Abstract
Because cardenolides specifically inhibit the Na+K+-ATPase, insects feeding on cardenolide-containing plants need to circumvent this toxic effect. Some insects such as the monarch butterfly rely on target site insensitivity, yet other cardenolide-adapted lepidopterans such as the oleander hawk-moth, Daphnis nerii, possess highly sensitive Na+K+-ATPases. Nevertheless, larvae of this species and the related Manduca sexta are insensitive to injected cardenolides. By radioactive-binding assays with nerve cords of both species, we demonstrate that the perineurium surrounding the nervous tissue functions as a diffusion barrier for a polar cardenolide (ouabain). By contrast, for non-polar cardenolides such as digoxin an active efflux carrier limits the access to the nerve cord. This barrier can be abolished by metabolic inhibitors and by verapamil, a specific inhibitor of P-glycoproteins (PGPs). This supports that a PGP-like transporter is involved in the active cardenolide-barrier of the perineurium. Tissue specific RT-PCR demonstrated expression of three PGP-like genes in hornworm nerve cords, and immunohistochemistry further corroborated PGP expression in the perineurium. Our results thus suggest that the lepidopteran perineurium serves as a diffusion barrier for polar cardenolides and provides an active barrier for non-polar cardenolides. This may explain the high in vivo resistance to cardenolides observed in some lepidopteran larvae, despite their highly sensitive Na+K+-ATPases.
Keywords: cardenolides, Daphnis nerii, Manduca sexta, P-glycoprotein, Na+K+-ATPase, resistance
1. Introduction
Over the course of evolution, plants have evolved a vast diversity of secondary plant compounds many of which act as chemical weapons against herbivores. In return, herbivores have developed strategies to overcome plant defences. Mechanisms of resistance in insects are numerous and include detoxification of toxins by enzymes, excretion, exclusion (gut barriers) and target site insensitivity [1,2].
In this study, we focus on insect resistance to plant-produced cardenolides (aka cardiac glycosides), a specific class of plant toxins [3,4]. Cardenolides are specific inhibitors of the Na+K+-ATPase, a ubiquitous animal enzyme that is essential for many physiological processes [5,6].
Several herbivorous insects, including the monarch butterfly (Danaus plexippus) not only feed on cardenolide-containing plants, but also sequester the toxins and thus derive protection against predators [7]. The Na+K+-ATPase of D. plexippus is altered by specific amino acid substitutions, which significantly reduce its cardenolide susceptibility (target site insensitivity; [8,9]). In earlier studies, however, we found that lepidopterans that are adapted to cardenolides sometimes possess cardenolide sensitive Na+K+-ATPases [10,11]. Moreover, among cardenolide-adapted Lepidoptera, the monarch butterfly actually seems to be an exceptional case [9,12,13].
In Lepidoptera, Na+K+-ATPase is predominantly expressed in the nervous tissue. The concomitant occurrence of dietary cardenolides in the caterpillars' haemolymph, therefore, renders the interface between insect blood and nervous tissue especially important [11]. The ventral nerve cord of insects is, like in other organisms, surrounded by the perineurium, a tissue maintaining ionic conditions required for the excitability of neurons, which may be different from the composition of the haemolymph (low K+ and high Na+ required in the extracellular space of the nerve cord versus an approximate 1 : 1 ratio in the haemolymph; [14]). Additionally, this tissue is believed to function as a blood–brain barrier for toxic plant compounds present in the herbivores' haemolymph [15]. However, thus far there is only limited functional evidence for such a protective function of the perineurium.
In this study, we test whether the perineurium can function as a barrier to dietary cardenolides absorbed into the haemolymph and thus potentially contribute to resistance to these toxins. We used the oleander hawk-moth (Daphnis nerii; figure 1a), a cardenolide specialist that feeds primarily on oleander (Nerium oleander), a plant rich in these toxins [16]. For comparison, we included Manduca sexta, a related species which is not adapted to cardenolides.
Figure 1.
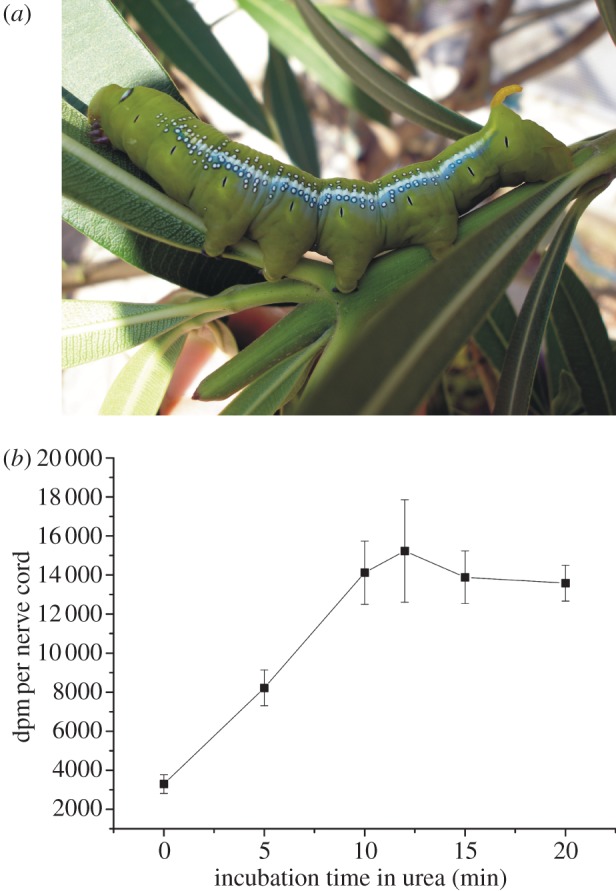
Binding of 3H-ouabain to the isolated nerve cord of D. nerii caterpillars: (a) caterpillar of the oleander hawk-moth (D. nerii). The major host plant of this species is N. oleander, whose marked toxicity is based on cardenolides. (b) Disruption of the perineurial barrier with urea in isolated nerve cords of D. nerii (each datapoint represents the mean (±s.d.) of three independent incubations, i.e. 18 caterpillars were used in total). (Online version in colour.)
Daphnis nerii caterpillars have relatively low levels of oleander cardenolides in their body (approx. 150–200 µg at maximum; [16]). Nonetheless, owing to their highly sensitive Na+K+-ATPase even minute amounts of cardenolides in the haemolymph can be fatal [10]. Because oleander and other cardenolide-containing plants have cardenolides with a wide range of polarity, we speculated that different mechanisms may be important in insect resistance to diverse cardenolides. For example, the perineurium forms a diffusion barrier for polar cardenolides as was shown for M. sexta [17]. Nonetheless, non-polar cardenolides that are able to use the transcellular pathway [18] might require an active barrier mechanism (i.e. efflux transporters). Both mechanisms are tested here by physiological experiments.
In the mammalian brain, P-glycoprotein (PGP) is one of the most important efflux transporters [19] with an amazingly wide substrate spectrum including the cardenolide digoxin [20]. This 170-kDa membrane bound protein, a member of the ABC (ATP-binding cassette)-transporter superfamily, extrudes xenobiotic compounds from cells driven by ATP hydrolysis. In M. sexta, PGP was already suggested to be involved in nicotine resistance [15]. In other insects, it is believed to mediate resistance to insecticides or xenobiotics [21,22]. We therefore tested whether a PGP-like transporter may be involved in the physiological blood–brain barrier of the hawk-moth nerve cord using the well-known PGP inhibitors quinidine and verapamil. Immunohistochemical assays with monoclonal antibodies were further used to visualize PGP as well as Na+K+-ATPase occurrence in the nerve cord. Moreover, an analysis of M. sexta expressed sequence tags (EST) data followed up by tissue-specific RT-PCR confirmed the occurrence of PGP-like transporters in the perineurium.
In summary, our investigations address the relative importance of passive and active mechanisms in protecting the hawk-moth nervous system from potent plant toxins.
2. Material and methods
(a). Radiochemicals and inhibitors
3H-ouabain (12 Ci mmol−1, dissolved in 9 : 1 ethanol : toluene, or 30 Ci mmol−1, dissolved in ethanol) was purchased from GE Healthcare (Freiburg, Germany) and Perkin Elmer (Rodgau, Germany). 3H-digoxin was purchased from Perkin Elmer (40 Ci mmol−1, dissolved in ethanol). Both ouabain and digoxin most likely do not occur in larval host plants of D. nerii, but were used owing to their commercial availability and strongly differing polarity. 2,4-dinitrophenol (2,4-DNP; Fluka, Taufkirchen, Germany), carbonyl cyanide 3-chlorophenylhydrazone (CCCP; Sigma, Taufkirchen, Germany), verapamil hydrochloride (Sigma) and quinidine (Sigma) were used as 0.05 M stock solutions in ethanol. In our binding experiments, we used 3H-cardenolide concentrations of 0.35 and 0.7 µM, respectively. We decided to use such low amounts because Rubin et al. [17] observed non-specific binding of 3H-ouabain to native nerve cords of Manduca at concentrations above 10 µM. We decided not to refer our disintegrations per minute (dpm) values to protein content throughout the experiments because protein determination proved to be dependent on storage time (at −20°C) post-experiment. Referring to nerve cords as experimental units, on the other hand, proved to be highly reliable because the (simultaneously determined) protein content of 18 D. nerii nerve cords (eight ganglia each, see below) averaged 63.01 µg with a standard deviation of 9.74. The small standard deviations of our treatment groups throughout the experiments give further evidence that this approach provides reliable data that are not biased by size differences. Therefore, the radioactivity measured in our experiments is expressed as dpm per nerve cord. All data used in the inhibitor experiments are provided in the electronic supplementary material.
(b). Diffusion barrier
To test for a diffusion barrier to polar cardenolides, we followed the experimental design described by Rubin et al. [17], who disrupted the perineurium of M. sexta by treatment with urea. Caterpillars of D. nerii (European origin) were raised on greater periwinkle (Vinca major), which is devoid of cardenolides, at 23°C (16 L : 8 D cycle). Prior to dissection, last instar caterpillars were chilled on ice and decapitated. Ventral nerve cords were removed, placed in cold incubation buffer (125 mM NaCl, 5 mM MgCl2, 0.5% bovine serum albumin (BSA) and 12.5 mM imidazole, pH 7.3), cleaned from adherent tissue and trimmed to a chain of eight ganglia plus intervening connectives (abdominal ganglia plus metathoracic ganglion; [23]). For each of three replicates a series of six caterpillars was used. One nerve cord of a series was used as a control and was kept in incubation buffer at room temperature for the duration of the urea treatment. The additional five nerve cords were immersed in 3 M urea in incubation buffer for 5, 10, 12, 15 or 20 min, respectively. The cords were then washed twice with incubation buffer for at least 5 min each. Following urea treatment, cords (including the control cord) were individually incubated in 100 µl incubation buffer with 0.7 µM 3H-ouabain for 1 h at 37°C. After incubation, cords were washed in an excess volume of 10 mM imidazole (pH 7.3) for 30 min on ice. Each cord was then transferred to 200 µl 0.2 M NaOH/1 per cent SDS and digested overnight. To 150 µl of this extract 3 ml liquid scintillation cocktail (Ultima Gold, Perkin Elmer) were added and radioactivity determined in a liquid scintillation counter (Wallac 1409, easy count mode). The remainder of each sample was stored at −20°C for later protein determination with the bicinchoninic acid (BCA) assay (Thermo Scientific) using BSA as a standard.
(c). Active barrier
(i). Manduca sexta
Eggs of M. sexta were kindly supplied by Dr. Markus Huß (University of Osnabrück). Caterpillars were reared on gypsy moth diet (MP Biomedicals) supplemented with streptomycin, chloramphenicol, methyl benzoate and formalin (26°C; 16 L : 8 D cycle). Only last instar caterpillars before reaching the wandering stage were used.
(ii). Daphnis nerii
Caterpillars of D. nerii (origin Thailand) were raised from eggs at 27°C at 13 L : 11 D cycle. Hatched caterpillars were initially fed with V. major later transferred (second instar) to N. oleander and raised to the last instar.
Nerve cords of both species were dissected as described above and maintained until incubation on ice in Manduca saline: 5.0 mM K2HPO4, 10.0 mM MgCl2, 1.0 mM CaCl2, 10.0 mM NaCl, 10.0 mM KOH, 7.4 mM L-proline, 7.7 mM tripotassium citrate, 2.8 mM disodium succinate, 2.0 mM glucose, 175.0 mM sucrose, 5.6 mM malic acid, 10.0 mM HEPES, pH 6.7 [24]. Again, the posterior eight ganglia were used. To test the hypothesis that the nerve cords of M. sexta and D. nerii possess an energy-driven barrier that prevents cardenolides from reaching the Na+K+-ATPase, the metabolic inhibitors 2,4-DNP and CCCP were applied. To test whether a PGP-like transporter is involved in this barrier, we used verapamil and quinidine that are well-known competitive PGP inhibitors [25]. All inhibitors were dissolved in ethanol and applied at a final concentration of 1 mM. Controls were incubated with an equivalent amount of ethanol. The concentration of 3H-digoxin in the assay was 0.35 µM (ethanol concentration 3.36%). Each nerve cord was incubated in a volume of 100 µl Manduca saline at 37°C. After 30 min, tubes were placed on ice, the radioactive solution was removed, 1 ml of cold 10 mM imidazole (pH 7.3) added and mixed by vortex stirring. After replacing the washing buffer once, tubes were inverted and kept on ice for 30 min. The short washing step was performed to remove adherent radioactive solution, whereas the long washing step was performed to remove unbound 3H-digoxin [17]. In an additional experiment (data not shown) we found that nearly all adhering radioactivity is removed from the tissue after the 30 min washing step. After washing, the samples were lysed and radioactivity counted as described above.
(d). Statistical analysis
If necessary, data were squared or log-transformed to achieve homogeneity of variances (Levene's test) and approximately normal distributions (Shapiro–Wilk). Data were analysed by ANOVA using a randomized block design with the experiment as blocking factor. Post hoc comparisons are based on Tukey's honestly significant difference (HSD) test. All statistical tests were performed with SPSS (Statistical Package for the Social Sciences, IBM).
(e). Comparison of digoxin versus ouabain permeability
This experiment was performed to demonstrate the different permeability of the perineurium of D. nerii caterpillars for ouabain and digoxin. As incubation buffer, physiological saline without energy sources (PBS: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4; [26]) was used, otherwise the assay followed the procedures described above. Control nerve cords were incubated in buffer with 0.7 µM 3H-digoxin or 3H-ouabain only. In parallel, nerve cords were incubated with the labelled compounds plus CCCP (1 mM). CCCP was added to disable active transport processes and get an estimate of the amount of cardenolides infiltrating the nerve cord by diffusion.
(f). Immunohistochemistry
(i). P-glycoprotein-like transporter
Nerve cords of chilled D. nerii caterpillars (last instar) were dissected and immersed in PBS. Tissues were fixed for 1 h at room temperature in Lana's fixative (15% picric acid, 4% paraformaldehyde (PFA) in 0.5 M sodium phosphate buffer, pH 7; [15]). After fixation, tissues were washed three times for 10 min each in PBS and successively cryoprotected in 5, 10 and 15 per cent sucrose in PBS for 1 h each. Following cryoprotection tissues were embedded in optimal cutting temperature (OCT) compound (Sakura, Alphen aan den Rijn, The Netherlands) frozen in isopentane in liquid nitrogen and stored at −80°C until sectioning. Sections of 16 µm were cut on a Leica CM 1950 cryostat and allowed to dry at room temperature. Slides were stored at −80°C until use. The anti-PGP antibody C-219 (Abcam, Cambridge, UK; dissolved in PBS) was applied at a concentration of 10 µg ml−1. In the control sections, the primary antibody was omitted. The primary antibody was detected with the NOVADetect DAB (3,3′-diaminobenzidine)-Substrate Kit (Dianova, Hamburg, Germany). Stained sections were shortly washed with deionized water, transferred into 80 per cent ethanol (via 60% ethanol) and mounted in Euparal. Sections of ganglia were inspected under a Zeiss Axioskop 2 and photographed with a Zeiss AxioCam colour camera.
(ii). Na+K+-ATPase
To visualize the target site of cardenolides in the hawk-moth ganglia (M. sexta) we followed the methods described in [27] and [11]. For the specific detection of Na+/K+-ATPase in paraffin sections, we used the monoclonal antibody α5 (developed by D.M. Fambrough, maintained and distributed by the Developmental Studies Hybridoma Bank, University of Iowa, USA).
(g). Molecular phylogenetic analyses
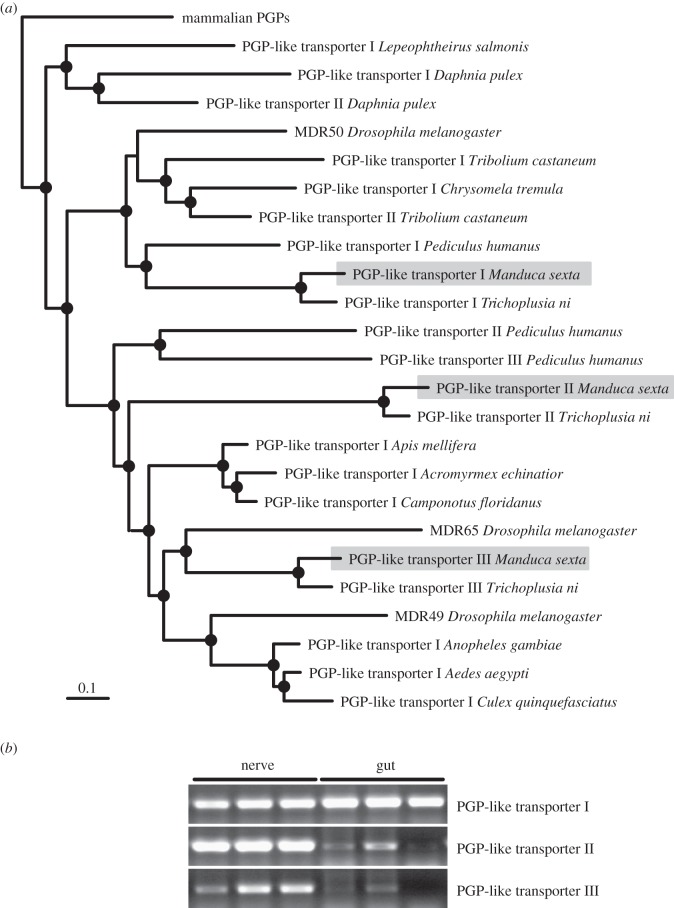
The coding sequences of three PGP-like transporters of Trichoplusia ni [28] were used to identify homologous M. sexta sequences in a collection of preassembled M. sexta ESTs (H. Vogel 2011, unpublished data). The corresponding amino acid sequences are given in the electronic supplementary material, figure S1. Available PGP-like transporters were downloaded from GenBank (accession date: May 2012), including the three PGP-like transporters of Drosophila melanogaster (MDR49, MDR50 and MDR65; [29,30]), as well as several PGP-like transporters of other insects and crustaceans. A complete sequence list including accession numbers is given in electronic supplementary material, table S1. The amino acid sequences were aligned with MAFFT using the G-INS-i routine [31] and the alignment was processed with Gblocks v. 0.91b [32]. Gblocks settings and the final alignment are given in electronic supplementary material, figure S2. Phylogenetic analyses were performed with MrBayes v. 3.1 [33] using the WAG model [34]. Metropolis-coupled Markov chain Monte Carlo sampling was performed with one cold and three heated chains. Two independent runs were performed for 1 million generations. Trees were sampled every 100th generation and posterior probabilities were estimated on the final 7500 trees (burnin = 2500). Mammal PGPs, which are known to be homologous to the PGP-like transporters of D. melanogaster [29,30] were used to root the phylogram for visualization purpose.
(h). RT-PCR
Total RNA was extracted from nerve cords (eight hindmost ganglia, tissues from 2–3 individuals pooled) and midguts of M. sexta caterpillars (last instar) with the RNeasy plus kit (Qiagen, Hilden, Germany). In both cases, three independent RNA extractions were performed (biological replicates). Amounts of RNA were assessed by reading the absorption at 260 nm and subsequently confirmed by denaturing gel electrophoresis. Equivalent amounts were transcribed into cDNA with Superscript III (Invitrogen, Darmstadt, Germany) using a combination of dT-17 and random hexamer primers. Amplification was performed using a standard protocol (Invitrogen Taq Polymerase; PCR: 95°C for 45 s, 52°C for 60 s, and 72°C for 60 s; 40 cycles). Gene specific oligonucleotide primers were: 5′-TTGACGGCAGTGTGACGATAG-3′ and 5′-CCTTCAGGAGACGTTTGCATC-3′ for M. sexta PGP-like transporter I, 5′-TCAAGATGTAGAGCCCGTGGT-3′ and 5′-CCAGCGGTAGTGAAGGTTGAG-3′ for M. sexta PGP-like transporter II, and 5′-TTCGGTGGCGCAGTTTATAGT-3′ and 5′-TCTTGTGCCCATCTTCTTTGC-3′ for M. sexta PGP-like transporter III. Primer specificity was confirmed by sequencing the corresponding PCR products (GATC, Konstanz, Germany).
3. Results
(a). Diffusion barrier
In our experiment with D. nerii caterpillars, we found a corresponding result to that described for M. sexta [17]: ouabain binding to the isolated D. nerii nerve cord linearly increased with time over the first 10 min of incubation in 3 M urea (figure 1b). After 10 min, the curve reached a plateau either indicating that a limit of permeabilization was achieved, or complete permeabilization of the diffusion barrier and saturation of the ouabain binding sites.
(b). Active barrier
In contrast to polar ouabain, the more lipophilic digoxin is known to permeate cell membranes [18]. Therefore, we assume non-polar cardenolides to necessitate active barrier mechanisms that prevent them from entering the nerve cord.
Application of the metabolic inhibitors (ionophores) 2,4-DNP and CCCP on the isolated nerve cord of M. sexta significantly enhanced tissue binding of 3H-digoxin (figure 2a) to 1.7-fold and 2.3-fold, respectively, of the untreated control. For D. nerii, we tested only the more effective inhibitor CCCP (figure 2c). Here, the increase of 3H-digoxin binding was even stronger than that in M. sexta (4.5-fold compared with 2.3-fold).
Figure 2.
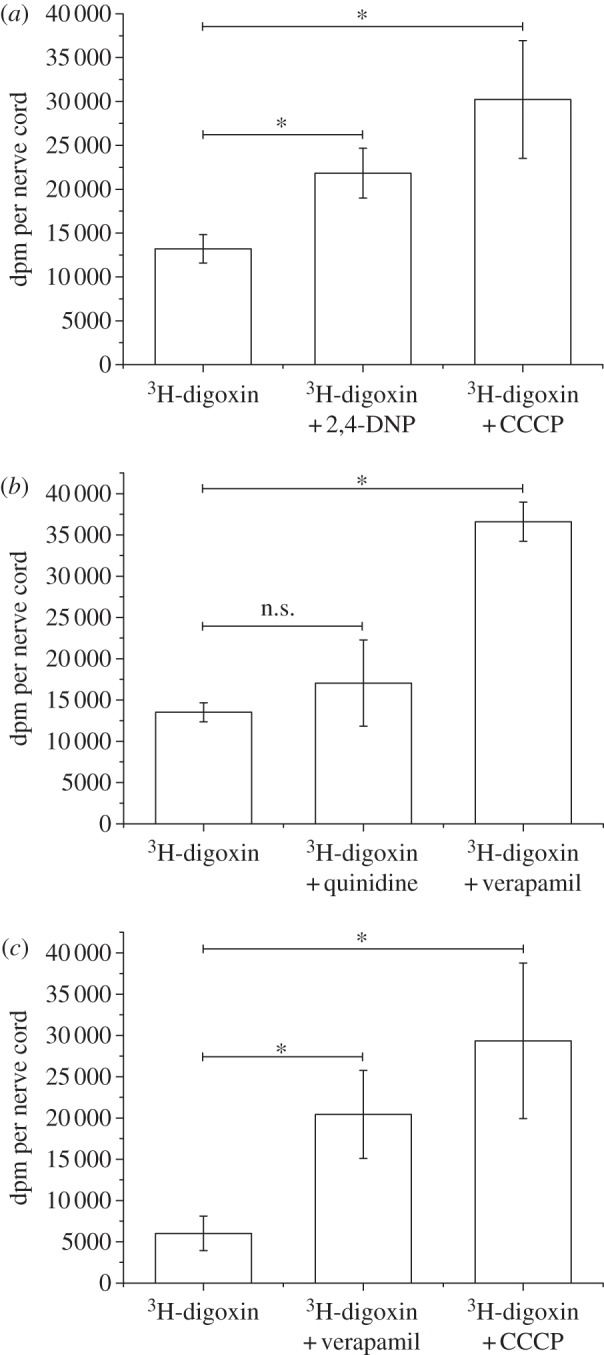
Influence of inhibitors on 3H-digoxin binding in isolated nerve cords: (a) effect of the metabolic inhibitors 2,4-DNP and CCCP on nerve cords of M. sexta (n = 5; mean±s.d.). (b) Effect of the PGP inhibitors quinidine and verapamil on nerve cords of M. sexta (n = 5; mean±s.d.). (c) Influence of the metabolic inhibitor CCCP and the PGP inhibitor verapamil on 3H-digoxin binding in isolated nerve cords of D. nerii (n = 6; mean±s.d.). Asterisks above horizontal lines indicate significant differences (p < 0.05, randomized block ANOVA with experiment as blocking factor, followed by Tukey HSD tests). Each data point represents the mean of 5 nerve cords per treatment (b and c) or 6 nerve cords per treatment (c) ±s.d.
To assess the involvement of a PGP-like transporter in the active barrier, the widely used PGP inhibitors verapamil and quinidine were tested for their effect on digoxin binding to the nerve cord. For M. sexta, verapamil enhanced 3H-digoxin binding to the nerve cord 2.7-fold. Quinidine produced a similar trend (figure 2b) which was, however, not significant. For D. nerii, only verapamil was applied which again significantly increased 3H-digoxin binding. Interestingly, as with CCCP, this effect was also stronger than in M. sexta (3.4-fold compared with 2.7-fold).
To exclude the possibility that verapamil simply permeabilizes the perineurium, M. sexta nerve cords were incubated with digoxin and digoxin plus verapamil or with ouabain and ouabain plus verapamil, respectively. If verapamil simply disrupted the passive diffusion barrier, the polar ouabain was also expected to gain access and bind to the nervous tissue to a larger extent upon verapamil treatment. This was, however, not the case (see the electronic supplementary material, figure S3).
(c). Comparison of digoxin versus ouabain permeability
When nerve cords of D. nerii were incubated with either cardenolide alone or in combination with CCCP, the latter significantly increased digoxin binding but not ouabain binding (figure 3). This supports our conclusion that digoxin can infiltrate the nerve cord by diffusion when active efflux carriers are disabled. The polar ouabain, however, is excluded by a diffusion barrier and its binding is not increased when the metabolic poison CCCP is applied. Interestingly, the active barrier was still functional in this experiment (and excluding most digoxin when not blocked by CCCP), although we here used PBS instead of Manduca saline. In contrast to the latter, the former lacks energy sources thus indicating that intrinsic energy levels are sufficient to maintain functionality of the efflux carriers for the duration of the experiment.
Figure 3.
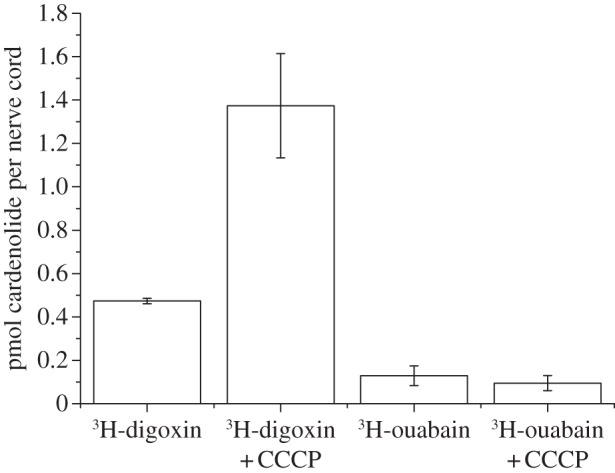
Diffusion of digoxin and ouabain into the nerve cord of D. nerii. As the two cardenolides have different specific activities, dpm values are not directly comparable. Data are, therefore, presented as pmol cardenolide/nerve cord (instead of dpm per nerve cord) to allow for quantitative comparisons. Nerve cords were incubated in equal concentrations of ouabain and digoxin, respectively (0.7 µM). Each data point is the mean of three nerve cords per treatment (i.e. 12 caterpillars were used in total).
(d). Immunohistochemical detection of a P-glycoprotein-like transporter
The widely used anti-PGP antibody C219 binds to a conserved epitope of the protein [35]. We applied this antibody to cryosections of ganglia from D. nerii caterpillars and found specific staining (brown precipitate) only in the periphery of the respective ganglion (figure 5a).
Figure 5.
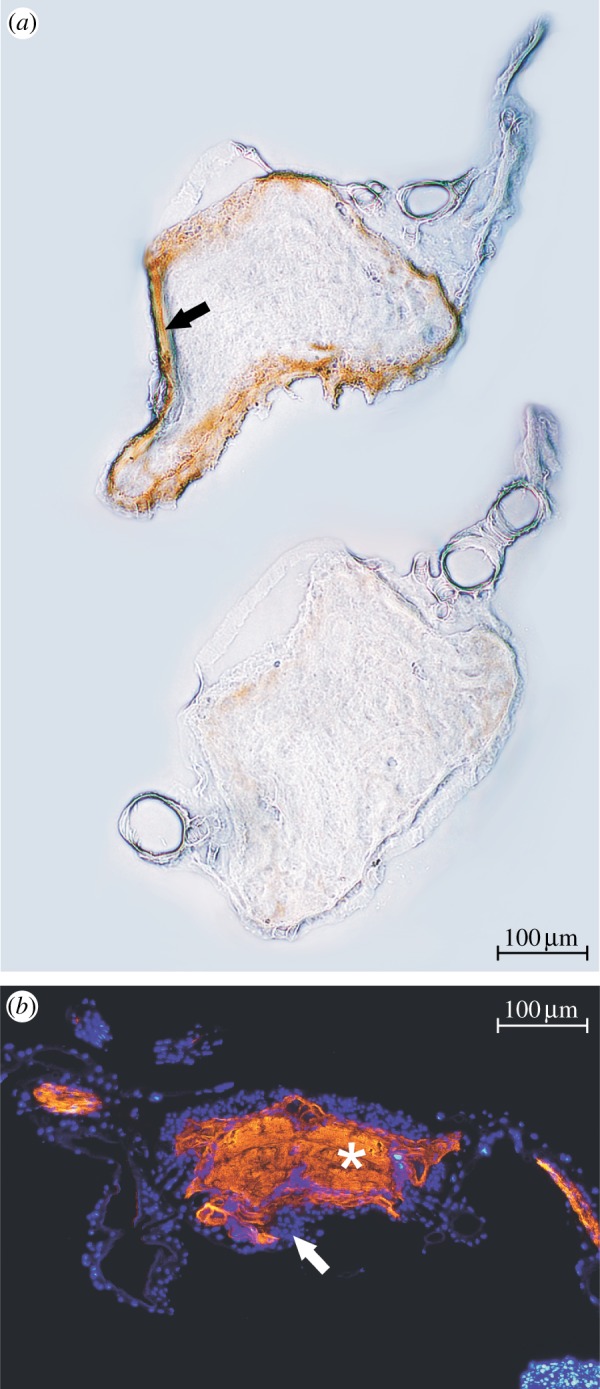
Immunohistochemical detection of a PGP-like transporter and Na+K+-ATPase on sections of larval lepidopteran ganglia: (a) Frozen section of a D. nerii ganglion treated with the anti-PGP antibody C219. Specific labelling (brown precipitation, black arrow) can be seen in the periphery of the ganglion. Above, treatment; below, control (primary antibody omitted). (b) Paraffin section of a ganglion of a M. sexta caterpillar. Orange (white asterisk): specific label of Na+K+-ATPase by the antibody α5. Blue (white arrow): nuclei stained with 4′,6-Diamidino-2-phenylindole dihydrochlorid (DAPI). (Online version in colour.)
(e). Identification of P-glycoprotein-like transporters expressed in the nerve cords
Three proteins with at least 75 per cent identity to either one of the PGP-like transporters of T. ni were identified in the ESTs of M. sexta. We here arbitrarily refer to these corresponding proteins as PGP-like transporters I, II, and III. The phylogenetic analysis (figure 4a) shows that the lepidopteran PGP-like transporters I form a monophyletic clade with D. melanogaster MDR50 and several other insect proteins (posterior probability 1.0). Lepidopteran PGP-like transporters III form a monophyletic clade with D. melanogaster MDR65 (posterior probability 0.97), which are in a sister group position with a monophyletic clade that among other insect proteins includes D. melanogaster MDR49 (posterior probability 1.0). Lepidopteran PGP-like transporters II are in a sister group position to a monophyletic clade, which comprises both D. melanogaster MDR49 and MDR65 (posterior probability 0.98). RT-PCR analyses show that all of the lepidopteran PGP-like transporters are expressed in the nerve cord of M. sexta, whereas in the gut a comparable strong expression appears to be restricted to PGP-like transporter I (figure 4b).
Figure 4.
Molecular characterization of M. sexta PGP-like transporters: (a) phylogenetic relationships among arthropod PGP-like transporters. Except for M. sexta, available sequences were retrieved from GenBank and one was arbitrarily chosen if several sequences per genus existed. In accordance with previous studies, the PGP-like transporters of D. melanogaster were named multi drug resistance (MDR) protein and the mammalian PGPs were used to root the phylogram [29,30]. Nodes with Bayesian posterior probabilities higher than 0.95 are indicated by filled black circles, and the scale bar equals 0.1 expected substitutions per site. (b) Detection of PGP-like transporter encoding mRNAs in nervous and midgut tissue of M. sexta (biological triplicates under identical PCR-conditions).
(f). Immunohistochemical detection of Na+K+-ATPase
Application of the monoclonal anti-Na+K+-ATPase antibody α5 revealed a strong signal in larval nerve cords of M. sexta ganglia (figure 5b). The occurrence of Na+K+-ATPase is apparently restricted to the neurons within the ganglion and no specific signal could be observed in the perineurium.
4. Discussion
In our study we focused on D. nerii and M. sexta, two closely related species that differ in their host plants and the secondary compounds they are typically exposed to. Whereas M. sexta is naturally not exposed to dietary cardenolides, D. nerii is an oleander specialist and encounters high concentrations of cardenolides of a wide polarity range in its natural diet, oleander. This species does not sequester cardenolides as do other specialists such as the monarch butterfly. The presence of only low amounts of cardenolides in the body [16] could be achieved by a relative impermeable gut membrane as has been observed in generalist insects such as Schistocerca and Periplaneta [1]. Such impermeability is not surprising in the case of polar cardenolides, which are unable to passively cross the gut membrane, yet in these species the guts are even impermeable to the markedly non-polar cardenolide digitoxin. As the Na+K+-ATPase of D. nerii is highly susceptible to cardenolides [10], additional mechanisms are needed to avoid even low amounts penetrating into the haemolymph.
An earlier study on cardenolide-adapted caterpillars revealed that Na+K+-ATPase is largely restricted to the caterpillars’ nervous tissue [11]. Accordingly, we found that D. nerii caterpillars can tolerate high levels of ouabain if injected into the larvae's body cavity [10]. We, therefore, postulated a mechanism that prevents cardenolides from reaching the Na+K+-ATPase within the nervous system and thus, focused here on the interface between cardenolide-containing haemolymph and the ventral nerve cord.
Our data revealed that ouabain gains access to the Na+K+-ATPase only when the nerve cord of D. nerii has been treated with urea. As urea is believed to disrupt the lepidopteran perineurium [17], our results provide evidence that the native, intact perineurium is not permeable for ouabain and we can assume that this applies also to other relatively polar cardenolides. Therefore, the target site of cardenolides, the Na+K+-ATPase, is shielded from polar cardenolides present in the haemolymph. We here observed a similar time-dependent increase of 3H-ouabain binding to the urea-treated nerve cord as described by Rubin et al. [17] for M. sexta to nerve cords of D. nerii, a species that actually has to cope with dietary cardenolides.
The diffusion barrier is probably constituted by the cells of the perineurium that form tight junctions [14] impeding the paracellular pathway for diffusing compounds. This barrier is most likely not selective for cardenolides, but represents a diffusion barrier for any polar compound. The ionic composition of the haemolymph would not be suitable for nervous function and thus, the perineurium is assumed to be responsible for the maintenance of the necessary ion concentrations in the nerve cord's extracellular space [14]. Therefore, the impermeability to ouabain of the perineurium of D. nerii is likely not a specialization. Nonetheless, the LD50 of injected ouabain for Schistocerca and Periplaneta is as low as 4.4 and 0.6 µg per individual, respectively [36]. This might mean that the perineuria of Schistocerca and Periplaneta are not as tight to ouabain as the one of the hawk-moths. However, the Na+K+-ATPase in these insects may not be restricted to the nervous tissue, but may also occur in other tissues, e.g. in the gut and the Malpighian tubules [37–39].
However, coping with dietary cardenolides also implies that non-polar forms have to be excluded from the nerve cord. To test for the existence of an active barrier for non-polar cardenolides in the hawk-moth perineurium, we used the relatively non-polar cardenolide digoxin, which is known to passively permeate cells. By the application of the ionophores CCCP and 2,4-DNP, we aimed at blocking the respiratory chain in our test tissue, thus interrupting the supply of ATP. Consistent with the notion of an active barrier that protects the Na+K+-ATPase, we found a higher binding of digoxin to the nerve cords when the metabolic inhibitors CCCP or 2,4-DNP were applied. This fits with the hypothesis of an active efflux mechanism: when the energy supply is depleted, digoxin can no longer be actively removed from the cells and reaches its target site.
Carriers of the PGP family are strong candidates to mediate the observed effect: in the mammalian brain they constitute the most important part of the blood–brain barrier by extruding infiltrating compounds [19]. Furthermore, Mayer et al. [40] demonstrated that PGP is responsible for excluding digoxin from the brain of wild-type mice. PGPs are members of the mdr gene family of which at least three genes are present in the Drosophila genome ([41] and references therein). In M. sexta (this study) and other lepidopteran species [28] three PGP-like transporters were identified which are homologous to those of D. melanogaster. All of these are expressed in the Manduca nerve cord, and are thus potential candidates for the efflux carriers evidenced here.
To test whether PGP-like transporters are involved in the energy-driven digoxin barrier, we incubated nerve cords of M. sexta and D. nerii with two of the most widely used PGP inhibitors, quinidine and verapamil. These compounds are known to elevate the plasma level of digoxin in humans when co-administered with this drug and this phenomenon is primarily attributed to the inhibition of PGP ([40], and references therein). Both in D. nerii and in M. sexta, the application of verapamil increased the amount of digoxin bound to the nervous tissue. These observations suggest that the efflux barrier for digoxin is mediated by a PGP-like transporter. When comparing the data of the two hawk-moth species, it is conspicuous that digoxin binding under control conditions is about twice as high in M. sexta as in D. nerii. At this point, however, it is difficult to judge whether this difference is due to quantitative or qualitative differences in the perineurial barrier of both species and there may also size differences between both species.
The presence of a PGP-like transporter is furthermore demonstrated by our immunohistochemical data that revealed specific binding of the anti-PGP antibody C219 in the periphery of D. nerii ganglia. The presence of this protein in the nervous system of M. sexta was already demonstrated [15] though on the whole, the data on the occurrence of PGP-like transporters in insects is limited. Our knowledge about the involvement of PGPs in the exclusion of plant compounds in herbivorous insects is still insufficient, yet the excretion of nicotine in Malpighian tubules of Manduca caterpillars [42] was already suggested to be based on a PGP homologue [43]. Especially, the wide substrate range suggests a potential key role for these transporters in the resistance of herbivorous insects to toxic secondary plant compounds. PGP-like transporters might, in addition, not only be part of the blood brain barrier, but also be responsible for rendering insect guts impermeable to some plant toxins. They could in theory enable generalist species to cope with a wide array of diverse toxic secondary plant compounds.
The mechanisms of cardenolide exclusion described here, however, may not be the only mode of resistance to cardenolides. It is known that cardenolides are metabolically modified within the insect body [16,44]. If regions of the molecule are affected, which mediate biochemical interactions, metabolism also results in detoxification. In addition, excretion by the Malpighian tubules can be expected to reduce haemolymph levels of cardenolides. The situation is further complicated by the fact that digoxin is not only substrate to PGP, but also to organic anion transporting polypeptides (Oatps) and potentially even additional carriers [45].
Thus, our picture of how specialists cope with cardenolides appears to be a diversity of strategies, with several often used together, but potentially with distinct combinations among different herbivores.
Acknowledgements
We want to express our sincere thanks to Heiko Vogel (Max Planck Institute for Chemical Ecology, Jena, Germany) for providing us with EST data on M. sexta. We are also indebted to Markus Huß and Martin Dransmann (University of Osnabrück, Germany) for the steady supply of eggs of Manduc a sexta. We furthermore thank Anurag Agrawal (Cornell University, Ithaca, USA) and Heiko Vogel for valuable comments on the manuscript and Lara Flacht (Hamburg, Germany) for technical support. The study was financially supported by a PhD scholarship of the Studienstiftung des deutschen Volkes to G.P., and by the Deutsche Forschungsgemeinschaft (Do527/5–1) to S.D.
References
- 1.Scudder GGE, Meredith J. 1982. The permeability of the midgut of three insects to cardiac glycosides. J. Insect Physiol. 28, 689–694 10.1016/0022-1910(82)90147-0 (doi:10.1016/0022-1910(82)90147-0) [DOI] [Google Scholar]
- 2.Despres L, David JP, Gallet C. 2007. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 22, 298–307 10.1016/j.tree.2007.02.010 (doi:10.1016/j.tree.2007.02.010) [DOI] [PubMed] [Google Scholar]
- 3.Luckner M, Wichtl M. 2000. Digitalis. Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft mbH [Google Scholar]
- 4.Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. 2012. Toxic cardenolides: chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol. 194, 28–45 10.1111/j.1469-8137.2011.04049.x (doi:10.1111/j.1469-8137.2011.04049.x) [DOI] [PubMed] [Google Scholar]
- 5.Schatzmann H-J. 1953. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv. Physiol. Pharmacol. Acta 11, 346–354 [PubMed] [Google Scholar]
- 6.Horisberger JD. 2004. Recent insights into the structure and mechanism of the sodium pump. Physiology 19, 377–387 10.1152/physiol.00013.2004 (doi:10.1152/physiol.00013.2004) [DOI] [PubMed] [Google Scholar]
- 7.Brower LP, Van Zandt Brower J, Corvino JM. 1967. Plant poisons in a terrestrial food chain. Proc. Natl Acad. Sci. USA 57, 893–898 10.1073/pnas.57.4.893 (doi:10.1073/pnas.57.4.893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaughan GL, Jungreis AM. 1977. Insensitivity of lepidopteran tissues to ouabain: physiological mechanisms for protection from cardiac glycosides. J. Insect Physiol. 23, 585–589 10.1016/0022-1910(77)90052-X (doi:10.1016/0022-1910(77)90052-X) [DOI] [Google Scholar]
- 9.Holzinger F, Wink M. 1996. Mediation of cardiac glycoside insensitivity in the monarch butterfly (Danaus plexippus): role of an amino acid substitution in the ouabain binding site of Na+K+-ATPase. J. Chem. Ecol. 22, 1921–1937 10.1007/BF02028512 (doi:10.1007/BF02028512) [DOI] [PubMed] [Google Scholar]
- 10.Petschenka G, Dobler S. 2009. Target-site sensitivity in a specialized herbivore towards major toxic compounds of its host plant: the Na+K+-ATPase of the oleander hawk moth (Daphnis nerii) is highly susceptible to cardenolides. Chemoecology 19, 235–239 10.1007/s00049-009-0025-7 (doi:10.1007/s00049-009-0025-7) [DOI] [Google Scholar]
- 11.Petschenka G, Offe JK, Dobler S. 2012. Physiological screening for target site insensitivity and localization of Na+/K+-ATPase in cardenolide-adapted Lepidoptera. J. Insect Physiol. 58, 607–612 10.1016/j.jinsphys.2011.12.012 (doi:10.1016/j.jinsphys.2011.12.012) [DOI] [PubMed] [Google Scholar]
- 12.Mebs D, Zehner R, Schneider M. 2000. Molecular studies on the ouabain binding site of the Na+, K+-ATPase in milkweed butterflies. Chemoecology 10, 201–203 10.1007/PL00001823 (doi:10.1007/PL00001823) [DOI] [Google Scholar]
- 13.Mebs D, Reuss E, Schneider M. 2005. Studies on the cardenolide sequestration in African milkweed butterflies (Danaidae). Toxicon 45, 581–584 10.1016/j.toxicon.2004.12.017 (doi:10.1016/j.toxicon.2004.12.017) [DOI] [PubMed] [Google Scholar]
- 14.Pichon Y, Sattelle DB, Lane NJ. 1972. Conduction processes in the nerve cord of the moth Manduca sexta in relation to its ultrastructure and haemolymph ionic composition. J. Exp. Biol. 56, 717–734 [DOI] [PubMed] [Google Scholar]
- 15.Murray CL, Quaglia M, Arnason JT, Morris CE. 1994. A putative nicotine pump at the metabolic blood–brain barrier of the tobacco hornworm. J. Neurobiol. 25, 23–34 10.1002/neu.480250103 (doi:10.1002/neu.480250103) [DOI] [PubMed] [Google Scholar]
- 16.Abe F, Yamauchi T, Minato K. 1996. Presence of cardenolides and ursolic acid from oleander leaves in larvae and frass of Daphnis nerii. Phytochemistry 42, 45–49 10.1016/0031-9422(95)00837-3 (doi:10.1016/0031-9422(95)00837-3) [DOI] [Google Scholar]
- 17.Rubin AL, Stirling CE, Stahl WL. 1983. 3H-ouabain binding autoradiography in the abdominal nerve cord of the hawk moth, Manduca sexta. J. Exp. Biol. 104, 217–230 [Google Scholar]
- 18.Petropoulos S, Gibb W, Matthews SG. 2010. Effect of glucocorticoids on regulation of placental multidrug resistance phosphoglycoprotein (P-gp) in the mouse. Placenta 31, 803–810 10.1016/j.placenta.2010.06.014 (doi:10.1016/j.placenta.2010.06.014) [DOI] [PubMed] [Google Scholar]
- 19.de Boer AG, van der Sandt ICJ, Gaillard PJ. 2003. The role of drug transporters at the blood–brain barrier. Annu. Rev. Pharmacol. Toxicol. 43, 629–656 10.1146/annurev.pharmtox.43.100901.140204 (doi:10.1146/annurev.pharmtox.43.100901.140204) [DOI] [PubMed] [Google Scholar]
- 20.Tanigawara Y, Okamura N, Hirai M, Yasuhara M, Ueda K, Kioka N, Komano T, Hori R. 1992. Transport of digoxin by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1). J. Pharmacol. Exp. Ther. 263, 840–845 [PubMed] [Google Scholar]
- 21.Lanning CL, Fine RL, Corcoran JJ, Ayad HM, Rose RL, Abou-Donia MB. 1996. Tobacco budworm P-glycoprotein: biochemical characterization and its involvement in pesticide resistance. Biochim. Biophys. Acta 1291, 155–162 10.1016/0304-4165(96)00060-8 (doi:10.1016/0304-4165(96)00060-8) [DOI] [PubMed] [Google Scholar]
- 22.Podsiadlowski L, Matha V, Vilcinskas A. 1998. Detection of a P-glycoprotein related pump in Chironomus larvae and its inhibition by verapamil and cyclosporin A. Comp. Biochem. Phys. B 121, 443–450 10.1016/S0305-0491(98)10137-2 (doi:10.1016/S0305-0491(98)10137-2) [DOI] [PubMed] [Google Scholar]
- 23.Johnston RM, Levine RB. 1996. Crawling motor patterns induced by pilocarpine in isolated larval nerve cords of Manduca sexta. J. Neurophysiol. 76, 3178–3195 [DOI] [PubMed] [Google Scholar]
- 24.Woods A, Chamberlin ME. 1999. Effects of dietary protein concentration on l-proline transport by Manduca sexta midgut. J. Insect Physiol. 45, 735–741 10.1016/s0022-1910(99)00050-5 (doi:10.1016/s0022-1910(99)00050-5) [DOI] [PubMed] [Google Scholar]
- 25.Horio M, Lovelace E, Pastan I, Gottesman MM. 1991. Agents which reverse multidrug-resistance are inhibitors of [3H]vinblastine transport by isolated vesicles. Biochim. Biophys. Acta 1061, 106–110 10.1016/0005-2736(91)90274-C (doi:10.1016/0005-2736(91)90274-C) [DOI] [PubMed] [Google Scholar]
- 26.Yasuhara JC, Baumann O, Takeyasu K. 2000. Localization of Na/K-ATPase in developing and adult Drosophila melanogaster photoreceptors. Cell Tissue Res. 300, 239–249 10.1007/s004410000195 (doi:10.1007/s004410000195) [DOI] [PubMed] [Google Scholar]
- 27.Patrick ML, Aimanova K, Sanders HR, Gill SS. 2006. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J. Exp. Biol. 209, 4638–4651 10.1242/jeb.02551 (doi:10.1242/jeb.02551) [DOI] [PubMed] [Google Scholar]
- 28.Labbé R, Caveney S, Donly C. 2011. Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol. Biol. 20, 243–256 10.1111/j.1365-2583.2010.01064.x (doi:10.1111/j.1365-2583.2010.01064.x) [DOI] [PubMed] [Google Scholar]
- 29.Wu CT, Budding M, Griffin MS, Croop JM. 1991. Isolation and characterization of Drosophila multidrug resistance gene homologs. Mol. Cell. Biol. 11, 3940–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerrard B, Stewart C, Dean M. 1993. Analysis of Mdr50: a Drosophila P-glycoprotein/multidrug resistance gene homolog. Genomics 17, 83–88 10.1006/geno.1993.1286 (doi:10.1006/geno.1993.1286) [DOI] [PubMed] [Google Scholar]
- 31.Katoh K, Kuma K-i, Toh H, Miyata T. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33, 511–518 10.1093/nar/gki198 (doi:10.1093/nar/gki198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17, 540–552 10.1093/oxfordjournals.molbev.a026334 (doi:10.1093/oxfordjournals.molbev.a026334) [DOI] [PubMed] [Google Scholar]
- 33.Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 10.1093/bioinformatics/17.8.754 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 34.Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699 10.1093/oxfordjournals.molbev.a003851 (doi:10.1093/oxfordjournals.molbev.a003851) [DOI] [PubMed] [Google Scholar]
- 35.van den Elsen JMH, Kuntz DA, Hoedemaeker FJ, Rose DR. 1999. Antibody C219 recognizes an α-helical epitope on P-glycoprotein. Proc. Natl Acad. Sci. USA 96, 13 679–13 684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore LV, Scudder GGE. 1986. Ouabain-resistant Na,K-ATPases and cardenolide tolerance in the large milkweed bug, Oncopeltus fasciatus. J. Insect Physiol. 32, 27–33 10.1016/0022-1910(86)90154-X (doi:10.1016/0022-1910(86)90154-X) [DOI] [Google Scholar]
- 37.Anstee JH, Bell DM. 1975. Relationship of Na+-K+-activated ATPase to fluid production by malpighian tubules of Locusta migratoria. J. Insect Physiol. 21, 1779–1784 10.1016/0022-1910(75)90240-1 (doi:10.1016/0022-1910(75)90240-1) [DOI] [PubMed] [Google Scholar]
- 38.Tolman JH, Steele JE. 1976. A ouabain-sensitive, (Na+-K+)-activated ATPase in the rectal epithelium of the american cockroach, Periplaneta americana. Insect Biochem. 6, 513–517 10.1016/0020-1790(76)90077-9 (doi:10.1016/0020-1790(76)90077-9) [DOI] [Google Scholar]
- 39.Peacock AJ. 1977. Distribution of Na+-K+-activated ATPase in the hindgut of two insects Schistocerca and Blaberus. Insect Biochem. 7, 393–395 10.1016/0020-1790(77)90043-9 (doi:10.1016/0020-1790(77)90043-9) [DOI] [Google Scholar]
- 40.Mayer U, Wagenaar E, Beijnen JH, Smit JW, Meijer DK, van Asperen J, Borst P, Schinkel AH. 1996. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr 1a P-glycoprotein. Br. J. Pharmacol. 119, 1038–1044 10.1111/j.1476-5381.1996.tb15775.x (doi:10.1111/j.1476-5381.1996.tb15775.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapadia MG, Lakhotia SC. 2005. Expression of mdr49 and mdr65 multidrug resistance genes in larval tissues of Drosophila melanogaster under normal and stress conditions. Cell Stress Chaperon 10, 7–11 10.1379/CSC-67R.1 (doi:10.1379/CSC-67R.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maddrell S, Gardiner B. 1976. Excretion of alkaloids by malpighian tubules of insects. J. Exp. Biol. 64, 267–281 [DOI] [PubMed] [Google Scholar]
- 43.Gaertner L, Murray C, Morris C. 1998. Transepithelial transport of nicotine and vinblastine in isolated malpighian tubules of the tobacco hornworm (Manduca sexta) suggests a P-glycoprotein-like mechanism. J. Exp. Biol. 201, 2637–2645 [DOI] [PubMed] [Google Scholar]
- 44.Seiber JN, Tuskes PM, Brower LP, Nelson CJ. 1980. Pharmacodynamics of some individual milkweed cardenolides fed to larvae of the monarch butterfly (Danaus plexippus L.). J. Chem. Ecol. 6, 321–339 10.1007/BF01402911 (doi:10.1007/BF01402911) [DOI] [Google Scholar]
- 45.Yao HM, Chiou WL. 2006. The complexity of intestinal absorption and exsorption of digoxin in rats. Int. J. Pharm. 322, 79–86 10.1016/j.ijpharm.2006.05.030 (doi:10.1016/j.ijpharm.2006.05.030) [DOI] [PubMed] [Google Scholar]