 42 patients who underwent IVUS; the remaining six patients declined CMR after initial consent.
Figure 1.
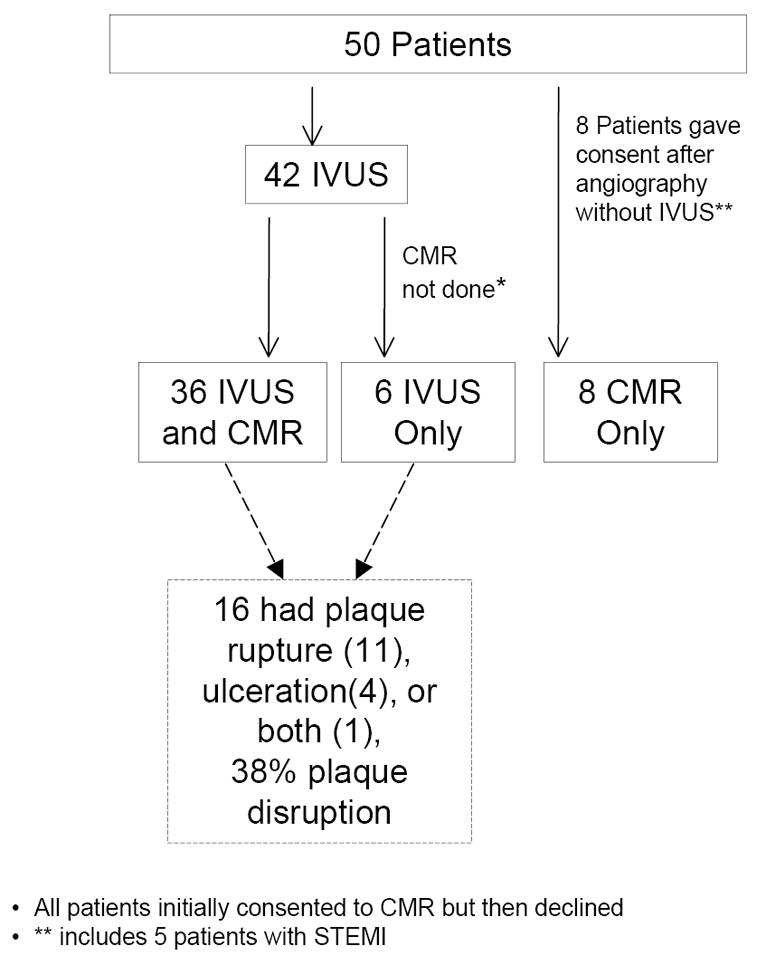
Patient flow and proportion with plaque disruption. *All patients initially consented to CMR but then declined. ** includes 5 patients with ST elevation myocardial infarction.
CMR=cardiac magnetic resonance imaging; IVUS=intravascular ultrasound
Patient characteristics (see Table 1)
Table 1.
Clinical Electocardiographic, Angiographic, Intravascular ultrasound and MRI Findings
| All Patients (n=50) | Plaque Disruption (n=16) | No Plaque Disruption (n=26) | p | LGE (n=17) | No LGE (n=27) | p | STE (n=11) | No STE (n=39) | p | Abnormal T2 (n=17) | Normal T2 (n=15) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean±SD) | 56.9±12.8 | 60.9±11.4 | 53.5±13.7 | 0.07 | 55.4±14.3 | 57.3±12.7 | 0.65 | 52.5±14.7 | 58.2±12.0 | 0.26 | 59.5±14.1 | 52.0±12.4 | 0.12 |
| Troponin, ng/mL (median, IQR) | 1.60 (0.44-4.31) | 1.49 (0.53,2.71) | 0.96 (0.14,4.13) | 0.75 | 3.13 (1.90,40.3) | 1.10 (0.37,1.98) | 0.01 | 1.40 (2.20,4.55) | 1.32 (0.16, 4.13) | 0.32 | 5.97(1.96, 11.0) | 0.66(0.16, 1.30) | <0.001 |
| HTN (%) | 30(60%) | 8(50%) | 17(65%) | 0.35 | 15(88%) | 16(59%) | 0.05 | 8(73%) | 22(56%) | 0.47 | 12(71%) | 12(80%) | 0.61 |
| DM (%) | 16(32%) | 7(44%) | 8(31%) | 0.35 | 6(35%) | 8(30%) | 0.75 | 3(27%) | 14(36%) | 0.17 | 6(35%) | 6(40%) | 0.99 |
| Prior MI (%) | 6(12%) | 1(6%) | 4(15%) | 0.69 | 2(12%) | 4(15%) | 0.73 | 2(18%) | 4(10%) | 0.85 | 3(18%) | 2(13%) | 0.99 |
| Smoking (%) | 9(18%) | 3(19%) | 7(27%) | 0.54 | 4 24%) | 4(15%) | 0.69 | 2(18%) | 8(21%) | 0.80 | 3(18%) | 2(13%) | 0.99 |
| BMI (median, IQR) | 26.2 (24.4,28.4) | 27.6 (24.3,33.7) | 0.52 | 27.4 (24,28.8) | 26.4 (26.4,30.7) | 0.78 | 25.1 (23.1,27.3) | 27.0 (24.4, 31.4) | 0.11 | 27.1 (24.8,28.1) | 31.4 (25.4,36.3) | 0.15 | |
| ECG Findings | |||||||||||||
| ST Elevation | 11(22%) | 2(13%) | 4(15%) | 5(29%) | 5(19%) | 0.47 | 11(100%) | 0(0%) | - | 4(24%) | 3(20%) | 0.99 | |
| ST Depression | 7(14%) | 2(13%) | 2(8%) | 0.63 | 0(0%) | 3(11%) | 0.23 | 0(0%) | 4(11%) | 0.56 | 1(6%) | 2(13%) | 0.59 |
| T Wave inversion | 22(44%) | 10(63%) | 10(38%) | 0.19 | 7(41%) | 14(52%) | 0.36 | 5(45%) | 17(44%) | 0.42 | 9(53%) | 4(27%) | 0.14 |
| LV Wall Motion | |||||||||||||
| Any abnormal segment | 26(52%) | 7(44%) | 14(54%) | 0.79 | 6(35%) | 7(26%) | 0.05 | 4(29%) | 20(52%) | 0.51 | 13(77%) | 5(33%) | 0.03 |
| LVEF (median, IQR) | 60(43,63) | 56(49,62) | 60(55,62) | 0.66 | 55(42,60) | 60(48,65) | 0.57 | 55(38,60) | 60(48,64) | 0.17 | 55(45,60) | 64(55,68) | 0.06 |
| Cath findings | |||||||||||||
| Worst % angiographic stenosis (median, IQR) | 20(0,40) | 40(30,45) | 0(0,20) | <0.001 | 15(0,30) | 30(0,40) | 0.16 | 30 (0,45) | 20 (0,37.5) | 0.47 | 30(15,40) | 10(0,30) | 0.10 |
| Absence of atherosclerosis | 15(30%) | 0(0%) | 13(50%) | 6(18%) | 6(22%) | 0.28 | 4(36%) | 11(28%) | 0.73 | 7(41%) | 11(73%) | 0.09 | |
| IVUS Findings | N=42 | N=42 | N=36 | N=42 | N=25 | ||||||||
| Plaque Disruption | 16(38%) | - | - | - | 1(6%) | 13(48%) | 0.003 | 2(13%) | 12(34%) | 0.13 | 6(43%) | 2(17%) | 0.20 |
| CMR findings | N=44 | N=36 | N=44 | N=44 | N=32 | ||||||||
| LGE (any) LGE type | 17(39%) | 1(7%) | 11(50%) | 0.03 | 17(39%) | 27(61%) | - | 7(47%) | 15(43%) | 0.80 | 8(47%) | 4(27%) | 0.29 |
| Transmural | 9 | 1 | 6 | 0.32 | 9 | 0 | - | 5 | 3 | 0.01 | 4 | 3 | 0.99 |
| Subendocardial | 1 | 0 | 0 | 1 | 1 | 0 | - | 1 | 0 | 1.0 | 0 | 1 | 0.49 |
| Centromyocardial | 2 | 0 | 2 | 0.67 | 2 | 0 | - | 0 | 2 | 1.0 | 1 | 0 | 0.99 |
| Subepicardial | 1 | 0 | 1 | 1 | 1 | 0 | - | 0 | 1 | 1.0 | 1 | 0 | 0.99 |
| “Mixed” pattern | 4 | 0 | 2 | 0.70 | 4 | 0 | - | 1 | 3 | 0.99 | 2 | 0 | 0.49 |
| T2 signal hyperintensity | N=32 | N=25 | N=32 | N=32 | |||||||||
| Present | 17(53%) | 6(75%) | 7(41%) | 0.20 | 8(67%) | 9(45%) | 0.29 | 4(57%) | 13(52%) | 1 | - | - | - |
| Absent | 15(47%) | 2(25%) | 10(59%) | 4(33%) | 11(55%) | 3(43%) | 12(48%) | - | - | - | |||
| Timing of Procedures | N=50 | N=16 | N=26 | N=17 | N=27 | N=11 | N=39 | N=17 | N=15 | ||||
| MI to IVUS (days) | 2(1,2) | 3.5(1,5) | 2(1,4) | 0.18 | 2(1,3.5) | 2.5(1,5) | 0.54 | 1(1,1) | 2(1,5) | 0.01 | 2(1,5) | 2(1,5) | 0.86 |
| MI to CMR (days) | 6(4,8) | 6(4,7) | 6(2,11) | 0.74 | 6.5(3,10) | 6(3,7) | 0.41 | 4(3,6) | 6(3,8) | 0.25 | 6.5(4 9) | 6(4.5,9) | 0.54 |
BMI=body mass index; ECG=electrocardiogram; LV=left ventricle; LVEF=left ventricular ejection fraction; CMR=cardiac magnetic resonance imaging; DM=diabetes mellitus; HTN=hypertension; IQR=interquartile range; IVUS=intravascular ultrasound; LGE=late gadolinium enhancement; MI=myocardial infarction
Mean age was 57±13 years; 46/50 (92%) presented with chest pain. Six (12%) had history of prior MI. Median peak troponin was 1.60ng/ml (interquartile range [IQR],0.44-4.31). 88% of patients had peak troponin >5 times the upper limit of normal (0.04 ng/ml). Thirty-four patients (68%) had an abnormal ECG, including ST elevation in 11 patients (22%), ST depression in 7 (14%), left bundle branch block in one (2%), and T wave inversion (≥2 contiguous leads) in 22(44%). Most patients (84%) were in Killip class I; 3 (6%) presented with or developed cardiogenic shock during the admission; 5 (10%) were in Killip class II. Average LVEF was 55%±14%. LVEF was ≤40% in 10 patients (20%). A suspected culprit vessel could be identified based on ECG, wall motion abnormalities and/or angiography in 23/50 patients (46%).
Angiographic findings
Fifteen patients (30%) had completely normal coronary angiography. Remaining patients had some degree of coronary stenosis, ranging from mild luminal irregularities to 45% diameter stenosis. The median worst diameter stenosis by visual estimation on angiography was 20%. Myocardial bridging was observed in two patients, both in the LAD.
Intravascular ultrasound findings
Sixteen of 42 patients (38%) undergoing IVUS at median 2 days after the onset of ischemic symptoms (IQR 1-2d) had plaque disruption. Twelve patients (29%) had plaque rupture, including two with multiple plaque ruptures and one with plaque rupture and a distinct plaque ulceration (see Figure 2). Four additional patients had plaque ulceration only (10%). The location of plaque rupture was the LAD in 7(58%), LCX in 3(25%) and both the LAD and LCX in 2(17%). The RCA was imaged in 5/42 patients. Plaque ulceration was located in the LAD in three cases and the LCX in two cases. Evaluation for plaque disruption (rupture or ulceration) was concordant in 92% of vessels; remaining vessels were resolved by consensus. Patients with plaque disruption had higher maximal angiographic diameter stenosis (median worst diameter stenosis 40% (IQR 30-45%) vs. 0% (IQR 0-20%) without plaque disruption, p<0.001). Plaque rupture was identified in normal appearing segments (see Figure 2) but not in patients with completely normal angiograms, i.e., without luminal irregularities. There was no relationship between plaque disruption and presence of LV wall motion abnormalities or ECG changes (See Table 1). Peak troponin was similar among those with and without plaque disruption (median peak troponin 1.49ng/ml vs. 0.96ng/ml, respectively, p=0.75). One patient had a non-flow limiting distal coronary dissection resulting from guidewire manipulation during IVUS that had no adverse consequences; the patient declined CMR.
Figure 2.
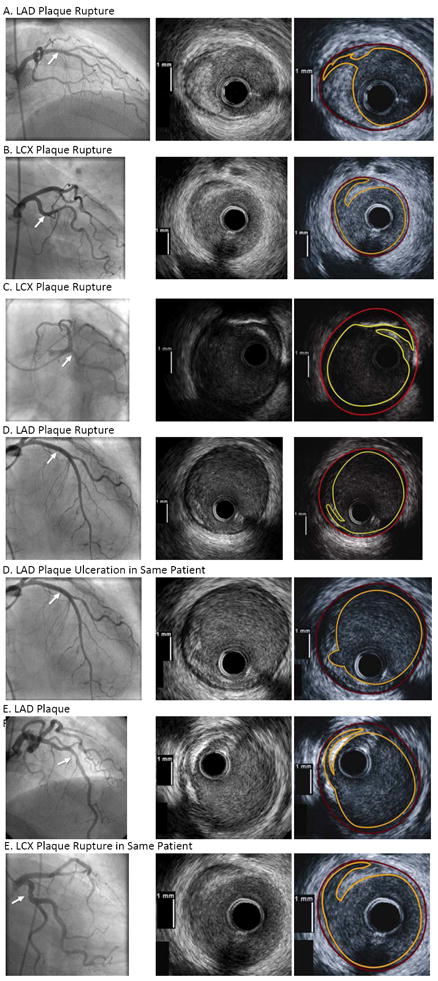
Representative angiographic and intravascular ultrasound (IVUS) images in patients with plaque disruption. The site of plaque rupture or ulceration is marked with an arrow on each angiogram. The artery with the abnormality is indicated for each patient. The right panel for each IVUS image shows the outline of the luminal border (yellow) and external elastic lamina (red) corresponding to each IVUS image directly to its left.
CMR findings
Among 44 patients who underwent CMR at median 6 days since symptom onset (IQR 4-8d), 17 (39%) had at least one area of LGE. Evaluation for LGE was concordant for 96% of segments; remaining segments were resolved by consensus. LGE was associated with higher peak troponin (median 3.13ng/ml vs. 1.10ng/ml, p=0.01) and abnormal LV wall motion (59% vs. 26%, p=0.05). (See Table 1) Ten of the 17 patients with LGE had a transmural or subendocardial pattern typical of an ischemic injury (59%), 3 had a subepicardial or midwall pattern typical of a non-ischemic insult (18%), and 4 had both an area of transmural LGE and a distinct area of midwall LGE (“mixed pattern”, 24%). (See Figure 3.) Patients with ST elevation were more likely to have an ischemic LGE pattern. A likely culprit vessel could be identified based on ECG, wall motion and/or angiography in 9 of 17 patients with LGE. The region of LGE corresponded to the territory of the suspected culprit in 8 of these 9 patients(89%). The median number of segments with LGE was 2 (IQR 1,3); however, often only part of a segment was involved (see Figures 3 & 4).
Figure 3.
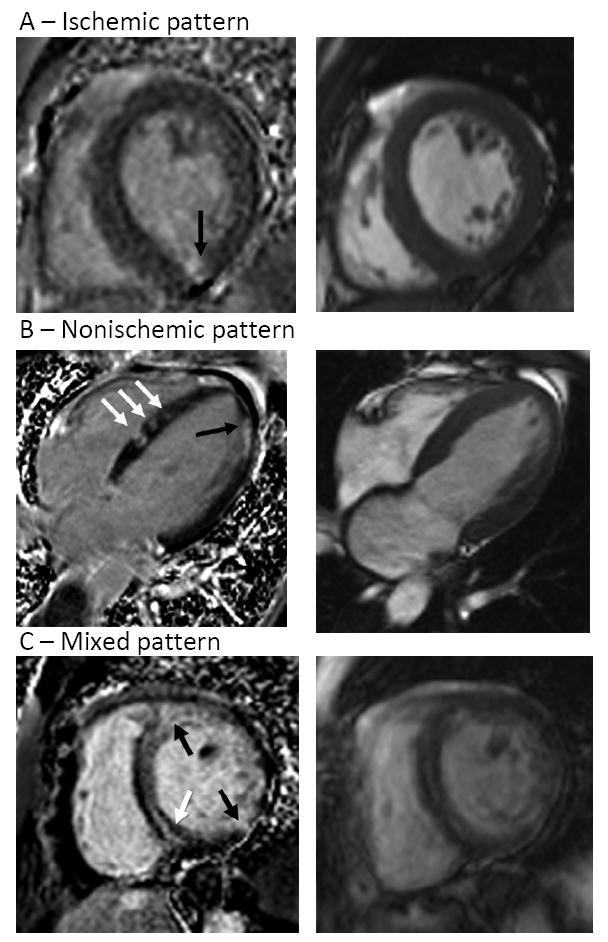
Representative CMR images showing late gadolinium enhancement (LGE) (left) with corresponding end-diastolic cine image (right)
Panel A: Small, nearly transmural LGE involving the mid inferior wall (arrow).
Panel B: Patchy areas of LGE throughout the left ventricle which are primarily midwall, with some septal areas extending to the right ventricular subendocardium (white arrows) and a nearly transmural area in the apical lateral wall (black arrow).
Panel C: Representative images showing a mixed pattern of LGE: multiple separate areas of enhancement demonstrating midwall (white arrow) and transmural (black arrows) involvement.
Figure 4.
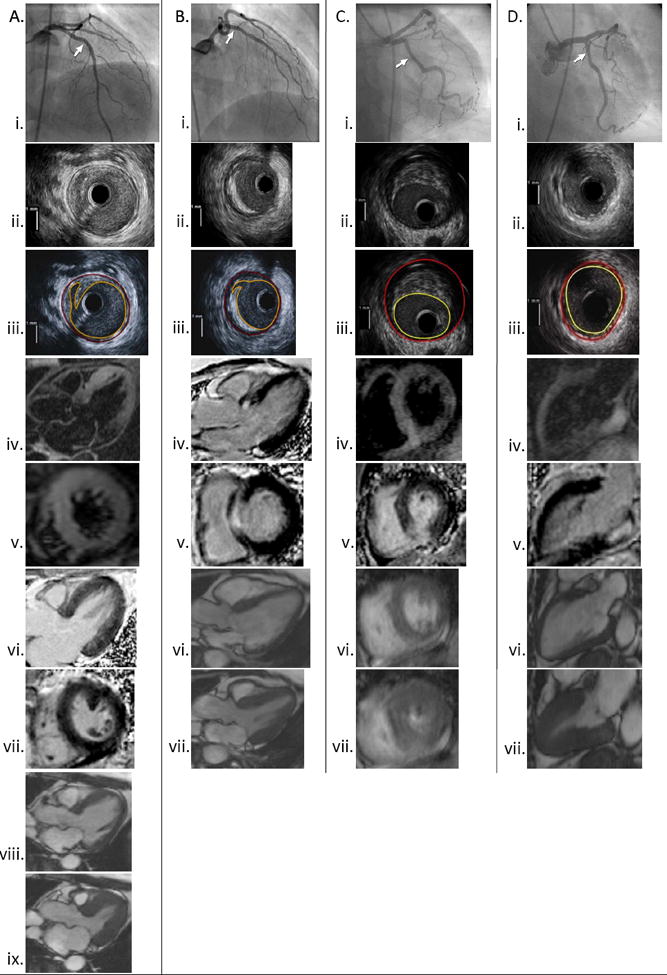
Co-localization of angiographic, IVUS and CMR images in patients with different constellations of findings. The site of IVUS imaging is marked on each angiogram.
A: Plaque rupture with increased T2 signal and absent LGE. Left coronary angiogram (i) with corresponding IVUS showing plaque rupture in the mid LAD (ii-iii). T2 signal hyperintensity is noted to involve the mid anterior and mid and apical anterior septal walls (iv-v). No LGE is noted within the myocardium in 2 planes (vi-vii). Normal LV function (viii-ix).
B: Plaque rupture with LGE. Angiogram of the left coronary artery (i) with corresponding IVUS showing plaque rupture in the proximal LAD (ii-iii). The site of the IVUS images is marked with an arrow. Subendocardial to transmural LGE involving the basal anteroseptal wall in shown in 3-chamber and short axis views (iv-v). T2-weighted imaging was not performed in this patient. Normal LV function (vi-vii).
C: Ischemic-type LGE without plaque rupture on IVUS. Left coronary angiogram (i). IVUS of the LCX showing mixed-fibrofatty plaque without rupture (ii-iii). The LAD and LCX were imaged with IVUS in this case. T2 signal hyperintensity of the mid inferior, mid inferoseptal and adjacent right ventricular walls near the right ventricular insertion (iv). Transmural LGE involving subsegments of the same region (v). Normal LV function (vi-vii)
D: Non-ischemic type LGE with minimal atherosclerosis on IVUS. Left coronary angiogram (i). Normal IVUS of the LCX (ii-iii). T2 signal hyperintensity of the basal inferior/inferolateral wall (iv). Focal area of mid-myocardial LGE involving the same region. (v) Normal LV function (vi-vii).
Among 32 patients who underwent T2-weighted imaging, 17 (53%) had abnormal T2 signal in at least one area. This included 9 patients without LGE and 8 with LGE (4 transmural, 2 midwall or subepicardial, 2 with “mixed” pattern). Evaluation for abnormal T2 was concordant for 93% of segments; remaining segments were resolved by consensus. Patients with abnormal T2 signal had higher peak troponin (median 5.97ng/ml vs. 0.66ng/ml, p<0.001 and were more likely to have abnormal LV wall motion (77% vs. 33%, p=0.03). Time from MI to CMR was not different between those patients with and without T2 signal hyperintensity. A likely culprit vessel could be identified in 10 of 17 patients with abnormal T2. In each of these patients, the region of abnormal T2 signal corresponded to the territory of the suspected culprit. The median number of segments with T2 hyperintensity was 4 (IQR 3,7).
Correlation of MRI and IVUS findings
See Figure 4 for angiographic, IVUS and CMR images from representative cases with different constellations of findings. Among 16 patients with plaque disruption on IVUS, 14 underwent CMR. Only one patient with plaque disruption had LGE, in an ischemic pattern. T2 hyperintensity was noted in 6 of 8 patients with plaque disruption in whom T2-weighted imaging was performed. The remaining patients with plaque disruption were enrolled before T2-weighted imaging was included in the study CMR protocol. Among 26 patients without plaque disruption, 22 underwent CMR and 11/22 had LGE (p=0.03 vs. patients with plaque disruption).
Patients with tako-tsubo cardiomyopathy
Four patients (8%) met criteria for tako-tsubo cardiomyopathy. One had 45% RCA stenosis and an angiographically normal LAD; one had 45% LAD stenosis and the other two had no angiographic stenoses. Two patients with tako-tsubo cardiomyopathy had plaque ulceration on IVUS, one in the LAD (see Figure 5) and the other in the left main. The other two patients had no atherosclerosis on angiography or IVUS. Three of the four patients with tako-tsubo cardiomyopathy underwent CMR; none had LGE. Two patients (one with plaque ulceration) who underwent T2-weighted imaging had signal hyperintensity in all mid and apical segments, a pattern now considered typical of tako-tsubo cardiomyopathy.15
Figure 5.
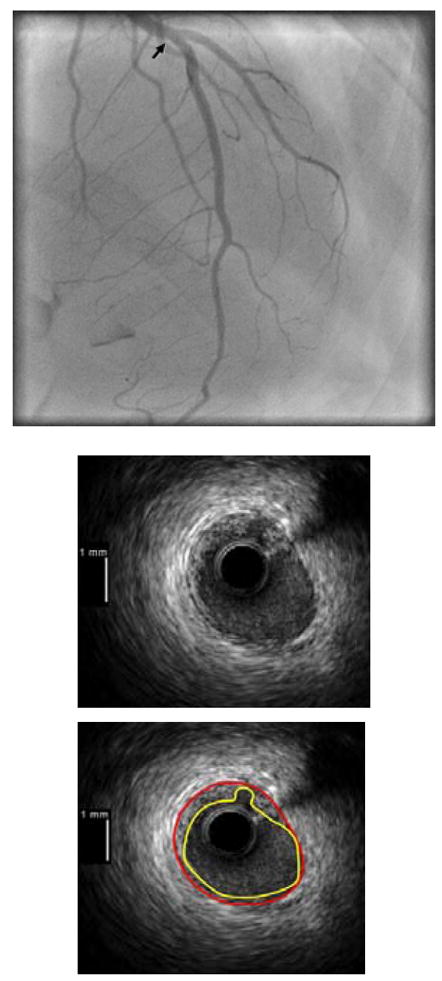
Angiographic and IVUS images from a patient with tako-tsubo cardiomyopathy and plaque ulceration. Angiogram shown in the top panel with arrow marking the site of the IVUS image shown in the lower panels, with and without contours outlining the intimal border (yellow) and external elastic lamina (red). Note that the LAD is a large vessel which wraps around the LV apex; this was also true of the other case of tako-tsubo cardiomyopathy with plaque ulceration in the left main coronary artery (not shown).
Discussion
In this study, the first prospective evaluation using IVUS and CMR in women presenting with acute MI without obstructive CAD at angiography, we have demonstrated that plaque disruption is a frequent finding. We have also shown that late gadolinium enhancement commonly identifies the location and pattern of myocardial damage and that T2 signal hyperintensity is frequently present, indicating acute myocardial edema. T2 signal hyperintensity was particularly common among women with plaque disruption but LGE was infrequent with plaque disruption. IVUS and CMR provided complementary information in this cohort and together revealed abnormalities in 35 of 50 patients (70%).
Plaque rupture or ulceration was identified by the blinded core laboratory in 38% of women undergoing IVUS in this study. Plaque disruption often occurred in segments which were angiographically normal but was not seen in patients with completely normal coronary angiograms, i.e., without luminal irregularities. The finding of plaque disruption in angiographically normal segments is consistent with prior reports in vivo using IVUS16 or during pathologic examination.17 Atherosclerotic plaques which are destined to cause late events have more severe plaque burden on IVUS than is usually appreciated on angiography.18 No clinical or electrocardiographic variables were associated with plaque disruption. The study population was similar in terms of age and proportion with ST elevation as compared to women with MI seen in our laboratories.7 As has been described in patients with obstructive CAD and MI,19 we found that some patients had multiple plaque ruptures, or rupture in one location and ulceration in another.
We have confirmed a long-held hypothesis about the etiology of MI with no obstructive CAD. There is every reason to believe that plaque ruptures in these women were etiologic for MI. Plaque disruption was accompanied by CMR evidence of myocardial edema in the majority of cases, and all patients had acute presentation with chest pain. The entry criteria for this study were created to select patients with acute MI who were referred for coronary angiography on clinical grounds. Plaque rupture is also known to occur in patients with stable angina16 and not all plaque rupture events are symptomatic. We did not include stable patients with non-obstructive CAD in this study as controls. However, a recent report of 100 women undergoing IVUS with stable chest pain and non-obstructive CAD on angiography did not identify plaque rupture or ulceration in any patient.20 Another series using IVUS in 55 patients with chest pain and normal coronary angiography also did not identify plaque rupture.21 Therefore it appears likely that plaque rupture is related to the acuity of the presentation and the degree of angiographic stenosis in stable patients. We hypothesize that plaque disruption as observed in this study was accompanied by distal embolization of atherothrombotic debris or platelet aggregates or transient or incomplete thrombosis with endogenous thrombolysis, leading to widely patent arteries by the time of angiography a median of 2 days after symptom onset.
Myocardial injury was confirmed in all patients by troponin elevation and was commonly observed on imaging studies, as evidenced by T2 signal hyperintensity in 53% of patients tested. LGE was associated with plaque disruption in only one case in this series, but T2 hyperintensity was common in patients with plaque disruption, seen in 75% of the patients examined. Myocardial edema is a known early consequence of ischemia.22 It is possible that earlier CMR would have shown LGE or T2 abnormalities in a greater proportion of patients, but we believe this is unlikely based on prior studies suggesting persistence of these abnormal findings, usually up to at least 2 weeks.22,23 Plaque disruption was not associated with evidence of more severe myocardial injury based on peak troponin or LGE. This suggests that endogenous thrombolysis and/or relief of superimposed vasospasm may have occurred before more extensive and irreversible myocardial injury was sustained, and that troponin elevation in these patients represents death of smaller groups of myocytes distributed throughout the territory at risk. The territory at risk was large in many cases, as can be appreciated from the representative angiograms and T2 weighted images. If hypoperfusion related to the culprit vessel had lasted for a longer period of time resulting in more necrosis, the vessel might have been expected to display significant stenosis at angiography, and therefore would have been excluded from this study. Of note, no patient with plaque disruption had a pattern which would suggest a non-ischemic mechanism, such as myocarditis.
The finding of plaque disruption in a patient with MI and non-obstructive CAD has implications for treatment. Such patients would presumably benefit from treatment with antiplatelet agents and statins. However, patients without obstructive CAD are less likely to be prescribed medical therapies for secondary prevention of MI, including aspirin, clopidogrel and statins.24 Patients with MI and no obstructive CAD have an approximately 2% risk of death or reinfarction over 6-12 months25,26 and a >15% readmission rate within 6 months.27 It is possible that women with disrupted plaque are at highest risk for events among those with MI and non-obstructive CAD, particularly if they are not afforded appropriate secondary prevention measures. This hypothesis remains speculative but employment of IVUS during angiography in patients with MI and no obstructive CAD with follow-up for outcomes would help to confirm it.
The most common LGE pattern was ischemic (transmural or subendocardial). Ischemic-type LGE without obstructive CAD has also been observed in heart failure patients.28, 29 Nearly one-third of patients with an ischemic LGE pattern had angiographically normal coronary arteries but all had some atherosclerosis on IVUS. Only one had plaque rupture. Review of LGE images shows that these were generally small infarctions, which may be surmised from the median peak troponin of 3.1ng/ml. We hypothesize that vasospasm of, embolism to or perhaps flush occlusion of a branch vessel was the cause of MI in these patients. The appearance of ischemic LGE in these patients is remarkably similar to that reported in a study examining embolic phenomena around the time of percutaneous coronary intervention, i.e., subendocardial-to-transmural LGE involving a small portion of the myocardial segment affected.30 Embolism in our cases could have originated from a proximal plaque disruption or a non-coronary source. A non-ischemic etiology, such as sarcoidosis or myocarditis, is also possible with an ischemic type LGE pattern. Women are much more likely than men to develop cardiac syndrome X, the stable ischemic syndrome due to endothelial and/or microvascular dysfunction without angiographically obstructive CAD. Vasospasm causing MI may represent an extreme form of endothelial dysfunction, perhaps linking the predominance of female patients in cardiac syndrome X and MI with no obstructive CAD.
The frequency of abnormal LGE in our cohort was similar to that reported in prior series of patients with chest pain, troponin elevation and no obstructive CAD.31-35 The distribution of patterns was different than in prior studies,32, 33 likely related to demographic and clinical characteristics. For example, studies reporting a higher frequency of non-ischemic LGE (e.g., myocarditis) included younger, predominantly male cohorts,31, 33, 34 often with a requirement for completely normal angiography, as opposed to the present series of women with a clinical diagnosis of MI. Some prior reports specifically excluded patients with a clinical diagnosis of MI. The one study which did include a predominantly female patient population found a higher rate of ischemic-type LGE, as did our study.35
Four patients in this study had LGE patterns that were not typical of ischemic or non-ischemic disease. Such mixed patterns have also been described in patients with heart failure undergoing CMR for etiologic diagnosis.28 The appearance of scans in individual patients may suggest a higher likelihood of ischemic or non-ischemic disease, or a combination of the two.
We identified plaque ulceration in two patients who met criteria for tako-tsubo cardiomyopathy, one with ulceration in the left main coronary artery and the other with ulceration in the LAD. Each of these patients had a large LAD which wrapped around the apex and could potentially account for a large area of wall motion abnormality. Plaque rupture has previously been reported in patients with tako-tsubo cardiomyopathy36, 37 but was not identified in a recent IVUS series.38 Most authors hypothesize that tako-tsubo cardiomyopathy is due to catecholamine toxicity, autonomic dysfunction and/or multivessel coronary spasm.9 However, the tako-tsubo LV dysfunction pattern does occur in anterior MI.39 Our findings support the concept that tako-tsubo cardiomyopathy may have several pathophysiologic mechanisms, including plaque disruption in the LAD or left main.
Limitations
The present study was a small, single center study limited to women. Men were not included because the prevalence of non-obstructive CAD is higher in women and because mechanisms may differ by sex. We therefore chose to focus on the larger population of women to allow for an adequate sample size, avoiding inclusion of a small number of men which might dilute the overall findings observed. We were not able to perform IVUS or CMR in all patients due to logistical problems and withdrawals of consent. Ideally, IVUS of all three coronary arteries would have been performed in all patients and this could have resulted in a higher proportion of patients with plaque disruption, or multiple plaque disruption, particularly considering the low rate of imaging of the RCA. Quantitative coronary angiography and virtual histology IVUS were not performed. Study enrollment started during a time when the importance of T2-weighted imaging in cardiac MR was just being realized; shortly thereafter, an optimized, robust T2-weighted technique was available and was incorporated into the CMR protocol. There was no control group within this study for comparison of plaque disruption frequency; the most scientifically appealing control group would have been stable women without obstructive CAD, who were not included due to potential risks of IVUS. Nonetheless, this is the largest prospective series of women with MI and no obstructive CAD in which CMR and IVUS data were gathered prospectively and interpreted by experienced observers in experienced laboratories by investigators who were blinded to clinical data. Thus we are able to offer important insights into the mechanisms of MI with non-obstructive CAD.
Conclusions
Plaque rupture and ulceration are common findings in women with MI and non-obstructive CAD on angiography. This proves a long-held hypothesis regarding the mechanism of MI with non-obstructive CAD. Based on our results, it is apparent that there is more than one clinical syndrome of MI with no obstructive CAD at angiography, with causes including occult plaque disruption, ischemic injury without plaque disruption, inflammatory or infiltrative etiologies, tako-tsubo cardiomyopathy, and possibly others which were not identified using the combination of IVUS and CMR. IVUS and CMR provide distinct, complementary information in patients with MI and no obstructive CAD, and could be considered for use in series to permit categorization of potential mechanisms in these patients. Such classification will facilitate future research into mechanisms and treatment of MI with non-obstructive CAD.
Short commentary.
There is no angiographically demonstrable obstructive coronary artery disease (CAD) in a substantial proportion of patients with myocardial infarction (MI), particularly women. Plaque rupture has long been hypothesized to be an etiology of MI with non-obstructive CAD. We sought to determine mechanism(s) of MI in this setting using intravascular ultrasound (IVUS) during angiography and cardiac magnetic resonance imaging (CMR) performed within one week. Fifty women were enrolled with median worst coronary angiographic stenosis of 20% and median peak troponin 1.60ng/ml. Plaque disruption (rupture and/or ulceration) was found on blinded core laboratory IVUS review in 38% of patients tested. Late gadolinium enhancement (LGE) was identified in 39% and abnormal T2 signal in 53% of women undergoing CMR. The most common LGE pattern was ischemic (transmural/subendocardial). Non-ischemic (midmyocardial/subepicardial) and mixed LGE patterns were also observed. T2 signal hypertensity was common and LGE was infrequent among patients with plaque disruption. We hypothesize that vasospasm of, embolism to or flush occlusion of a branch vessel caused MI in patients with an ischemic LGE pattern but without plaque disruption. IVUS and CMR provided complementary information in this cohort and together revealed abnormalities in 35/50 patients (70%). In this study, the first prospective evaluation using IVUS and CMR in women with acute MI and without obstructive CAD at angiography, we have demonstrated that plaque disruption is a frequent finding. We have also shown that LGE commonly identifies the location and pattern of myocardial damage and that acute myocardial edema is frequently present in these patients.
Acknowledgments
The authors thank the study coordinators, Chao Wang and Arline Roberts, RN, and the participants.
Funding Sources: Doris Duke Charitable Foundation Clinical Scientist Development Award, New York University-Health & Hospital Corporation Clinical and Translational Sciences Institute (NIH-NCRR 1UL1RR029893) and New York University General Clinical Research Center (NIH-NCRR M01RR0096).
Footnotes
Conflict of Interest Disclosures: none
References
- 1.Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, VandeWerf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, VandeWerf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 3.Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, Ohman EM, Newby LK, Peterson ED, Hochman JS. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results of the CRUSADE quality improvement initiative. Am Heart J. 2009;158:688–694. doi: 10.1016/j.ahj.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Chokshi NP, Iqbal SN, Berger RL, Hochman JS, Feit F, Slater JN, Pena-Sing I, Yatskar L, Keller NM, Babaev A, Attubato MJ, Reynolds HR. Sex and race are associated with the absence of epicardial coronary artery obstructive disease at angiography in patients with acute coronary syndromes. Clin Cardiol. 2010;33:495–501. doi: 10.1002/clc.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nageh T, Sherwood RA, Wainwright RJ, Shah AM, Thomas MR. The clinical relevance of raised cardiac troponin I in the absence of significant angiographic coronary artery disease. Int J Cardiol. 2005;100:325–330. doi: 10.1016/j.ijcard.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights From the NHLBI-Sponsored WISE Study: Part II: Gender Differences in Presentation, Diagnosis, and Outcome With Regard to Gender-Based Pathophysiology of Atherosclerosis and Macrovascular and Microvascular Coronary Disease. J Am Coll Cardiol. 2006;47:S21–29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 7.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: An autopsy study. Am Heart J. 2011;161:681–688. doi: 10.1016/j.ahj.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. ACC Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS) J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 11.Maehara A, Mintz GS, Bui AB, Walter OR, Castagna MT, Canos D, Pichard AD, Satler LF, Waksman R, Suddath WO, Laird JR, Jr, Kent KM, Weissman NJ. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J Am Coll Cardiol. 2002;40:904–910. doi: 10.1016/s0735-1097(02)02047-8. [DOI] [PubMed] [Google Scholar]
- 12.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, Young JB, Nissen SE. High Prevalence of Coronary Atherosclerosis in Asymptomatic Teenagers and Young Adults: Evidence From Intravascular Ultrasound. Circulation. 2001;103:2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Lee VS, Chung YC, Babb JS, Simonetti OP. Myocardial infarction: optimization of inversion times at delayed contrast-enhanced MR imaging. Radiology. 2004;233:921–926. doi: 10.1148/radiol.2333032004. [DOI] [PubMed] [Google Scholar]
- 14.Schvartzman PR, Srichai MB, Grimm RA, Obuchowski NA, Hammer DF, McCarthy PM, Kasper JM, White RD. Nonstress delayed-enhancement magnetic resonance imaging of the myocardium predicts improvement of function after revascularization for chronic ischemic heart disease with left ventricular dysfunction. Am Heart J. 2003;146:535–541. doi: 10.1016/S0002-8703(03)00318-1. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Aty H, Cocker M, Friedrich MG. Myocardial edema is a feature of Tako-Tsubo cardiomyopathy and is related to the severity of systolic dysfunction: insights from T2-weighted cardiovascular magnetic resonance. Int J Cardiol. 2009;132:291–293. doi: 10.1016/j.ijcard.2007.08.102. [DOI] [PubMed] [Google Scholar]
- 16.Hong M-K, Mintz GS, Lee CW, Lee B-K, Yang T-H, Kim Y-H, Song J-M, Han K-H, Kang D-H, Cheong S-S, Song J-K, Kim J-J, Park S-W, Park S-J. The Site of Plaque Rupture in Native Coronary Arteries: A Three-Vessel Intravascular Ultrasound Analysis. J Am Coll Cardiol. 2005;46:261–265. doi: 10.1016/j.jacc.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 17.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of Vulnerable/Unstable Plaque. Arterioscler Thromb Vac Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Maehara A, Lansky AJ, deBruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW. A Prospective Natural-History Study of Coronary Atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 19.Rioufol G, Finet G, Ginon I, Andre-Fouet X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF, Tabib A. Multiple Atherosclerotic Plaque Rupture in Acute Coronary Syndrome: A Three-Vessel Intravascular Ultrasound Study. Circulation. 2002;106:804–808. doi: 10.1161/01.cir.0000025609.13806.31. [DOI] [PubMed] [Google Scholar]
- 20.Khuddus MA, Pepine CJ, Handberg EM, BaireyMerz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD. An Intravascular Ultrasound Analysis in Women Experiencing Chest Pain in the Absence of Obstructive Coronary Artery Disease: A Substudy from the NHLBI–Sponsored WISE. J Interv Cardiol. 2010;23:511–519. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge J, Erbel R, Gerber T, Görge G, Koch L, Haude M, Meyer J. Intravascular ultrasound imaging of angiographically normal coronary arteries: a prospective study in vivo. Br Heart J. 1994;71:572–578. doi: 10.1136/hrt.71.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards NC, Routledge H, Steeds RP. T2-weighted magnetic resonance imaging to assess myocardial oedema in ischaemic heart disease. Heart. 2009;95:1357–1361. doi: 10.1136/hrt.2009.169961. [DOI] [PubMed] [Google Scholar]
- 23.Dall’Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD, Kellman P, Francis JM, Forfar C, Prendergast B, Banning AP, Channon KM, Kharbanda RK, Neubauer S, Choudhury RP. Dynamic Changes of Edema and Late Gadolinium Enhancement after Acute Myocardial Infarction and Their Relationship to Functional Recovery and Salvage Index. Circ Cardiovasc Imaging. 2011;4:228–236. doi: 10.1161/CIRCIMAGING.111.963421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of Secondary Prevention Therapies in Patients With Nonobstructive Coronary Artery Disease Identified During Cardiac Catheterization. Circ Cardiovasc Qual Outcomes. 2010;3:632–641. doi: 10.1161/CIRCOUTCOMES.109.906214. [DOI] [PubMed] [Google Scholar]
- 25.Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, Simoons ML, Akkerhuis M, Ohman EM, Kitt MM, Vahanian A, Ruzyllo W, Karsch K, Califf RM, Topol EJ. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. Circulation. 2000;102:1101–1106. doi: 10.1161/01.cir.102.10.1101. [DOI] [PubMed] [Google Scholar]
- 26.Bugiardini R, Manfrini O, DeFerrari GM. Unanswered Questions for Management of Acute Coronary Syndrome: Risk Stratification of Patients With Minimal Disease or Normal Findings on Coronary Angiography. Arch Intern Med. 2006;166:1391–1395. doi: 10.1001/archinte.166.13.1391. [DOI] [PubMed] [Google Scholar]
- 27.Patel MR, Chen AY, Peterson ED, Newby LK, Pollack CV, Jr, Brindis RG, Gibson CM, Kleiman NS, Saucedo JF, Bhatt DL, Gibler WB, Ohman EM, Harrington RA, Roe MT. Prevalence, predictors, and outcomes of patients with non-ST-segment elevation myocardial infarction and insignificant coronary artery disease: results from the CRUSADE initiative. Am Heart J. 2006;152:641–647. doi: 10.1016/j.ahj.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 28.Senthilkumar A, Majmudar MD, Shenoy C, Kim HW, Kim RJ. Identifying the etiology: a systematic approach using delayed-enhancement cardiovascular magnetic resonance. Heart Fail Clin. 2009;5:349–367. vi. doi: 10.1016/j.hfc.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCrohon JA, Moon JCC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJS, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 30.Ricciardi MJ, Wu E, Davidson CJ, Choi KM, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103:2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- 31.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 32.Baccouche H, Mahrholdt H, Meinhardt G, Merher R, Voehringer M, Hill S, Klingel K, Kandolf R, Sechtem U, Yilmaz A. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur Heart J. 2009;30:2869–2879. doi: 10.1093/eurheartj/ehp328. [DOI] [PubMed] [Google Scholar]
- 33.Zaldumbide EL, Pérez-David E, Larena JA, delCastillo SV, RumorosoCuevas JR, Onaindía JJ, LekuonaGoya I, García-Fernández MA. The Value of Cardiac Magnetic Resonance in Patients With Acute Coronary Syndrome and Normal Coronary Arteries. Revista Española de Cardiología. 2009;62:976–983. doi: 10.1016/s1885-5857(09)73263-3. [DOI] [PubMed] [Google Scholar]
- 34.Stensaeth K, Fossum E, Hoffmann P, Mangschau A, Klow N. Clinical characteristics and role of early cardiac magnetic resonance imaging in patients with suspected ST-elevation myocardial infarction and normal coronary arteries. Int J Cardiovasc Imaging. 2011;27:355–365. doi: 10.1007/s10554-010-9671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christiansen JP, Edwards C, Sinclair T, Armstrong G, Scott A, Patel H, Hart H. Detection of Myocardial Scar by Contrast-Enhanced Cardiac Magnetic Resonance Imaging in Patients With Troponin-Positive Chest Pain and Minimal Angiographic Coronary Artery Disease. Am J Cardiol. 2006;97:768–771. doi: 10.1016/j.amjcard.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Ibanez B, Navarro F, Cordoba M, M-Alberca P, Farre J. Tako-tsubo transient left ventricular apical ballooning: is intravascular ultrasound the key to resolve the enigma? Heart. 2005;91:102–104. doi: 10.1136/hrt.2004.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syed I, Prasad A, Oh J, Martinez M, Feng D, Motiei A, Glockner J, Breen J, Julsrud P. Apical ballooning syndrome or aborted acute myocardial infarction? Insights from cardiovascular magnetic resonance imaging. Int J Cardiovasc Imaging. 2008;24:875–882. doi: 10.1007/s10554-008-9320-6. [DOI] [PubMed] [Google Scholar]
- 38.Haghi D, Roehm S, Hamm K, Harder N, Suselbeck T, Borggrefe M, Papavassiliu T. Takotsubo Cardiomyopathy Is Not Due to Plaque Rupture: An Intravascular Ultrasound Study. Clin Cardiol. 2010;33:307–310. doi: 10.1002/clc.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chao T, Lindsay J, Collins S, Woldeyes L, Joshi SB, Steinberg DH, Satler LF, Kent KM, Suddath WO, Pichard AD, Waksman R. Can Acute Occlusion of the Left Anterior Descending Coronary Artery Produce a Typical “Takotsubo” Left Ventricular Contraction Pattern? Am J Cardiol. 2009;104:202–204. doi: 10.1016/j.amjcard.2009.03.018. [DOI] [PubMed] [Google Scholar]


