Abstract
Adipose-resident T-cells (ARTs) regulate metabolic and inflammatory responses in obesity, but ART activation signals are poorly understood. Here, we describe class II major histocompatibility complex (MHCII) as an important component of high-fat diet (HFD)-induced obesity. Microarray analysis of primary adipocytes revealed that multiple genes involved in MHCII antigen processing and presentation increased in obese women. In mice, adipocyte MHCII increased within two weeks HFD, paralleling increases in pro-inflammatory and decreases in anti-inflammatory ART markers, and preceding adipose tissue macrophage (ATM) accumulation and pro-inflammatory M1 polarization. Mouse 3T3-L1 and primary adipocytes activated T-cells in an antigen-specific, contact-dependent manner, indicating adipocyte MHCII is functional. HFD-fed MHCII−/− mice developed less adipose inflammation and insulin resistance than wild-type mice, despite developing similar adiposity. These investigations uncover a mechanism whereby a HFD-induced adipocyte/ART dialogue involving MHCII instigates adipose inflammation and, together with ATM MHCII, escalates its progression.
Keywords: Obesity, adipose tissue, MHCII, T-cell activation
While changes in adipose tissue T-cells and macrophages induced by high fat diet (HFD) are recognized as important determinants of adipose metabolism, pro-inflammatory cytokine secretion, and whole body insulin sensitivity (Finlay and Cantrell, 2011; Olefsky and Glass, 2010; Weisberg et al., 2003; Xu et al., 2003), the nature and source of early signals that trigger the pro-inflammatory phenotype in adipose tissue remain unclear. Adipose inflammation results from the integration of multiple interactions among adipocytes and immune cells. In obesity, adipocytes secrete less anti-inflammatory adiponectin and increase expression of pro-inflammatory factors (TNFα, IL-6, RBP4, ANGPTL2), macrophage chemoattractants (MCP-1 and NAMPT), and leptin to induce pro-inflammatory immune cell responses (Fernandez-Riejos et al., 2010; Lord et al., 1998; Ouchi et al., 2011). ATMs reciprocally secrete cytokines (TNFα, IL-1β) that can alter adipocyte lipid storage, lipolysis, insulin responses, glucose metabolism and adipokine production leading to ectopic fat deposition and systemic inflammation that results in whole-body insulin resistance (Guilherme et al., 2008). Attenuating these inflammatory responses by macrophage-specific IKKβ and JNK knockout or whole-body TNFα neutralization attenuates insulin resistance, hepatic steatosis and atherosclerosis, while mutations that increase inflammatory responses or polarization to a pro-inflammatory M1 phenotype have the opposite effect (Arkan et al., 2005; Chawla, 2010; Han et al., 2013; Hevener et al., 2007; Hotamisligil et al., 1993; Lumeng et al., 2007; Olefsky and Glass, 2010). These observations highlight the importance of the adipocyte/macrophage dialogue. Adipocyte death has been proposed to promote recruitment of pro-inflammatory macrophages into adipose tissue, since M1-like ATMs form characteristic ‘crown-like structures’ around dying hypertrophic adipocytes that accumulate with obesity (Sun et al., 2011). Recent results, however, indicate that ATMs surrounding adipocytes are primarily M2 macrophages (Fischer-Posovszky et al., 2011) and can accumulate in the absence of adipocyte death (Feng et al., 2011), suggesting that cells other than apoptotic adipocytes regulate ATM accumulation and M1 polarization in obesity.
Adipose-resident T-cells (ARTs) are increasingly recognized to modulate adipose tissue inflammation and systemic insulin action, interacting with both adipocytes and macrophages (Kintscher et al., 2008; Nishimura et al., 2009; Wu et al., 2007). HFD challenge increases adipose CD8+ T-cells (Finlay and Cantrell, 2011; Nishimura et al., 2009) and CD4+ TH1 cells, leading to increases in ART IFNγ production, which can promote adipocyte lipolysis and ATM M1 polarization (Finlay and Cantrell, 2011; Strissel et al., 2010; Yang et al., 2010), and decreases CD4+ TH2 and TREG cells, which neutralize inflammation and produce IL-4 to promote M2 polarization (Biswas and Mantovani, 2010; Tiemessen et al., 2007). Increasing adipose TREG numbers or levels of IL-10, an anti-inflammatory cytokine, can decrease M1 ATMs, systemic insulin resistance and liver steatosis (Feuerer et al., 2009; Winer et al., 2009). Activation of naïve CD4+ T-cells to secrete cytokines and proliferate requires recognition of a specific peptide antigen:MHCII complex on an antigen presenting cell (APC) in conjunction with co-stimulatory signals. Macrophages and dendritic cells are professional APCs; thus, ATMs could be responsible for activation of CD4+ T-cells in inflamed adipose tissue. However, several studies have detected changes in ART composition that precede changes in ATM number and polarization, so the mechanism regulating ART responses to HFD remains a compelling question (Lumeng et al., 2009).
To define novel adipocyte changes that are associated with obesity and could trigger changes in immune cell phenotypes, we microarray-profiled purified human adipocytes isolated from surgical adipose biopsies of obese and lean women. Surprisingly, expression of the class II transactivator (CIITA), the master transcriptional regulator of the MHCII pathway, and multiple MHCII-family genes including co-stimulators were markedly increased in adipocytes of obese subjects. Similar increases were observed in mouse adipocytes isolated after only two weeks of HFD challenge. These changes paralleled ART activation, but preceded changes in macrophage abundance or polarization. Moreover, adipocytes were found to potently activate T-cells in an antigen- and contact-dependent manner that was enhanced in obesity, and MHCII-deficiency attenuated inflammatory and metabolic responses to HFD. We propose that activation of adipocyte adaptive immunity regulates changes in ART sub-types early in HFD challenge, triggering a pro-inflammatory dialogue between adipocytes and ARTs that then promotes ATM recruitment and M1 polarization. Ultimately both adipocytes and ATMs contribute to T-cell activation later during HFD. These results underscore a role for the adipocyte in instigating adipose inflammation, provide a time course of adipose immune cell changes in response to HFD challenge, and emphasize an essential role of adaptive immunity in obesity that has therapeutic implications.
RESULTS
Obesity is associated with increased MHCII in primary human and mouse adipocytes, but not in ATMs
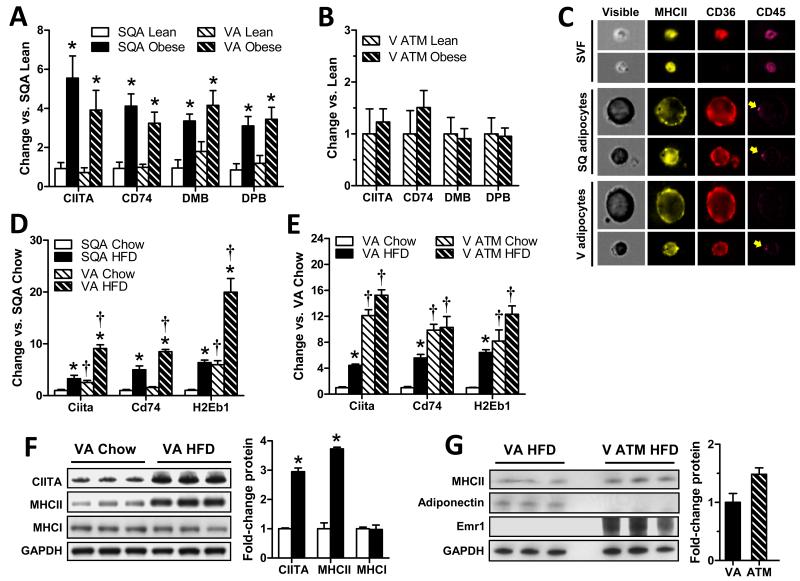
To identify adipocyte genes impacted by obesity, adipocytes were isolated from surgical adipose biopsies of 44 women, CD45-immunoprecipitated to remove residual leukocyte contamination. Microarray analysis was performed to identify genes differentially expressed (>1.5-fold absolute change, p<0.05) in subcutaneous (SQ) adipocyte RNA isolated from lean and obese post-menopausal women (Table S1). Gene set enrichment analysis performed on this data found that 9 of the top 10 up-regulated pathways (Table S2) demonstrated substantial overlap due to shared expression of multiple MHCII genes; and that 11 of 15 genes involved in MHCII antigen presentation, 3 of 5 involved in MHCII antigen processing, and the costimulatory molecule CD86 increased with obesity (Table 1). In contrast, there were no increases in MHCI-related genes in the microarray. Confirming these results, RT-PCR analysis of CIITA, the MHCII invariant chain peptide CD74, and the MHCII proteins HLA-DMB and HLA-DBP in SQ and visceral (V) adipocytes of lean and obese post-menopausal women revealed 3- to 6-fold increases with obesity (Fig. 1A). However, no similar changes were detected in ATMs of lean vs. obese subjects (Fig. 1B). Expression of the major costimulatory molecules CD80 and CD86 was also increased in adipocytes of obese vs. lean women (Fig S1A). Low pressure, image-capture flow cytometry analyses of human SQ and V adipocyte and SVF samples were performed to confirm adipocyte MHCII expression and purity. Cells in adipocyte fractions were morphologically distinct and uniformly expressed the fatty acid transporter CD36 (Fig.1C), used as a marker of adipocyte integrity, but not the pan-leukocyte marker CD45 (<0.1% CD45+ cell detected, Fig. S2). MHCII+ SVF cells were smaller, translucent, and highly expressed both MHCII and CD45, with a subpopulation also expressing CD36.
Table 1. Human microarray data.
Microarray analyses revealed that multiple MHCII genes were upregulated in adipocytes from 7 obese versus 7 lean post-menopausal women shown as relative fold-change. Bolded red text denotes genes with greater than 1.5-fold change and p<0.05 by T-test.
| GENE ID | FC | p-value | DESCRIPTION |
|---|---|---|---|
|
| |||
| MHC gene pathway transcription | |||
|
| |||
| CIITA | 1.03 | 0.872 | class II transactivator |
| RFX5 | 0.97 | 0.921 | regulatory factor X (RFX) 5 |
| RFXANK | 0.57 | 0.069 | RFX-associated ankyrin-containing protein |
| RFXAP | 0.79 | 0.506 | RFX-associated protein |
| NFYA | 1.22 | 0.246 | nuclear transcription factor Y alpha |
| NFYB | 1.04 | 0.914 | nuclear transcription factor Y beta |
| NFYC | 0.75 | 0.354 | nuclear transcription factor Y gamma |
| CREB1 | 0.53 | 0.008 | cAMP responsive element binding protein 1 |
|
| |||
| MHCII-mediated antigen presentation | |||
|
| |||
| CD74 | 2.69 | 0.002 | MHCII invariant chain |
| HLA-DMA | 1.16 | 0.44 | HLA-DMA |
| HLA-DMB | 2.65 | 0.001 | HLA-DMB |
| HLA-DOA | 1.38 | 0.239 | HLA-DOA |
| HLA-DOB | 1.08 | 0.781 | HLA-DOB |
| HLA-DPA1 | 2.14 | 0.016 | HLA-DPA1 |
| HLA-DPB1 | 1.93 | 0.035 | HLA-DPB1 |
| HLA-DQA1 | 1.92 | 0.012 | HLA-DQA1 |
| HLA-DQA2 | 2.03 | 0.052 | HLA-DQA2 |
| HLA-DQB1 | 1.08 | 0.575 | HLA-DQB1 |
| HLA-DRA | 2.56 | 0.003 | HLA-DRA |
| HLA-DRB1 | 2.29 | 0.006 | HLA-DRB1 |
| HLA-DRB3 | 2.58 | 0.007 | HLA-DRB3 |
| HLA-DRB4 | 2.97 | 0.001 | HLA-DRB4 |
| HLA-DRB5 | 1.83 | 0.047 | HLA-DRB5 |
|
| |||
| MHCII antigen processing | |||
|
| |||
| CTSB | 1.81 | 0.02 | cathepsin B |
| CTSL1 | 0.58 | 0.126 | cathepsin L1 |
| CTSS | 2.06 | 0.011 | cathepsin S |
| IFI30 | 2.13 | 0.027 | interferon, gamma-inducible protein 30 |
| LGMN | 1.73 | 0.085 | legumain |
|
| |||
| Co-stimulatory molecules | |||
|
| |||
| CD80 | 0.84 | 0.626 | CD80 molecule |
| CD86 | 2.40 | 0.001 | CD86 molecule |
|
| |||
| MHCI-mediated antigen presentation | |||
|
| |||
| B2M | 1.22 | 0.42 | B2M:beta-2-microglobulin |
| HLA-A | 1.07 | 0.766 | HLA-A |
| HLA-B | 0.85 | 0.49 | HLA-B |
| HLA-C | 1.53 | 0.158 | HLA-C |
| HLA-E | 1.19 | 0.51 | HLA-E |
| HLA-F | 1.44 | 0.092 | HLA-F |
| HLA-G | 0.97 | 0.909 | HLA-G |
Fig. 1. Adipocyte MHCII mRNA and protein expression is markedly increased with obesity.
RT-PCR analysis of MHCII family genes in A) subcutaneous (SQA) and visceral (VA) adipocytes and B) SQ ATMs of lean and obese women (N=5-19/group). C) Representative flow cytometry images of human SVF and adipocyte samples hybridized with fluorescent-antibodies specific for human MHCII (pan HLA-DR), CD36 and CD45. Arrows indicate non-specific signal from residual magnetic beads not removed during the CD45-depletion procedure. RT-PCR of MHCII family genes in D) SQ and V adipocytes and E) V adipocytes and V ATMs (N=4/group) of male C57BL/6 mice fed 3 months chow (lean) or HFD (obese). Western blot analysis of MHC family proteins in F) V adipocytes from lean and obese male C57BL/6 mice and G) V adipocytes and V ATMs from obese male C57BL/6 mice (N=3 samples (2-3 mice/sample)/group; p<0.05 vs. matching lean (*) or SQA (†) sample by Mann-Whitney (A-E) or Welch’s T-test (F,G). Each mouse sample represents pooled material obtained from 4 (adipocytes) or 8 (ATMs) mice. Data represent Means±SEM.
Similar MHCII expression was observed in obese mouse models. Both SQ and V adipocytes of male C57BL/6 mice with diet-induced obesity demonstrated increased MHCII expression, more so in V than SQ adipocytes (Fig. 1D). CD45-depleted V adipocytes expressed more MHCII mRNA (H2-Eb1, 6-fold) and protein (4-fold) than lean mice (Fig. 1E-F) with increased costimulatory molecule expression (Fig. S1B). In contrast, there was no change in MHCI mRNA (not shown) or protein (Fig. 1F). ATM CIITA mRNA expression modestly increased with obesity in these mice, but ATM H2-Eb1 and CD74 mRNA expression did not significantly increase. Adipocyte MHCII mRNA (H2-Eb1) expression in HFD-fed mice thus approached that found in ATM of chow- and HFD-fed mice, resulting in a non-significant difference in MHCII protein expression in adipocytes vs. ATMs of HFD-fed mice (Figs. 1E, G). Similar results were found in middle-aged male Ldlr−/− mice fed western diet; these mice develop obesity-related complications closely resembling human nonalchoholic steatohepatitis and atherosclerosis (Collins et al., 2009; Gupte et al., 2010). Here, again, there was increased MHCII (H2EB and H2DMA) expression in adipocytes, but not ATMs, with HFD (Fig. S1C).
Leptin stimulates IFNγ secretion from T-cells to induce adipocyte MHCII expression
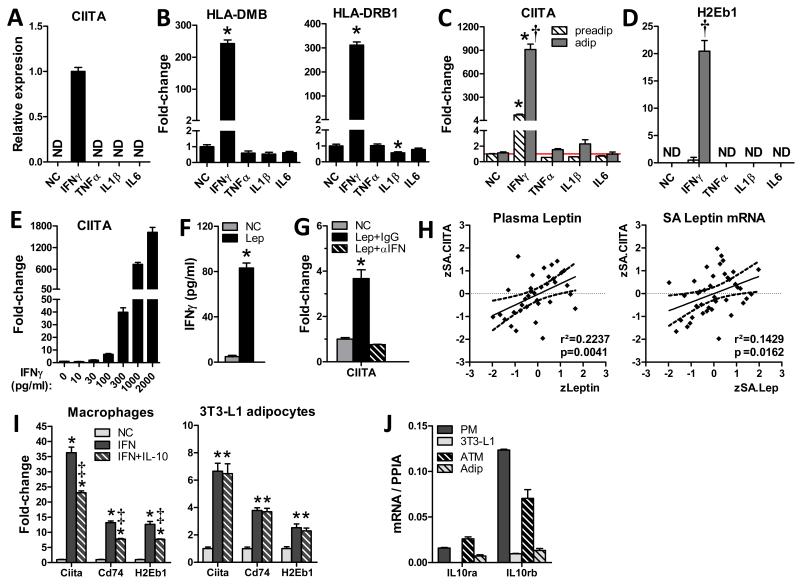
Adipose tissue produces multiple pro-inflammatory factors that are up-regulated in obesity, including IFNγ, a known regulator of CIITA expression and an important contributor to adipose inflammation and metabolic responses to HFD (Rocha et al., 2008). To investigate transcriptional regulation of adipocyte CIITA, differentiated human primary adipocytes were incubated with cytokines known to increase with obesity. Among these cytokines, only IFNγ induced CIITA and MHCII expression (Fig. 2A-B). Similar results were found with differentiated mouse 3T3-L1 adipocytes (Fig. 2C-D), where MHCII induction was markedly lower (~10%) in 3T3-L1 preadipocytes, indicating a preferential response in differentiated adipocytes. IFNγ, but not other cytokines (not shown), also stimulated MHCII (H2Eb1) in differentiated primary mouse adipocytes, such that adipocyte CIITA expression was increased 2-fold by 30pg/ml IFNγ and progressively increased thereafter (Fig. 2E).
Fig. 2. Leptin-induced IFNγ from T-cells stimulates adipocyte MHCII expression.
MHCII family gene induction in A,B) differentiated primary human adipocytes and C,D) mouse 3T3-L1 preadipocytes and adipocytes cultured for 24hrs with PBS (NC, vehicle), IFNγ, TNFα, IL-1β or IL-6. E) IFNγ dose-response of CIITA expression in 3T3-L1 adipocytes. F) IFNγ secretion by C57BL/6 splenic T-cells incubated 24hrs with 1μg/ml leptin. G) 3T3-L1 adipocyte MHCII induction by supernatant of splenic T-cells incubated with PBS (NC), leptin + control IgG (IgG), or leptin + IFNγ-neutralizing antibody (αIFN). H) Correlation of z-skew normalized human SQ adipocyte CIITA mRNA (zSA.CIITA) with plasma leptin (zLeptin) and SQ adipocyte leptin mRNA (zSA.Lep). (N=35-40; dashed line indicates the 95% confidence interval of the regression line). I) MHCII expression in peritoneal macrophages (PM) and 3T3-L1 adipocytes treated 24hrs with PBS (NC) or IFNγ ± 4hrs pre-treatment with IL-10. J) IL-10Rα/β expression in PMs, 3T3-L1 adipocytes and mouse primary ATM and adipocytes. Data represent Means±SEM. (A-G, I-J: N= 2-4/group; p<0.05 vs. matching NC (*), or preadipocyte(†) or PM (‡) treatment group by T-test or 1-way ANOVA.)
CD4+ TH1 T-cells secrete IFNγ upon leptin stimulation (Lord et al., 1998). Accordingly, we found that leptin markedly induced IFNγ secretion from splenic T-cells and supernatant from these cells induced CIITA expression in 3T3-L1 adipocytes in a manner that was completely attenuated by prior addition of IFNγ-neutralizing antibody (Fig. 2F-G). These results suggest adipocyte-derived leptin can stimulate CD4+ TH1 IFNγ secretion to induce adipocyte MHCII expression. Moreover, human SQ adipocyte CIITA mRNA expression correlated with both SQ adipocyte leptin expression and plasma leptin levels (Fig. 2H), suggesting that this mechanism may be operative in human adipose.
IL-10 modifies macrophage, but not adipocyte, responses to IFNγ
IL-10 is an anti-inflammatory cytokine that can attenuate macrophage MHCII expression (de Waal Malefyt et al., 1991). However, unlike macrophages, co-induction with IL-10 did not attenuate IFNγ-induced MHCII expression in adipocytes (Fig. 2I). This adipocyte IL-10 resistance was likely due to low IL-10Rα and IL-10Rβ expression: both 3T3L1 and primary mouse adipocytes had substantially lower expression of these receptors than mouse peritoneal macrophages or ATMs (Fig. 2J). Thus, differential MHCII responses in adipocytes and ATMs during obesity may result from differential responses to increased adipose tissue IL-10 expression, which occurs in obesity (see below).
Adipocyte MHCII expression is an early response to diet-induced obesity
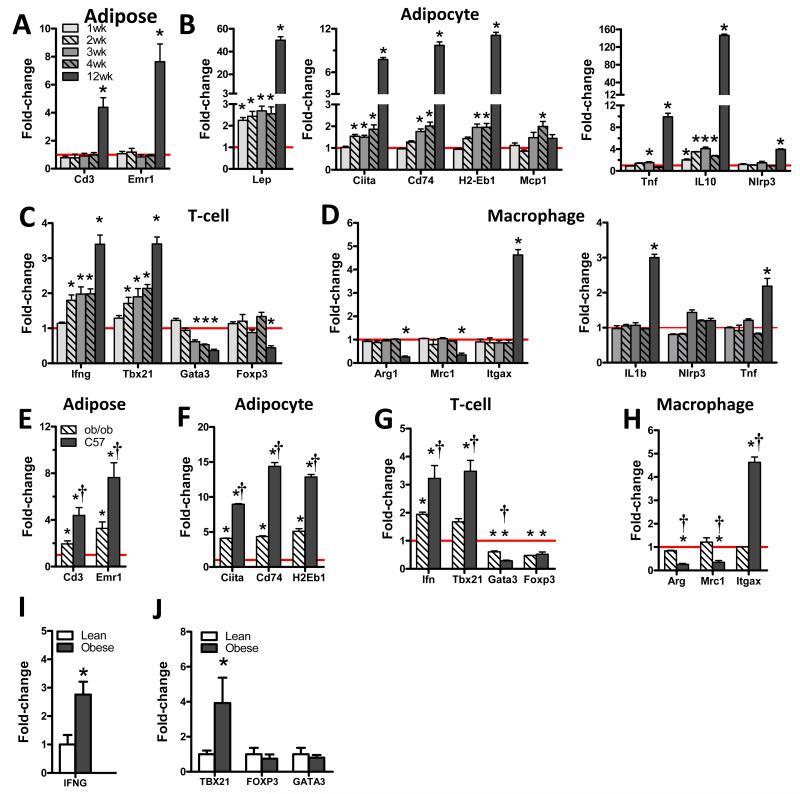
To determine the time course of adipose gene expression and mouse phenotypic changes, RNA was isolated from adipose tissue, adipocytes, ARTs and ATMs of C57BL/6 mice fed 1 to 12 weeks of chow or HFD and RT-PCR analyzed for altered expression of cell lineage and phenotype marker genes. HFD-fed mice gained weight at twice the rate of chow-fed mice, predominantly as adipose tissue, developing significantly increased fasting glucose and insulin within 2 weeks of HFD (Fig. S3A-B). Adipose tissue expression of T-cell (CD3) and macrophage (Emr1) marker genes did not increase until after 4 weeks HFD (Fig. 3A). However, expression of several adipocyte genes exhibited progressive increases: leptin increased within 1 week of HFD followed by MHCII family genes at 2 weeks HFD, and both gene sets increased markedly by 12 weeks (Fig. 3B). Adipocyte IL-10 changes paralleled those of leptin increasing by 4-fold during the first 4 weeks of HFD, and nearly 150-fold by 12 weeks. Adipocyte macrophage chemoattractant protein-1 (MCP-1) gene expression did not increase until 4 weeks of HFD, consistent with delayed macrophage accumulation (adipose expression of Emr1). Adipocyte expression of TNFα, implicated to mediate insulin resistance in obesity (Hotamisligil, 1999), and NLRP3, which forms an inflammasome to activate caspase-1 for the cleavage of pro-IL-1β to mature IL-1β (Wen et al., 2012), increased only at 12 weeks (Fig. 3B). ARTs also demonstrated early changes: expression of the pro-inflammatory TH1 marker genes Tbx21 and Ifng increased within 2 weeks of HFD, corresponding to the development of glucose intolerance and insulin resistance (Fig. S3C-E), followed by reduced expression of anti-inflammatory TH2 (Gata3 and IL-13) and Treg (Foxp3) marker genes (Fig. 3C). Changes in ATM polarization with HFD occurred after T-cell changes (Fig. 3D), as indicated by increased expression of the M1 polarization marker Itgax (CD11c) and decreased expression of the anti-inflammatory M2 markers Arg1 and Mrc1 at 12 weeks HFD. ATM cytokine expression paralleled changes in polarization markers, with IL-1β and TNFα gene expression increasing only at 12 weeks of HFD although, unlike adipocytes, ATM NLRP3 expression did not increase with HFD (Fig. 3D). Insulin tolerance tests and HOMA-IR measurements performed at 4 weeks of HFD, prior to adipose macrophage infiltration or ATM inflammatory changes, indicated the presence of insulin resistance, suggesting that early systemic metabolic changes occur prior to detectable ATM changes (Fig. S3E-F).
Fig. 3. Adipocyte MHCII and ARTs changes occur early after HFD, while ATM changes occur later.
RT-PCR analysis of A) adipose tissue, B) CD45-depleted adipocytes, C) ARTs and D) ATMs of male C57BL/6 mice fed HFD for 1, 2, 3, 4 or 12 weeks; and E) adipose tissue, F) CD45-depleted adipocytes, G) ARTs and H) ATMs of similar weight male ob/ob mice and 12wk HFD-fed C57BL/6 mice. (N=4-8/group, p<0.05 and >1.5-fold absolute change vs. matching chow control (*) or ob/ob mouse (†) expression by Mann-Whitney tests.) ART and ATM samples represent pooled material obtained from 4 (adipocytes) or 8 (ARTs and ATMs) mice. All data are normalized against the expression of matching chow-fed lean control mice (red line). RT-PCR analysis of human SVF expression of I) IFN and J) CD3-normalized expression of the ART lineage markers TBX21, FOXP3 and GATA3 (N=6-15, *p<0.05 by Welch’s T-test.) Data represent Means±SEM.
Since findings outlined above pointed to an early role for leptin in ART activation, adipose phenotypes of C57BL/6 mice fed 3months HFD and C57BL/6-background leptin-deficient (ob/ob) mice of similar weight (C57 40.3g vs. ob/ob 44.6g, p<0.05) were compared to evaluate the impact of leptin on adipose inflammation. Despite greater adiposity (ob/ob 52.5% fat vs. C57 32.0% fat), adipose tissue of ob/ob mice expressed less CD3 and Emr1 than HFD-fed C57BL/6 mice, indicating reduced immune cell content (Fig. 3E). Adipocytes of ob/ob mice also expressed less MHCII than HFD-fed C57BL/6 mice, while their ARTs expressed less Tbx21 and Ifng, and their ATMs revealed less M1 polarization (Fig. 3F-H), indicating an attenuation of the pro-inflammatory impact of obesity in leptin-deficient ob/ob mice. However, anti-inflammatory TH2 (Gata3), Treg (Foxp3), and M2 macrophage (Arg1 and Mrc1) markers similarly decreased in both groups (Fig. 3G-H), suggesting different mechanisms may impact these pro- and anti-inflammatory changes. These data suggest that loss of leptin-stimulated IFNγ expression attenuates adipose adaptive immune mechanisms and M1 polarization.
In human SVF, obese vs. lean subjects had greater expression of IFNγ (Fig. 3I) and the TH1 marker, TBX21, (Fig. 3J), but no differences in TH2 (GATA3) or Treg (FOXP3) markers.
Adipocyte-mediated CD4 T-cell activation is MHCII and antigen-dependent
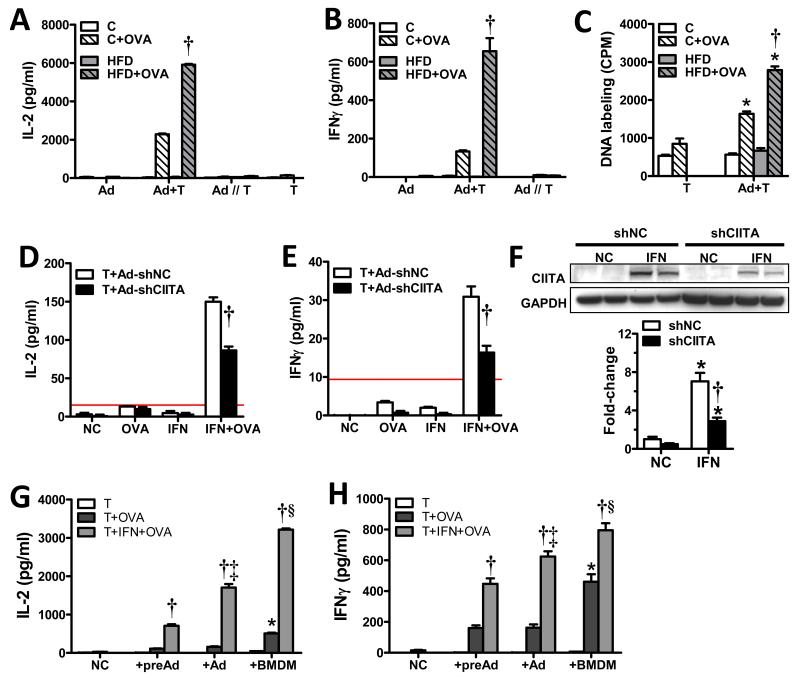
CD4+ T-cells secrete IL-2 to induce antigen-specific T-cell clonal expansion upon interaction with MHCII-presented antigens. To test the ability of primary mouse adipocytes to activate CD4+ T-cells, CD45-depleted adipocytes isolated from lean and obese LDLR−/− mice were cultured with or without splenic T-cells specific for MHCII-presented ovalbumin, in the presence or absence of ovalbumin. Isolated adipocytes and T-cells were co-cultured or cultured in wells separated by a cytokine-permeable membrane to determine the contact-dependence of adipocyte-mediated T-cell activation. After 48h, cell culture supernatants were analyzed for cytokines involved in T-cell activation and pro-inflammatory TH1 cell polarization. IL-2 was not significantly secreted by adipocytes or T-cells cultured separately; however, adipocyte and T-cell co-cultures exhibited a dramatic increase in IL-2 production in an antigen-dependent manner (Fig. 4A). The ~3-fold difference between IL-2 secretion in obese vs. lean mouse adipocyte co-cultures corresponded with adipocyte MHCII protein differences observed in these mice (Fig. 1D). IFNγ, which is predominantly secreted by TH1 cells after MHCII-restricted activation, was also increased (~5-fold) in obese vs. lean co-cultures (Fig. 4B), but was almost undetectable in separate adipocyte or T-cell cultures (not shown). IL-4, primarily secreted by TH2 cells, was essentially undetectable in this assay (not shown). Adipocytes of HFD-fed mice were also found to induce more antigen-dependent T-cell DNA replication than those of chow-fed mice (Fig. 4C).
Fig. 4. Adipocytes can present MHCII antigens to activate CD4+ T-cells.
Chow- and HFD-fed C57BL/6J mouse adipocytes (Adip) and ovalbumin (OVA)-specific CD4+ T-cells (T), were cultured alone or co-cultured in direct contact (Adip+T) or in separate chambers of a transwell plate (Adip//T). Cultures were incubated 48hrs ±OVA, after which supernatants were analyzed for A) IL-2 and B) IFNγ or C) incubated with 3H-thymidine for 16hrs to assess antigen-stimulated T-cell replication. (N=2-3/group, 3 mice/adipocyte sample, p<0.05 vs. T-cells±OVA and adipocytes without OVA (*) or matching chow (†) control by T-test.) 3T3-L1 adipocyte cultures expressing negative control (shNC) or Ciita (shCiita) shRNAs were treated 24hrs ± INFγ, co-cultured for 48hrs with CD4+ T-cells of OVA-immunized mice ±OVA, and then analyzed for D) IL-2 and E) IFNγ secretion, and analyzed for F) CIITA protein expression. (N=2-3/group, p<0.05 vs. matching NC (*) or matching shNC (†) expression by T-test.) Red line indicates the concentration of the lowest ELISA standard. C5BL/6J mouse preadipocytes (preAd), differentiated adipocytes (Ad) and BMDMs treated with or without IFNγ, incubated ±OVA for and OVA-specific T-cells for 48hrs and assessed for F) IL-2 and G) IFNγ production. (N=4/group, p<0.05 vs. matching T+OVA(†) by t-test or vs. preAd (*) or preAd and Ad (‡) by ANOVA). Data represent Means±SEM.
Studies were also performed with 3T3-L1 adipocytes stably transduced with CIITA-shRNA or negative control (NC)-shRNA lentiviral constructs to verify that adipocyte MHCII expression was required for T-cell activation. MHCII expression was induced by IFNγ treatment and both IFNγ-treated and untreated cells were co-cultured with splenic T-cells of haplotype-matched DHA1 mice immunized with chicken ovalbumin. Cell culture supernatants were collected after 48h of co-culture, and analyzed for IL-2 and IFNγ concentrations. Strong cytokine responses were induced only in IFNγ-treated adipocytes incubated with ovalbumin (Fig. 4D-E), while cytokine induction was attenuated ~50% in adipocytes expressing CIITA-shRNA, corresponding to the degree of CIITA knockdown (Fig. 4F). Neither ovalbumin- nor IFNγ-treatment alone was sufficient to stimulate IL-2 or IFNγ secretion. Finally, unstimulated primary mouse preadipocytes and adipocytes induced less T-cell activation (~30%) than BMDMs, and activity markedly increased in all cell types after IFNγ exposure (Fig. 4G-H). Taken together, these data indicate that 1) adipocytes can function as antigen presenting cells to induce CD4+ T-cell activation and polarization, 2) this activity increases with obesity in correspondence with increasing CIITA and MHCII mRNA and protein expression, and 3) adipocyte activation of T-cells approaches that of BMDMs.
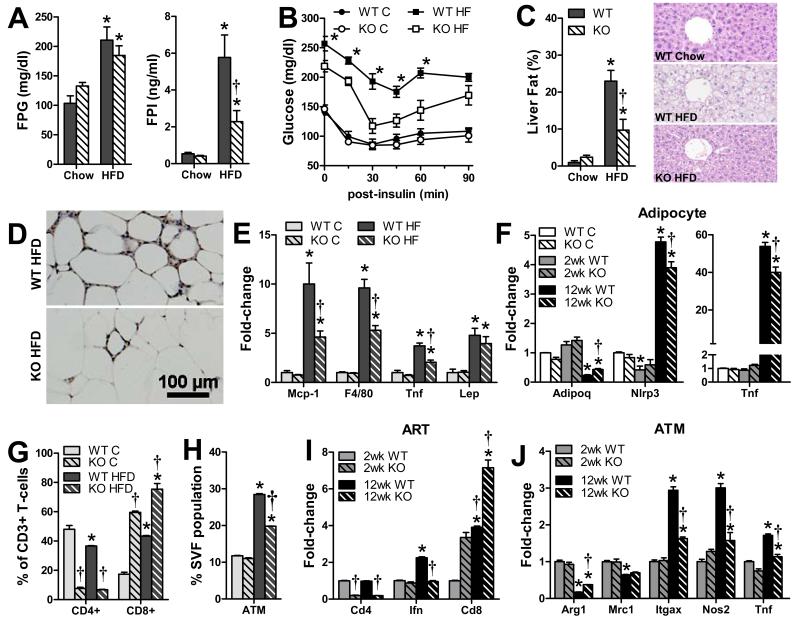
MHCII-deficient mice have less adipose inflammation and are more insulin sensitive when fed HFD
To determine the importance of MHCII-mediated mechanisms in metabolic responses to caloric excess, MHCII-deficient (H2A−/−) and WT mice were fed chow or HFD for 3 months and analyzed for whole adipose, adipocyte, ART, ATM, and metabolic differences. Both H2A−/− and WT mice developed similar adiposity on chow and HFD, although H2A−/− mice had slightly lower starting body weights, gained slightly less weight than WT mice when fed either chow or HFD, and had reduced plasma leptin (Fig. S4A-D). Although HFD-fed H2A−/− mice had similar fasting plasma glucose and adiposity as WT mice, H2A−/− mice had lower fasting insulin levels, greater insulin sensitivity, and, consistent with these metabolic changes, less liver steatosis than WT mice (Fig. 5A-C). RT-PCR analysis also found reduced expression of the macrophage marker Emr1 in adipose tissue of HFD-fed H2A−/− mice, in agreement with reduced macrophage accumulation by immunohistology, and less mRNA expression of the pro-inflammatory cytokines MCP-1 and TNFα with no difference in leptin expression (Fig. 5 D-E). RT-PCR analysis found that adipocytes isolated from HFD-fed H2A−/− mice did not markedly differ at 2 weeks HFD, but by 12 weeks expressed more adiponectin and less Tnf and Nlrp3 than those of WT mice, indicating reduced adipocyte inflammation (Fig. 5F). Flow cytometry of SVF from H2A−/− mice revealed reduced CD4+ T-cells and increased CD8+ T-cells (Fig. 5G), as previously reported for H2A−/− mice (Madsen et al., 1999). HFD challenge decreased CD4+ ARTs and increased CD8+ ARTs in SVF of WT mice, and further increased CD8+ ARTs in SVF of H2A−/− mice, with no effect on CD4+ ARTs. ATM abundance was similar in SVF of both chow-fed mouse groups, but increased more in SVF of HFD-fed WT vs. H2A−/− mice (Fig. 5H). Similarly, ARTs of H2A−/− mice expressed less Cd4 and more Cd8 at 2 and 12 weeks HFD than WT mice; IFNγ expression was similar at 2 weeks but half that of WT mice at 12 weeks (Fig. 5I). ATMs did not differ at 2 weeks, but revealed an attenuation of the M1 polarization profile that occurred in WT mice, with increased expression of the M2 marker Arg1 and reduced expression of the M1 markers Itgax, Nos2 and Tnf at 12 weeks (Fig. 5J). Taken together these results demonstrate that MHCII-deficiency attenuates adipose inflammatory and metabolic responses to HFD, indicating an important role for MHCII and activation of CD4+ T-cells in the proinflammatory response of adipose tissue to HFD.
Fig. 5. HFD-fed MHCII-deficient mice have less adipose inflammation and insulin resistance.
A) fasting plasma glucose and insulin levels, B) intraperitoneal insulin tolerance test data of H2A−/− and WT mice fed 3 months of chow or HFD (N=6-7/group) and liver steatosis analyzed by C) NMR- and hematoxylin and eosin staining. Adipose inflammation was assessed by D) F4/80 immunhistochemistry and E) RT-PCR, while relative F) adipocyte changes were analyzed by RT-PCR. SVF G) ART and H) ATM abundance was assessed by flow cytometry, and I) ART and J) ATM fractions were analyzed by RT-PCR. (N=4-6/group). ART and ATM samples represent pooled material obtained from 4 (adipocytes) or 8 (ARTs and ATMs) mice. (p<0.05 vs. genotype-matched (*) or diet-matched (†) expression by T-test).
Because of the improved metabolic responses to HFD in H2A−/− mice, we investigated insulin responses in primary adipocytes isolated from H2A−/− vs. WT mice. Adipocytes of HFD-fed H2A−/− mice revealed greater insulin-stimulated glucose uptake than those of WT mice. Although there was little difference in insulin-stimulated pAkt/Akt protein ratios, H2A−/− adipocytes demonstrated less repression of the adipogenic and insulin-sensitizing transcription factors Pparg, Irs1 and Pik3r3, which binds Irs1 during insulin-stimulation (Fig. S4E-G). In contrast, attenuation of IFNγ-stimulated MHCII expression by shRNA mediated Ciita knockdown, did not alter insulin-mediated glucose uptake or isoproterenol-induced lipolysis in 3T3-L1 adipocytes (Fig. S4H-I). Thus, MCHII-deficiency does not appear to impact adipocyte metabolism through intracellular-mediated effects, but likely exerts its beneficial effects by decreasing local inflammation.
H2A−/− mice were additionally studied at 4 weeks HFD, when adipocytes and T-cells instigate adipose inflammation in WT mice. At this time there were no differences in body weight, plasma lipids in HFD-fed WT and H2A−/− mice (Fig. S5A-B); however, the H2A−/− mice were more insulin sensitive than WT mice (Fig. S5C). Thus, loss of adipocyte MHCII may impact systemic insulin resistance early after HFD administration.
DISCUSSION
Cytokine production triggered by innate immune processes in both ATMs and adipocytes drives adipose tissue inflammation in obesity and regulates the accumulation and polarization of ATMs (Anderson et al., 2010; Olefsky and Glass, 2010). However, the contributions of adaptive immunity to the establishment of inflamed adipose tissue are much less clear. A previous microarray study reported increased expression of components of the MHCII pathway in obese vs. lean adipose tissue, but no investigations have precisely delineated the functional importance of this pathway (Klimcakova et al., 2011). Observations that T-cells accumulate in adipose tissue during HFD to influence ATM phenotypes and insulin sensitivity and that the adipose CD4+ T-cell receptor repertoire is restricted, suggestive of selection for specific antigen recognition (Feuerer et al., 2009; Winer et al., 2009), emphasizes the potential importance of this pathway. We now report that both SQ and V adipocytes of obese humans and mice, but not lean equivalents, express all MHCII components required for antigen presentation and that primary mouse adipocytes can induce antigen-specific CD4+ T-cell activation. Surprisingly, adipocytes isolated from obese mice revealed MHCII mRNA levels approaching those of ATMs after 12 weeks of HFD, while western blot analysis detected similar adipocyte and ATM MHCII protein levels, confirming that adipocytes express substantial levels of MHCII protein. Moreover, mature adipocytes could activate T-cells nearly as well as macrophages. Leptin, known to promote T-cell proliferation and cytokine secretion (Lord et al., 1998), increased within one week of HFD challenge, and adipocyte MHCII and co-stimulatory molecule expression increased within two weeks of HFD, corresponding to increases in adipose tissue pro-inflammatory TH1 cell differentiation and IFNγ expression and preceding decreases in TH2 and TREG markers. ATM infiltration and M2 to M1 polarization did not occur until well after these ARTs changes, and ATM MHCII expression did not change with HFD. Together these data suggest that induction of adipocyte MHCII contributes to the early activation and polarization of ARTs after HFD challenge. However, the lack of an adipocyte-specific MHCII knockout mouse precludes a definitive conclusion. Nevertheless our investigation suggests adaptive immunity is necessary for adipose inflammation.
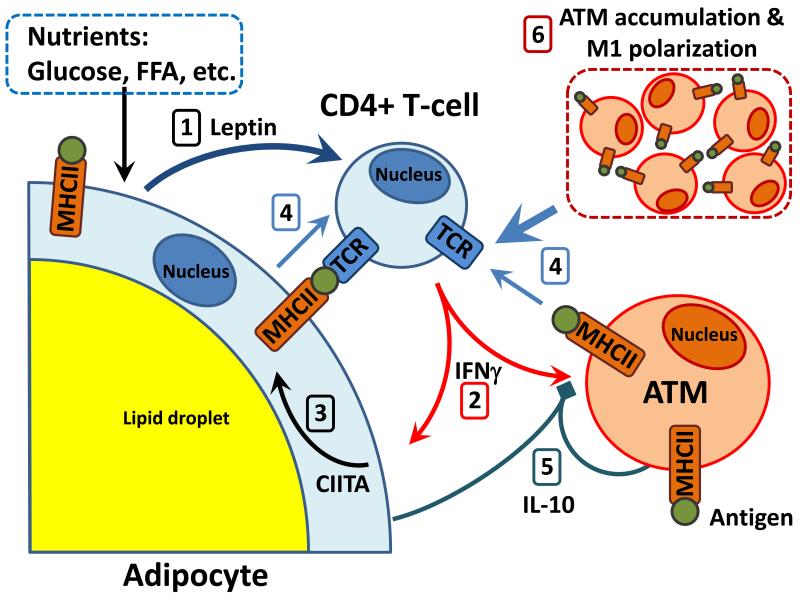
Leptin is an important contributor to the initiation of the adipose inflammatory cascade. Leptin receptor expression is prominent on human ARTs and increases with obesity (Duffaut et al., 2009). Leptin signaling induces T-cell IFNγ secretion stimulating TH1 and repressing TH2 differentiation (Lord et al., 1998). We found that supernatant of leptin-treated T-cells enhanced CIITA expression in 3T3-L1 adipocytes, but this effect was completely attenuated in the presence of IFNγ-blocking antibodies. Compared to other cytokines, IFNγ substantially promoted adipocyte MHCII expression and antigen-specific T-cell activation. Indeed, adipose tissue IFNγ expression and circulating levels increase with obesity and promote macrophage M1 polarization (Pacifico et al., 2006; Rocha et al., 2008), while IFNγ-deficient mice develop less insulin resistance and steatosis than WT mice (Wong et al., 2011), emphasizing the importance of IFNγ in adipose inflammation. Thus, we propose that leptin stimulates ART IFNγ production, increasing adipocyte MHCII to promote TH1 differentiation, thereby resulting in an escalating cycle of adipocyte/T-cell inflammation, as depicted in Fig. 6. Accordingly, leptin-deficient ob/ob mice have less ART IFNγ and adipocyte MHCII expression and fewer adipose TH1 cells and M1 ATMs than similar-weight HFD-fed WT mice. In humans, adipocyte CIITA expression correlates with both plasma leptin concentration and adipocyte leptin gene expression, and SVF IFNγ expression was 3-fold elevated in obese vs. lean subjects with increased TH1 marker expression, consistent with this mechanism.
Fig. 6. Model of adipose tissue cell interactions during HFD-induced inflammation.
Nutrient excess increases 1) adipocyte leptin (1 week), inducing 2) CD4+ ART IFNγ expression (2 weeks) to stimulate 3) adipocyte CIITA and MHCII expression (2-3 weeks). 4) MHCII antigen presentation by adipocytes, or other APCs, stimulates CD4+ ART proliferation and differentiation. 5) Increased adipocyte IL-10, starting at 1 week HFD, may attenuate pro-inflammatory ATM polarization and APC function. By 12 weeks HFD challenge, ART activation/proliferation stimulates 6) ATM accumulation and polarization to escalate adipose inflammation.
Our study is the first to demonstrate that adipocytes can directly activate T-cells via the MHCII pathway which is enhanced in obesity. Several cell types can express MHCII and function as APCs upon exposure to IFNγ in addition to macrophages and dendritic cells, the so called professional APCs (Razakandrainibe et al., 2012). Here, we showed that adipocytes of obese mice activated T-cells to a greater extent than those of lean mice and, consistent with an APC role, adipocytes of obese humans and mice exhibited marked increases in expression of two major costimulatory molecules, CD80 and CD86, found exclusively on cells that stimulate T-cell proliferation. Preadipocytes and adipocytes have previously been reported to acquire other phenotypic traits commonly associated with macrophages. Charrière et al showed that preadipocyte microarray profiles were closer to macrophages than to adipocytes and that preadipocytes demonstrated phagocytic activity and macrophage-specific protein expression similar to peritoneal macrophages (Charriere et al., 2003). Meijer et al (Meijer et al., 2011) also reported that human primary preadipocytes and adipocytes express cytokines, MHCII genes and acute phase proteins, many of which were increased by LPS stimulation. However, these studies were performed using primary pre-adipocyte cell lines before and after in vitro differentiation, not ex vivo adipocytes, and these authors did not examine the ability of these cells to activate T-cells.
We emphasize that our model does not exclude a role for ATM-dependent antigen presentation in T-cell activation in inflamed adipose tissue, but we suggest that such events occur later in the inflammatory cascade. In contrast to adipocytes and T-cells, ATMs were unexpectedly quiescent during the first 4 weeks of HFD challenge, revealing no major changes in candidate pro-inflammatory cytokines. Moreover, MHCII expression was not different in ATMs of obese vs. lean humans or mice. These differences between adipocyte and ATM MHCII induction may be explained by their diverse responses to IL-10, which attenuates both macrophage MHCII antigen presentation and cytokine production (Turner et al., 2010). In contrast, we observed no effect of IL-10 on IFNγ-induced MHCII expression in 3T3L1 or primary adipocytes due to greatly reduced IL-10 receptor expression compared to macrophage expression. Both adipocyte and ATM IL-10 expression increased with time on HFD: adipocyte expression increased 2-fold within 1 week of HFD and 150-fold by 12 weeks HFD, while ATM expression significantly increased at 12 weeks HFD (not shown). These increases in adipose IL-10 early in HFD-induced obesity may thus suppress APC activity of ATMs, but not adipocytes, as shown in Fig. 6. However, during prolonged caloric excess, progressive increases in T-cell IFNγ secretion and other factors, such as macrophage lipid accumulation (McGillicuddy et al., 2009; Prieur et al., 2011), likely promote ATM M1 polarization and macrophage APC activity. Studies in both humans and mice demonstrate that systemic insulin resistance can develop prior to macrophage changes possibly due to increased secretion of the T-cell cytokine IFNγ, which can directly impair insulin action (McGillicuddy et al., 2009), or due to lipotoxic effects resulting from increases in circulating free fatty acids (Lara-Castro and Garvey, 2008; Samuel and Shulman, 2012). Thus, adipocyte MHCII expression may contribute to CD4+ ART activation and IFNγ production early in HFD, while adipose IL-10 can suppress ATM adaptive immune activity. Both adipocytes and ATMs had similar MHCII expression with 3 months HFD. At this time, newly infiltrated ATMs are reported to comprise as much as 50% of the adipose cell population (Weisberg et al., 2003); since ATM MHCII expression did not increase with HFD, an enhanced number of ATMs appears necessary to increase ATM T-cell activation. Our data suggests both adipocytes and ATMs contribute to T-cell activation late in HFD (Fig. 6).
In order to test the role of adaptive immunity in mediating adipose inflammatory responses, we administered HFD to MHCII-deficient H2A−/− vs. WT mice. Despite similar adiposity, HFD-fed H2A−/− mice had less insulin resistance and steatosis than WT controls, and less ART IFNγ mRNA expression and reduced ATM accumulation and M1 polarization. Even at 4 weeks HFD, prior to adipose macrophage infiltration, these mice were more insulin sensitive than WT mice, suggesting that deficiency of adipocyte MHCII may have contributed to improved insulin action. Loss of APC function by both adipocytes and macrophages impaired TH1 cell differentiation and substantially reduced adipose inflammatory responses to HFD. This finding was quite unexpected since low CD4+T-cells and increased CD8+T-cells, which characterize H2A−/− mice, have been reported to occur in obesity and mediate insulin resistance, and depletion of CD8+T-cells has been shown to improve insulin sensitivity (Nishimura et al., 2009). Thus, despite a T-cell profile that would predict insulin resistance, loss of MHCII improved metabolic responses. Of note, adipocytes of HFD-fed H2A−/− mice had increased adiponectin, but decreased pro-inflammatory cytokine expression compared to WT mice. While adipocytes from HFD-fed H2A−/− vs. WT mice were more insulin sensitive, there was no difference in insulin responses in 3T3L1 adipocytes with and without CIITA knockdown. These data suggest the pro-inflammatory milieu, rather than direct loss of MHCII, impacts adipocyte insulin responses.
Our investigation highlights a role of adaptive immunity in the development and progression of obesity-induced adipose inflammation and insulin resistance. Intriguingly, other groups have reported that CD4+ ARTs express a restricted subset of T-cell receptors (Feuerer et al., 2009; Winer et al., 2009), suggesting that ARTs may recognize specific antigens in fat that regulate adaptive immune responses to HFD, dictating T-cell maturation and lineage commitment (McDevitt, 2000). These observations suggest the adipocyte or other cells in the adipose microenvironment are the source of these antigens. Nothing is known, however, about the nature of antigens that activate ARTs. However, several mechanisms could produce novel antigens during obesity. For example, post-translational modifications, such as palmitoylation and oxidation, could directly alter adipose-derived proteins or alter their folding and processing to produce new antigenic variants; alternative splicing could produce new antigenic protein isoforms; stress-induced protease activities could alter protein processing to produce new antigenic peptides; and ER stress induced protein misfolding could alter posttranslational modification and proteolytic processing to produce new antigens. Our findings, together with current observations in the field, provide a compelling rationale to identify adipose antigens altered in obesity, since vaccine-based strategies to induce immune tolerance against these antigens could represent a new therapeutic approach to attenuate inflammatory-driven complications of caloric excess.
MATERIALS AND METHODS
Adipocyte and SVF RT-PCR and flow cytometry analyses
Adipocytes and SVF were isolated as previously described (Halleux et al., 1999), then fractionated with biotinylated antibodies (eBioscience, San Diego CA) and streptavidin-Dynabeads (Invitrogen, Grand Island, NY). CD45-depeleted adipocytes were processed with RNeasy Lipid Tissue Mini kits (Qiagen, Valencia, CA). ATMs and ARTs were isolated from SVF with biotinylated F4/80 and CD3d antibodies and RNA extracted with Trizol Reagent (Invitrogen). RNA was reverse-transcribed and amplified with Taqman mRNA Reverse Transcription Kits and primer/probes (Applied Biosystems, Carlsbad, CA). RNA expression was normalized to 36B4 (adipose or adipocytes) or PPIA (ARTs and ATMs).
CD45-depleted adipocytes and SVF were hybridized with CD45-PE, CD36-AF647 and HLA-DR-APC-eF780 antibodies (eBioscience) and analyzed on an Imagestreamx system (Amnis, Seattle, WA). Mouse SVF fractions were hybridized with mouse CD3-Pacific Blue, CD4-FITC and CD8-Pe-Cy7 antibodies or F4/80-APC and CD11b-FITC antibodies and analyzed on a BD LSRFortessa using FACSDiva Software (BD Biosciences, San Jose, CA) to detect CD3+CD4+ and CD3+CD8+ ARTs and F4/80+CD11b+ ATMs, using appropriate compensation controls to set gates.
Mouse Studies
Male ob/ob (B6.Lepob/J), B6.Cg-Tg(TcraTcrb), DBA/1J, H2A−/− (B6.129S2-H2dlAb1-Ea/J) and diet-induced obese (DIO) and lean C57BL/6J mice were from The Jackson Laboratory (Bar Harbor, ME). H2A−/− and C57BL6/J mice were randomly assigned to chow (8904, Harlan Teklad) or HFD (60% kcal fat; D12492, Research Diets, New Brunswick, NJ) as indicated, and analyzed for food intake (BioDAQ, Research Diets), NMR-determined body and liver fat (Echo Medical Systems, Houston, TX), overnight-fasted blood glucose (One-Touch, Lifescan, Milpitas, CA) and plasma insulin (Millipore, Billerica, MA). ITTs were performed on 4-hour-fasted, non-anesthetized mice, using tail vein blood samples obtained 0, 15, 30, 45, 60, and 90 minutes after intraperitoneal insulin injection. All animal procedures were conducted in a viral pathogen–free facility at The Methodist Hospital Research Institute in accordance with institutional animal care and use committee guidelines.
Cell Culture
HEK293T and 3T3-L1 cells were obtained from American Type Culture Collection (ATCC, Bethesda, MD). Primary human preadipocytes were purchased from Zen-bio (Research Triangle Park, NC), and cultured according to the supplier’s protocol. C57BL/6-Tg(TcraTcrb) mouse T-cells were isolated with a Pan T Cell isolation Kit (Miltenyi Biotec). Cell cultures methods are described in Supplemental Material.
Western Blots
Western blots were performed as described (Deng et al., 2006), using primary MHCII, F4/80 (Abcam, Cambridge, UK), MHCI, CIITA (Santa Cruz Biotechnology, Santa Cruz, CA), adiponectin and GAPDH (Chemicon, Billerica, MA) and secondary (Cell Signaling, Danvers, MA), with densitometry analyzed using NIH ImageJ software.
Histology and Immunohistochemistry
Mouse epididymal white adipose tissue and liver samples were fixed in 10% Formalin-PBS overnight, then paraffin-embedded, sectioned and hemotoxylin and eosin stained. Sections were hybridized with mouse F4/80 antibody (Abcam), developed using Histomouse MAX kits (Invitrogen), and imaged using a Nikon 90i system and NIS-Elements software (Nikon, Melville, NY).
Statistics
All data are presented as means±SEM and sample sizes are reported in figure legends. Prism 5.0 software (Graphpad, San Diego, CA) was used for all statistical analyses. Differences between groups were analyzed using Mann-Whitney U-tests, Student’s or Welch’s T-tests as indicated by data normality, variation and statistical power.
Supplementary Material
HIGHLIGHTS.
Obesity enhances MHCII expression in primary human and mouse adipocytes
Adipocytes activate CD4+ ARTs via MHCII and leptin to induce adipose inflammation
Macrophage changes in adipose follow adipocyte and T-cell interactions during HFD
Adaptive immune mechanisms are essential to obesity-induced adipose inflammation
Acknowledgments
This paper is dedicated to the late John Baxter for his enduring friendship and support. We thank Keyun Chen and Dr. Qiang Tong (Baylor College of Medicine) for assistance with the glucose uptake experiment.
Grant or fellowship support: This work was supported by a generous grant from the MacDonald Foundation, a gift from the Zucker Family and NIH grant R24DK087723 to WAH; NIH grants R01CA121225 and U54CA149196 to SW; NIH grants R01CA101795, R0CA116408 and R01CA121191 to RFW; and ADA fellowship ADA 7-07-CVD-12 to TD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tuo Deng, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA.
Christopher J. Lyon, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
Laurie J. Minze, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
Jianxin Lin, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA.
Jia Zou, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA.
Joey Z. Liu, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
Yuelan Ren, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA.
Zheng Yin, The Methodist Hospital Research Institute, Department of Systems Medicine and Bioengineering, Weill Cornell Medical College, Houston, TX, USA.
Dale J. Hamilton, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
Patrick R. Reardon, Department of Surgery, The Methodist Hospital, Houston, TX, USA
Vadim Sherman, Department of Surgery, The Methodist Hospital, Houston, TX, USA.
Helen Y. Wang, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
Kevin J. Phillips, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
Paul Webb, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA.
Stephen TC. Wong, The Methodist Hospital Research Institute, Department of Systems Medicine and Bioengineering, Weill Cornell Medical College, Houston, TX, USA
Rong-fu Wang, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA.
Willa A. Hsueh, The Methodist Hospital Research Institute, Center for Diabetes Research and Center for Inflammation and Epigenetics in The Methodist Diabetes and Metabolism Institute, Weill Cornell Medical College, Houston, TX, USA
REFERENCES
- Anderson EK, Gutierrez DA, Hasty AH. Adipose tissue recruitment of leukocytes. Current opinion in lipidology. 2010;21:172–177. doi: 10.1097/MOL.0b013e3283393867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Charriere G, Cousin B, Arnaud E, Andre M, Bacou F, Penicaud L, Casteilla L. Preadipocyte conversion to macrophage. Evidence of plasticity. The Journal of biological chemistry. 2003;278:9850–9855. doi: 10.1074/jbc.M210811200. [DOI] [PubMed] [Google Scholar]
- Chawla A. Control of macrophage activation and function by PPARs. Circulation research. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circulation research. 2009;104:e42–54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. The Journal of experimental medicine. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng T, Shan S, Li PP, Shen ZF, Lu XP, Cheng J, Ning ZQ. Peroxisome proliferator-activated receptor-gamma transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology. 2006;147:875–884. doi: 10.1210/en.2005-0623. [DOI] [PubMed] [Google Scholar]
- Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenes C, Lafontan M, Galitzky J, Bouloumie A. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- Feng D, Tang Y, Kwon H, Zong H, Hawkins M, Kitsis RN, Pessin JE. High-fat diet-induced adipocyte cell death occurs through a cyclophilin D intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60:2134–2143. doi: 10.2337/db10-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, Sanchez-Margalet V. Role of leptin in the activation of immune cells. Mediators of inflammation. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nature reviews. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152:3074–3081. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature reviews. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Liu JZ, Ren Y, Minze LJ, Wiles JR, Collins AR, Lyon CJ, Pratico D, Finegold MJ, Wong ST, et al. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology (Baltimore, Md. 2010;52:2001–2011. doi: 10.1002/hep.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halleux CM, Declerck PJ, Tran SL, Detry R, Brichard SM. Hormonal control of plasminogen activator inhibitor-1 gene expression and production in human adipose tissue: stimulation by glucocorticoids and inhibition by catecholamines. J Clin Endocrinol Metab. 1999;84:4097–4105. doi: 10.1210/jcem.84.11.6127. [DOI] [PubMed] [Google Scholar]
- Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science (New York, N.Y. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of clinical investigation. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. Journal of internal medicine. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (New York, N.Y. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- Klimcakova E, Roussel B, Marquez-Quinones A, Kovacova Z, Kovacikova M, Combes M, Siklova-Vitkova M, Hejnova J, Sramkova P, Bouloumie A, et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J Clin Endocrinol Metab. 2011;96:E73–82. doi: 10.1210/jc.2010-1575. [DOI] [PubMed] [Google Scholar]
- Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinology and metabolism clinics of North America. 2008;37:841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nature medicine. 2009;15:846–847. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C, Mathis D, Fugger L. Mice lacking all conventional MHC class II genes. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10338–10343. doi: 10.1073/pnas.96.18.10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt HO. Discovering the role of the major histocompatibility complex in the immune response. Annual review of immunology. 2000;18:1–17. doi: 10.1146/annurev.immunol.18.1.1. [DOI] [PubMed] [Google Scholar]
- McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, Smyth EM, Reilly MP. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. The Journal of biological chemistry. 2009;284:31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der Leij RJ, van der Want H, Kroesen BJ, et al. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PloS one. 2011;6:e17154. doi: 10.1371/journal.pone.0017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature medicine. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nature reviews. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico L, Di Renzo L, Anania C, Osborn JF, Ippoliti F, Schiavo E, Chiesa C. Increased T-helper interferon-gamma-secreting cells in obese children. European journal of endocrinology / European Federation of Endocrine Societies. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- Prieur X, Mok CY, Velagapudi VR, Nunez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O’Rahilly S, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razakandrainibe R, Pelleau S, Grau GE, Jambou R. Antigen presentation by endothelial cells: what role in the pathophysiology of malaria? Trends in parasitology. 2012;28:151–160. doi: 10.1016/j.pt.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circulation research. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring, Md. 2010;18:1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. The Journal of clinical investigation. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JJ, Foxwell KM, Kanji R, Brenner C, Wood S, Foxwell BM, Feldmann M. Investigation of nuclear factor-kappaB inhibitors and interleukin-10 as regulators of inflammatory signalling in human adipocytes. Clinical and experimental immunology. 2010;162:487–493. doi: 10.1111/j.1365-2249.2010.04260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nature immunology. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nature medicine. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N, Fam BC, Cempako GR, Steinberg GR, Walder K, Kay TW, Proietto J, Andrikopoulos S. Deficiency in interferon-gamma results in reduced body weight and better glucose tolerance in mice. Endocrinology. 2011;152:3690–3699. doi: 10.1210/en.2011-0288. [DOI] [PubMed] [Google Scholar]
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.