Abstract
Interleukin-4 (IL-4) and interleukin-10 (IL-10) are key cytokines whose increased production during systemic HIV infection has been associated with decreased cellular immunity during AIDS. We examined whether HIV-induced stimulation of IL-4 or IL-10 production leads to increased susceptibility to AIDS-related human cytomegalovirus retinitis. It was confirmed that there were increased amounts of IL-4 and IL-10 mRNA levels in mice with MAIDS of 10 weeks duration when most susceptible to MCMV retinitis. Surprisingly, however, MCMV-infected eyes of IL-4 −/− and IL-10 −/− mice with MAIDS of 8 weeks duration exhibited retinitis and infectious virus equivalent to that observed in MCMV-infected eyes of wild-type mice with MAIDS. We conclude that neither IL-4 nor IL-10 alone play a role in increased susceptibility to MAIDS-related MCMV retinitis, but may work collectively with other retrovirus-induced immunosuppressive factors to allow for retinal disease.
Keywords: cytomegalovirus, retinitis, AIDS, interleukin-4, interleukin-10, MAIDS
Introduction
The immune response is tightly regulated by different CD4+T-helper cell subsets. Mosmann and coworkers1,2 initially demonstrated that T-cell clones exhibit at least one of two distinct cytokine production profiles, including CD4+ T-helper type 1 (Th1) cells that produce Th1 cytokines such as interleukin-2 (IL-2) and interferon-γ (IFN-γ), and CD4+ T-helper type 2 (Th2) cells that produce Th2 cytokines such as IL-4, IL-6, and IL-10.2,3 In general, cytokines produced by Th1 cells stimulate macrophage and CD8+ T-cell activation, thereby promoting a response biased toward traditional cell-mediated immunity.2,4 In sharp contrast, cytokines produced by Th2 cells activate eosinophils and helper T-cell function for immunoglobulin 1 (IgG1) and immunoglobulin E (IgE) antibody production, thereby promoting a response biased toward traditional humoral immunity and suppression of cellular immunity.2,4 While the forces that determine the dominance of Th1 versus Th2 cells are not completely understood, it is clear that Th1 cells predominate in immunologically normal persons.3 During HIV infection, however, and the ensuing immune dysregulation that results during progression of AIDS, a unique shift takes place whereby a Th2 response ultimately predominates over a Th1 response.5 Thus, cellular immunity is greatly diminished and eventually lost during HIV-induced immunosuppression. In addition, during AIDS there is increased predisposition to a wide spectrum of opportunistic diseases including a sight-threatening retinitis caused by human cytomegalovirus (HCMV).6,7
AIDS-related HCMV retinitis presents clinically as a slowly progressive retinal disease characterized by full-thickness retinal necrosis that is associated with cytomegalic cells.6 While use of antiretroviral drugs (ARV) has significantly reduced the number of new cases of AIDS-related HCMV retinitis in the United States,8 its incidence in the HIV/AIDS populations of other countries remains high, largely due to the relative unavailability of ARV. For example, a conservative estimate of the incidence of AIDS-related HCMV retinitis in the HIV/AIDS populations of Thailand and Africa remains 33% and 20%, respectively.9–11 Thus, vision loss and blindness due to AIDS-related HCMV retinitis remains a major ophthalmologic challenge worldwide.
IL-4 and IL-10 are key Th2 cytokines whose production is stimulated systemically during AIDS (and therefore during AIDS-related HCMV retinitis) as a result of HIV-induced Th2 dominance. IL-4 is an immunomodulatory Th2 cytokine that promotes a number of diverse immunological functions through binding to its two-chain receptor (IL4R) that is expressed on both immune and non-immune cells.12,13 Although the immunological outcome(s) of IL-4 secretion varies remarkably depending on effector cell, target cell, and the microenvironment in which IL-4 is secreted,12,14,15 IL-4 promotes a number of diverse immunological functions that impact macrophage differentiation, the differentiation of CD4+ T cells into Th2 cells, and the inhibition of secretion of various inflammatory cytokines.3 In comparison, IL-10 is an anti-inflammatory Th2 cytokine that inhibits the production of INF-γ as well as other Th1 cytokines,3,16 an accomplishment achieved through binding of IL-10 to its two-chain receptor composed of an alpha and beta subunit.16,17
Of greater significance, however, are observations that CD8+ T-cell-mediated cytotoxicity is remarkably diminished at times of increased IL-4 and/or IL-10 production. For example, increased IL-4 production during HIV infection results in conversion of cytotoxic CD8+ T cells to CD8− T cells that also produce more IL-4, further suppressing the Th1 response.12,18,19 HIV infection also results in IL-10-producing CD8+ T cells that exhibit reduced cytolytic activity to HIV as well as other viruses including HCMV.20 Overexpression of IL-4 in mice also results in increased Fas ligand (FasL) expression on T cells and a concomitant decrease in perforin production, an important observation that suggests that IL-4 favors Fas/FasL-mediated cytotoxicity over perforin-mediated cytotoxicity.21 Similar results have also been observed during over-expression of IL-10.22,23 Taken together, these findings suggest a pivotal association between increased IL-4 and/or IL-10 production during retrovirus-induced immunosuppression and suppressed cellular immunity, especially cellular immunity involving perforin-mediated cytotoxicity.
We have been investigating the immunological aspects of the pathogenesis of AIDS-related HCMV retinitis using a well-characterized experimental mouse model of murine cytomegalovirus (MCMV) retinitis that develops in C57BL/6 mice with MAIDS,6 a murine retrovirus-induced immunodeficiency syndrome that remarkably mimics HIV-induced AIDS in humans.6 Importantly, like patients with AIDS, mice with MAIDS undergo a profound shift in cytokine production by CD4+ T-helper cells from a Th1 dominant phenotype to a Th2 dominant phenotype that commences 3 to 4 weeks after retrovirus infection.6 This critical immunological event during MAIDS leads to a progressive immunodeficiency characterized by abnormal CD8+ T-cell responses ∼8 weeks after retrovirus infection, that ultimately results in high susceptibility to several opportunistic diseases including MCMV retinitis. It is noteworthy that MAIDS-related MCMV retinal disease presents with histopathologic features at 8 to 10 days after MCMV infection that closely resemble those observed in AIDS-related HCMV retinitis, ie, development of a full-thickness retinal necrosis with cytomegalic cells that replaces the normal retinal architecture.6
Using our MAIDS model of MCMV retinitis, we demonstrated previously that loss of the perforin cytotoxic pathway is responsible for increased susceptibility to MCMV retinitis during MAIDS,24 and this increased susceptibility can be reversed by immunotherapy with the Th1 cytokine IL-2.25 Moreover, MCMV-infected eyes of MAIDS animals susceptible to MCMV retinitis contain high amounts of IL-4.26 These observations, coupled with those from other laboratories showing that either IL-4 or IL-10 favor the Fas/FasL cytotoxic pathway over the perforin cytotoxic pathway,21–23,27–31 lead to the attractive hypothesis that an increase in systemic production of IL-4 or IL-10 during retrovirus-induced immunosuppression is responsible for increased susceptibility to MCMV retinitis during MAIDS. To our surprise, however, this hypothesis proved to be incorrect since mice with MAIDS deficient in either IL-4 or IL-10 exhibited a frequency of retinitis, a severity of retinitis, and intraocular amounts of infectious virus equivalent to those found in wild-type mice with MAIDS.
Methods and Materials
Animals
Wild-type female C57BL/6 mice, IL-4 −/− female mice on a C57BL/6 background, and IL-10 −/− female mice on a C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in pathogen-free conditions, allowed unlimited access to food and water, and maintained in alternating 12-hour light/dark cycles. All animal procedures were conducted in accordance with Georgia State University Institutional Animal Care and Use Committee (IACUC) policies as well as the ARVO Statement for Use of Animals in Ophthalmic and Vision Research.
Viruses
Stocks of MCMV (Smith strain, American Type Culture Collection, Manassas, VA) and the murine retrovirus mixture (LP-BM5) (kindly provided by the AIDS Research and Reference Reagent Program, Germantown, MD) were prepared and stored as previously described.32
Induction of MAIDS
MAIDS was induced in 3-week-old wild-type mice, IL-4 −/− mice, and IL-10 −/− mice with the LP-BM5 retrovirus mixture by intraperitoneal injection as described previously.32 Mice with MAIDS of 4-weeks duration (MAIDS-4), 8-weeks duration (MAIDS-8), and 10-weeks duration (MAIDS-10) were used throughout the investigation.
Subretinal inoculation with MCMV
Eyes of wild-type MAIDS-8 mice, IL-4 −/− MAIDS-8 mice, and IL-10 −/− MAIDS-8 mice were inoculated with MCMV as described previously.32 Briefly, 2 μL of maintenance medium containing approximately 104 to 105 plaque-forming units (PFU) of MCMV was injected subretinally into the left eye of each mouse. The right eye of each mouse was injected subretinally with 2 μL of maintenance medium only and served as control for all investigations.
Quantitative real-time RT-PCR assay
Total ribonucleic acid (RNA) was extracted from whole splenic cells and from whole eyes using Trizol (Invitrogen, Grand Island, NY) coupled with the PureLink Micro-to-Midi Total RNA Purification System (Invitrogen, Grand Island, NY) following manufacturer’s instructions. Extracted total RNA was stored at −80 °C prior to processing. Extracted total RNA was subjected to reverse transcription following the SuperScript III First-Strand System for RT-PCR (Invitrogen) protocol. Complimentary DNA (cDNA) was stored at −20 °C prior to processing. cDNA from whole splenic cells and whole eyes from all animals was subjected to quantitative real-time RT-PCR to determine the amount of transcripts specific for murine IL-4, murine IL-10, murine perforin, murine granzyme B, and murine FasL (Qiagen, Valencia, CA). Murine GAPDH cDNA (Qiagen) served as endogenous control. Briefly, 1.2 μL of cDNA was added to a reaction mixture of 15 μL of Power SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA), 3 μL of primers for each gene (see Table 1), 9.9 μL of double-distilled water, and 0.9 μL of dimethylsulfoxide (Sigma, St. Louis, MO) for a total volume of 30 μL per reaction. Parameters for each quantitative RT-PCR assay cycle were 15 minutes at 95 °C, 15 seconds at 94 °C, 31 seconds at 55 °C, and 35 seconds at 72 °C for a total of 45 cycles. Transcription levels were determined utilizing the 7500 Fast Real-Time PCR System (Applied Biosystems), and average threshold cycles (Ct) were determined using the 7500 Fast Real-Time PCR System software (Applied Biosystems).
Table 1.
Sequences, annealing temperature, and expected fragment size of primers used in real-time RT-PCR assay.
| Gene (accession number) | Source | Primer sequence | Annealing temperature (°C) | Amp length (bp) |
|---|---|---|---|---|
| IL-4 (NM_021283) | Qiagen | QuantiTect primer assay | 55 | 104 |
| IL-10 (NM_010548) | Qiagen | QuantiTect primer assay | 55 | 103 |
| Perforin (NM_011073) | Qiagen | QuantiTect primer assay | 55 | 123 |
| Granzyme B (NM_013542) | Qiagen | QuantiTect primer assay | 55 | 149 |
| Fas Ligand (NM_010177) | Qiagen | QuantiTect primer assay | 55 | 99 |
ELISA
Whole splenic cells and whole eyes from all animals were collected, individually stored in liquid nitrogen, thawed, individually homogenized in 1.0 mL of phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Sigma), and individually stored at −20 °C prior to performance of ELISA. At time of ELISA, homogenates were thawed, sonicated, clarified by centrifugation, and the resulting supernatants were subjected to ELISA for quantification of murine IL-4 and IL-10 protein using the commercially available kit provided by eBioscience (San Diego, CA) according to manufacturer’s instructions. Total protein for each sample was determined using the Bradford Protein Assay (Biorad, Hercules, CA).
Histopathology
Whole eyes collected from all animals were immediately fixed in 10% buffered formalin (Electron Microscopy Sciences, Hartford, PA) for at least 48 hours at 4 °C, frozen in Optimal Cutting Temperature (OCT) medium (Thermo Scientific, Rochester, NY), cut into 8-μm sections using a Shandon Special Motorized Electronic Cryotome (Thermo Scientific), and sections were collected onto positively charged microscope slides (Thermo Scientific). Hematoxylin and eosin staining was performed as previously described32 with minor modifications. Ocular sections were scored for frequency and severity of retinitis using a scoring system established by us previously.32
Recovery and quantification of infectious MCMV
Whole MCMV-infected eyes were collected from wild-type MAIDS-8 mice, IL-4 −/− MAIDS-8 mice, and IL-10 −/− MAIDS mice at 10 days after sub-retinal injection and individually stored in liquid nitrogen prior to processing. At time of quantitative plaque assay, individual eyes were thawed, individually homogenized on ice in 1.0 mL of cold Delbecco’s Minimal Essential Medium (DMEM) (Cellgro, Manassas, VA), and clarified by centrifugation. Tenfold dilutions of the resulting supernatants were titered onto monolayers of mouse embryo fibroblast (MEF) cells in 6-well plates, allowed to adsorb for 1 hour at 37 °C in a humidified atmosphere of 5% CO2, overlaid with 1.0 mL DMEM, and incubated for 5 days at 37 °C in a humidified atmosphere of 5% CO2. Individual plaques were counted using an inverted light microscope, and results were expressed as the number of PFU per mL per whole eye (PFU/mL/eye).
Statistical analysis
All quantitative data obtained from quantitative real-time RT-PCR assay and ELISA were expressed as means ± standard error of the mean (SEM) and standard deviation (SD), respectively. At least two independent experiments per performed for each study. Statistical analysis was performed using the Wilcoxon-rank sum or Student t-test. A P value of <0.05 was considered significant.
Results
Quantification of splenic IL-4 and IL-10 messenger RNA levels in wild-type C57BL/6 mice during progression of MAIDS
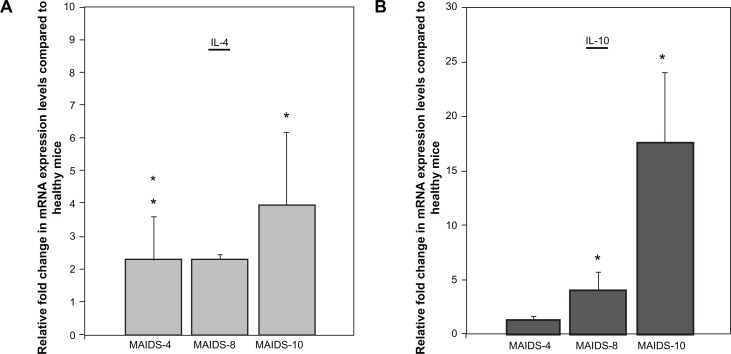
Early work by Gazzinelli and colleagues33,34 demonstrated that progression of MAIDS is associated with a shift in cytokine production by splenic CD4+ T cells from a Th1 profile to a Th2 profile as seen in HIV-infected patients with AIDS,5 and this shift commences at ∼3 weeks after retrovirus infection.6 We have shown previously that IL-4 messenger RNA (mRNA) levels increase significantly within the ocular compartment of MCMV-infected eyes of mice with MAIDS.26 Here, we sought to confirm that in the absence of MCMV infection our animals also exhibit a systemic increase in Th2 cytokines during the progression of MAIDS, especially for the Th2 cytokines IL-4 and IL-10 that have been associated with dampening of cellular immunity.21,24,27,28,31 Initial experiments using quantitative RT-PCR assay were therefore performed to measure IL-4 and IL-10 mRNA levels within splenic cells collected from wild-type C57BL/6 mice with MAIDS of 4-weeks duration (MAIDS-4), 8-weeks duration (MAIDS-8), and 10-weeks duration (MAIDS-10), but without ocular MCMV infection. Results are shown in Figure 1. Whereas splenic IL-4 mRNA levels increased nearly 2-fold in MAIDS-4 and MAIDS-8 animals, MAIDS-10 animals showed a significant 4-fold increase in splenic IL-4 mRNA levels (Fig. 1A). In comparison, splenic IL-10 mRNA levels also increased during progression of MAIDS, but this increase was evident later in the course of MAIDS and far greater than that seen for splenic IL-4 mRNA. Whereas no significant increase in IL-10 mRNA was observed in splenic cells collected from MAIDS-4 animals, splenic IL-10 mRNA levels were ∼4-fold and ∼17-fold higher in MAIDS-8 and MAIDS-10 animals, respectively (Fig. 1B). Thus, as expected,26,32,33 our mice with MAIDS did indeed exhibit increased systemic production of IL-4 and IL-10 mRNAs during progression of retrovirus-induced immunosuppression as measured using splenic cells, although the increase in splenic IL-10 mRNA levels was far greater than that of splenic IL-4 mRNA levels. Nonetheless, a significant increase in systemic mRNA levels to both Th2 cytokines was observed during MAIDS-8 and MAIDS-10, times during the course of retrovirus-induced immunosuppression when mice become susceptible to MCMV retinitis following subretinal infection.26,32
Figure 1.
IL-4 and IL-10 mRNA levels in whole splenic cells during progression of MAIDS (A) IL-4 mRNA levels in whole splenic cells during MAIDS progression versus healthy controls, P < 0.05 (n = 5) Error bars = Standard Error of Mean (SEM) of three independent experiments. Asterisks indicate statistical significance. (B) IL-10 mRNA levels in whole splenic cells during MAIDS progression versus healthy controls, P ≤ 0.03 (n = 5), error bars = SEM of two independent experiments.
Induction of MAIDS in mice deficient in IL-4 or IL-10
To determine if systemic reduction of IL-4 or IL-10 might lead to increased resistance to MCMV retinitis during MAIDS as hypothesized, we first attempted to induce MAIDS in IL-4 −/− mice and IL-10 −/− mice. Since IL-4 and IL-10 gene-deficient mice are not available, we elected to use IL-4 −/− and IL-10 −/− mice for these studies that possess a targeted mutation of the IL-4 gene or IL-10 gene. This mutation results in the production of truncated, non-functional IL-435 or IL-1036 protein products. Groups of IL-4 −/− and IL-10 −/− mice were therefore infected with the immunosuppressive retrovirus mixture LP-BM5, housed for 8 weeks, and assessed for development of MAIDS using criteria established by us previously.32 All retrovirus-infected IL-4 −/− and IL-10 −/− mice exhibited physical and immunological features consistent with development of MAIDS (data not shown), and were designated as IL-4 −/− MAIDS-8 mice and IL-10 −/− MAIDS-8 mice.
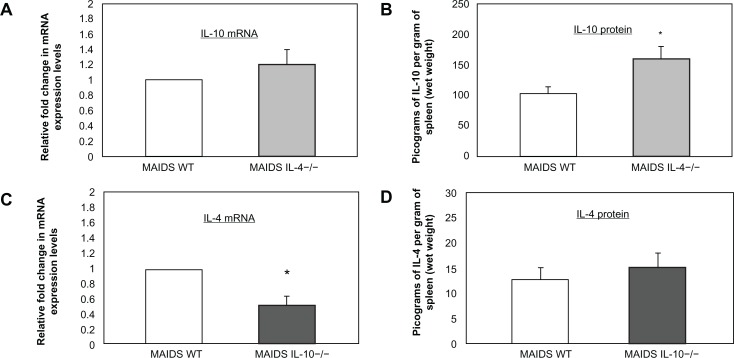
Additional studies were performed to confirm that splenic IL-10 mRNA levels were not affected in IL-4 −/− MAIDS-8 mice following subretinal MCMV infection. Conversely, splenic IL-4 mRNA levels were not affected in IL-10 −/− MAIDS-8 mice following subretinal MCMV infection. This finding was of interest since Green and coworkers37 noted that IL-10 −/− mice infected with LP-BM5 exhibited exaggerated disease development when compared with wild-type LP-BM5-infected C57BL/6 mice, an outcome that might affect splenic IL-4 mRNA production. Results are shown in Figure 2. While equivalent amounts of IL-10 mRNA levels were observed in splenic cells collected from wild-type MAIDS-8 mice and IL-4 −/− MAIDS-8 mice following intraocular MCMV infection, IL-10 protein levels were significantly increased in IL-4 −/− MAIDS-8 mice. In comparison, while splenic IL-4 mRNA levels were reduced by ∼50% in IL-10 −/− MAIDS-8 mice when compared with wild-type MAIDS-8 mice following intraocular MCMV infection, nearly equivalent amounts of IL-4 protein were found within splenic cells of both animal groups.
Figure 2.
IL-10 mRNA and IL-10 protein levels in whole splenic cells of IL-4 −/− MAIDS-8 mice versus respective controls and IL-4 mRNA and IL-4 protein levels in whole splenic cells of IL-10 −/− MAIDS-8 mice versus respective controls. (A) IL-10 mRNA levels in whole splenic cells of wild-type MAIDS versus IL-4 −/− MAIDS-8 mice, P = 0.432 (n = 10) Error bars = SEM of two independent experiments. (B) IL-10 protein levels in whole splenic cells of wild-type MAIDS versus IL-4 −/− MAIDS-8 mice, P ≤ 0.02 (n = 10), error bars = Standard Deviation (SD) of two independent experiments. Asterisks indicate statistical significance. (C) IL-4 mRNA in whole splenic cells of wild-type MAIDS versus IL-10 −/− MAIDS-8 mice, P ≤ 0.04 (n = 5) Error bars = SEM of one experiment. Asterisks indicate statistical significance. (D) IL-4 protein in whole splenic cells of wild-type MAIDS versus IL-10 −/− MAIDS-8 mice P = 0.939 (n = 8, wild-type MAIDS-8 mice; n = 10, IL-10 −/− MAIDS-8 mice), error bars = SD of one experiment.
Quantification of amounts of infectious MCMV within eyes of wild-type MAIDS-8 mice, IL-4 −/− MAIDS-8 mice, and IL-10 −/− MAIDS-8 mice following subretinal MCMV infection
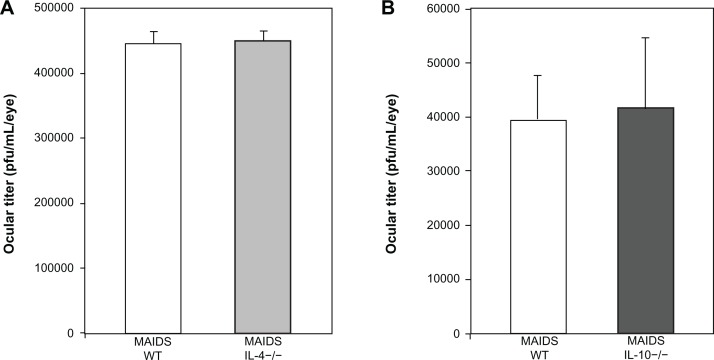
We have shown previously that the amounts of infectious MCMV within the ocular compartments of mice with MAIDS increase remarkably during development of retinitis following subretinal MCMV injection.6,26,32 Moreover, since increased IL-4 and IL-10 production would tend to delay virus clearance by dampening of CD8+ T-cell cytotoxicity, we sought to determine if loss of IL-4 or IL-10 would affect virus replication during MAIDS. Eyes of IL-4 −/− MAIDS-8 mice, IL-10 −/− MAIDS-8 mice, and wild-type MAIDS mice were therefore infected with MCMV by subretinal injection, collected 10 days later, and subjected to standard plaque assay for quantification and comparison of amounts of infectious virus. As shown in Figure 3, equivalent amounts of infectious virus were found in MCMV-infected eyes of IL-4 −/− MAIDS-8 mice, IL-10 −/− MAIDS-8 mice, and their respective wild-type MAIDS-8 controls. Thus, systemic loss of IL-4 or IL-10 during MAIDS did not appear to impact virus replication significantly within the eye, either positively or negatively.
Figure 3.
Ocular MCMV titer in wild-type MAIDS-8 mice, IL-4 −/− MAIDS-8 mice, and IL-10 −/− MAIDS-8 mice. (A) Average virus titer (expressed as PFU/eye/mL) in whole eyes of wild-type MAIDS-8 mice versus IL-4 −/− MAIDS-8 mice at 10 days after subretinal MCMV infection P = 0.658 (n = 5), error bars = SEM of one experiment. (B) Average virus titer (expressed as PFU/eye/mL) in whole eyes of wild-type MAIDS-8 mice versus IL-10 −/− MAIDS-8 mice at 10 days after subretinal MCMV infection P = 0.800 (n = 4), error bars = SEM of one experiment.
Frequency and severity of MCMV retinitis in IL-4 −/− MAIDS-8 mice and IL-10 −/− MAIDS-8 mice following subretinal MCMV infection
Since systemic loss of IL-4 or IL-10 had no effect of the amount of virus within the eyes of MAIDS-8 mice that have been shown previously by us to be susceptible to retinitis,6,32 we sought to determine whether systemic loss of IL-4 or IL-10 would result in increased resistance to retinitis as hypothesized. In separate experiments, IL-4 −/− MAIDS-8 mice, IL-10 −/− MAIDS-8 mice, and their respective wild-type MAIDS-8 controls were infected with MCMV by subretinal inoculation. Ten days later, all eyes were collected, analyzed histopathologically, and scored for frequency and severity of necrotizing retinitis using a scoring system described previously.32 Results are shown in Table 2. As expected, MCMV-infected eyes of wild-type MAIDS-8 control mice were indeed susceptible to retinitis as indicated by frequencies of retinitis of 78% and 100% in separate experiments (average = 89%). In sharp opposition to our hypothesis, however, MCMV-infected eyes collected from IL-4 −/− MAIDS-8 mice and IL-10 −/− MAIDS-8 mice exhibited a frequency of retinitis equivalent to that observed in control animals. Whereas 89% of MCMV-infected eyes of IL-4 −/− MAIDS-8 mice showed retinitis, 85% of IL-10 −/− MAIDS-8 mice also showed retinitis. Although frequency of retinitis was unaffected in MAIDS animals with a systemic loss of IL-4 or IL-10, it was possible that loss of these Th2 cytokines would result in a decrease in severity of retinal disease. This was not the case (Table 2). When scored for severity of retinitis,32 a statistical difference was not observed when MCMV-infected eyes of IL-4 −/− MAIDS-8 mice or MCMV-infected eyes of IL-10 −/− MAIDS-8 mice were compared with MCMV-infected eyes of their respective controls.
Table 2.
Frequency and severity of MCMV necrotizing retinitis in wild-type MAIDS-8 mice, IL-4 −/− MAIDS-8 mice, and IL-10 −/− MAIDS-8 mice at day 10 after subretinal MCMV infection.
| Group | Frequency of necrotizing retinitis (retinitis/total) | Severity score |
|---|---|---|
| Wild-type MAIDS-8 | 78% (7/9) | 2.94 (n = 7) |
| IL-4 −/− MAIDS-8 | 89% (8/9) | 3.24 (n = 8) |
| Wild-type MAIDS-8 | 100% (4/4) | 3.72 (n = 4) |
| IL-10 −/− MAIDS-8 | 85% (6/7) | 3.14 (n = 6) |
Quantification of splenic perforin, granzyme B, and FasL mRNA levels in IL-4 −/− MAIDS-8 mice and IL-10 −/− MAIDS-8 mice following subretinal MCMV infection
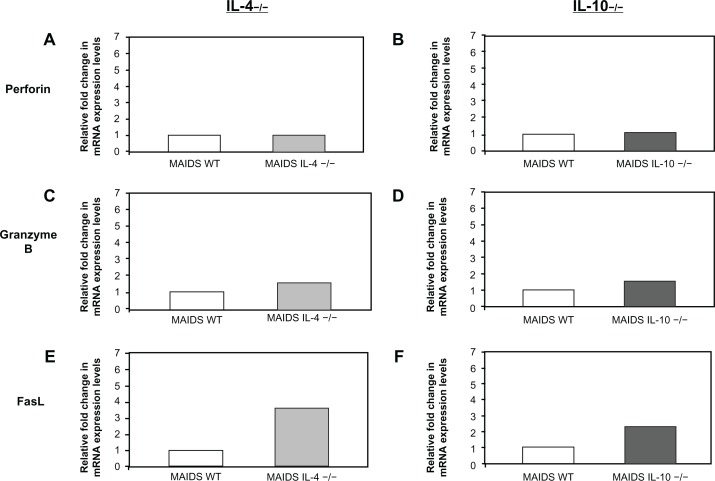
Since MCMV-infected eyes of mice with MAIDS deficient in systemic IL-4 or IL-10 failed to show a decrease in frequency or severity of retinitis as predicted, we were interested in knowing the fate of mRNAs of key molecules involved in CD8+ T-cell cytotoxicity in animals unable to produce functional systemic IL-4 or IL-10. These included perforin and granzyme B mRNAs associated with the perforin cytotoxic pathway3 and FasL mRNA associated with the Fas/FasL cytotoxic pathway.3 This finding was accomplished by measurement of perforin, granzyme B, and FasL mRNAs originating from splenic cells collected from IL-4 −/− MAIDS-8 mice and IL-10 −/− MAIDS-8 mice following subretinal MCMV infection, and comparing these amounts by quantitative RT-PCR assay with those obtained from wild-type MAIDS-8 mice following subretinal MCMV infection. As shown in Figure 4, amounts of splenic perforin and granzyme B mRNAs remained relatively unchanged in mice with MAIDS deficient in systemic IL-4 or IL-10 production when compared with wild-type MAIDS animals. Moreover, MAIDS mice deficient in systemic IL-4 or IL-10 production showed a significant increase in splenic FasL mRNA production, which was an unexpected finding.
Figure 4.
Perforin, granzyme B, and FasL mRNA levels in whole splenic cells of IL-4 −/− MAIDS-8 mice and IL-10 −/− MAIDS-8 mice versus respective controls. (A) Perforin mRNA levels in splenic cells of wild-type MAIDS-8 mice versus IL-4 −/− MAIDS-8 mice; P = 0.780 (n = 5), error bars = SEM of two independent experiments. (B) Perforin mRNA levels in splenic cells of wild-type MAIDS-8 mice versus IL-10 −/− MAIDS-8 mice; P = 0.49 (n = 5), error bars = SEM on one experiment. (C) Granzyme B mRNA levels in splenic cells of wild-type MAIDS-8 mice versus IL-4 −/− MAIDS-8 mice; P = 0.691 (n = 5), error bars = SEM of two independent experiments. (D) Granzyme B mRNA levels in splenic cells of wild-type MAIDS-8 versus IL-10 −/− MAIDS-8 mice; P = 0.22 (n = 5), error bars = SEM of one experiment. (E) FasL mRNA in splenic cells of wild-type MAIDS-8 mice versus IL-4 −/− MAIDS-8 mice; P ≤ 0.01 (n = 5), error bars = SEM of two independent experiments. (F) FasL mRNA in splenic cells of wild-type MAIDS-8 mice versus IL-10 −/− MAIDS-8 mice; P ≤ 0.007 (n = 5), error bars = SEM of one experiment.
Note: Asterisks indicate statistical significance.
Discussion
Since its appearance as a major cause of vision loss and blindness within the United States nearly 30 years ago, AIDS-related HCMV retinitis has become well characterized clinically and histologically.6,38 Despite many years of extensive clinical and laboratory investigation, however, a number of basic issues related to the virology, immunology, and pathogenesis of this sight-threatening disease within the unique immunosuppressive environment of HIV infection and disease remain unresolved. Among these unresolved issues is the lack of a crisp and comprehensive understanding of the basic immunological changes that take place during retrovirus-induced immunosuppression, especially those involved during loss of cellular immunity. Toward this end, we previously used our MAIDS model of MCMV retinitis to show that the perforin-mediated cytotoxic pathway is more important than the Fas/FasL-mediated cytotoxic pathway for protection against MAIDS-related MCMV retinitis.24 We24 and others39 showed that loss of the perforin cytotoxic pathway results in an increased susceptibility to MCMV-induced disease, including retinitis. Resistance to MCMV-related disease, however, could be restored by immunotherapy with the Th1 cytokine IL-2,25 a cytokine that has robust effects on cytotoxic T cell and natural killer (NK) cell functions.40 Unanswered in these investigations, however, was an understanding of the precise mechanism(s) by which the perforin cytotoxic pathway is diminished during MAIDS in favor of the Fas/FasL cytotoxic pathway. These findings, coupled with the fact that progression of MAIDS is also associated with a prominent shift in cytokine production by CD4+ T cells from a Th1 profile to a Th2 profile6 (Fig. 1) as seen during AIDS,5 lead to the attractive hypothesis that IL-4 or IL-10, both Th2 cytokines that have been shown to be involved in downregulation of cellular immunity,21,24,27,28,31 play key roles in the pathogenesis of MAIDS-related MCMV retinitis.
Surprisingly, results reported herein suggest that this is not the case, and our hypothesis is incorrect. In fact, MCMV-infected eyes of mice with MAIDS deficient in systemic IL-4 or IL-10 displayed a frequency of retinitis, a severity of retinitis, and intraocular amounts of virus equivalent to those found in MCMV-infected eyes of wild-type mice with MAIDS.
That IL-4 or IL-10 should play a pivotal role in loss of cellular immunity during the pathogenesis of MAIDS-related MCMV retinitis was a reasonable prediction. In addition to studies showing that increased IL-4 or IL-10 production during HIV infection results in decreased cytotoxic CD8+ T-cell activity against many viruses including HCMV,18–20,41 numerous in vitro and in vivo studies have also noted an association between elevated levels of the Th2 cytokine IL-4 and decreased CD8+ T-cell-mediated cytotoxicity, all associated with an increase in FasL expression and a concomitant decrease in perforin and granzyme B expression. For example, Kienzle and colleagues27,28 demonstrated that incubation of naïve mouse CD8+ T cells with high concentrations of IL-4 resulted in a population of T cells with significantly lower expression of CD8 and reduced cytotoxic ability. Aung and coworkers21 infected mice with an IL-4-expressing respiratory syncyital virus (RSV) recombinant and observed a subsequent increase in expression of FasL on CD4+ and CD8+ T cells coupled with alteration of the mechanism of CD8+ T-cell cytotoxicity from a perforin-mediated pathway to a favored Fas/FasL-mediated pathway. Moreover, systemic treatment of mice with anti-IL-4 antibody resulted in decreased morbidity following challenge with wild-type RSV due to an enhanced CD8+ cytotoxic response that lead to more effective virus clearance.21 Similar findings were reported by Jackson and colleagues42 for mousepox virus infection. These workers observed suppressed CD8+ T-cell cytotoxicity as well as suppressed NK-cell cytotoxicity in mice infected with an IL-4-expressing mousepox virus, outcomes that resulted in increased virus-induced mortality.
Despite this convincing body of evidence, we did not observe increased resistance to MCMV retinitis in mice with MAIDS in the absence of functional IL-4 or IL-10 production as hypothesized. Without functional IL-4 or IL-10, two Th2 cytokines thought to dampen cellular immunity during retrovirus-induced immunosuppression,18–20,23,41 we predicted a resurgence in CD8+ T-cell cytotoxicity that would provide protection against onset and progression of MCMV-induced retinal disease, possibly through stimulation of the perforin cytotoxic pathway associated with a concomitant decline in the Fas/FasL cytotoxic pathway. Surprisingly, this was not the case. In fact, loss of IL-4 or IL-10 during MAIDS resulted in no significant change in splenic mRNA levels for perforin or granzyme B, two key molecules involved in the perforin cytotoxic pathway, when compared with wild-type mice with MAIDS. More importantly, an increase in splenic mRNA levels for FasL was observed following loss of either IL-4 or IL-10 during MAIDS when compared with wild-type mice with MAIDS. One explanation for these unexpected results is that while IL-4 or IL-10 may work individually to suppress cellular immunity during MAIDS, they also function collectively toward this end. Our findings suggest that loss of IL-4 or IL-10 during MAIDS is an independent event that fails to affect production of the other Th2 cytokine. Thus, loss of one of these Th2 cytokines does not necessarily lead to a significant biological outcome as measured in our studies by a change in frequency or severity of MAIDS-related MCMV retinitis. One experimental approach to test this hypothesis would be to perform parallel studies using mice with MAIDS that suffer systemic loss in both IL-4 and IL-10 production. Unfortunately, all commercially available double knockout mice at present exist only on a BALB/c background, and MAIDS cannot be induced in BALB/c mice.43 Alternatively, we could reduce systemic levels of both IL-4 and IL-10 in mice with MAIDS using an antibody approach to neutralize their functions, but pilot studies to reduce systemic levels of IL-4 alone in mice with MAIDS using anti-IL-4 antibody have proven to be ineffective, resulting in only ∼50% reduction in splenic levels of IL-4 mRNA and protein (data not shown). Thus, future studies will be oriented toward production of IL-4 and IL-10 double knockout mice on a C57BL/6 background to explore the exciting possibility that these Th2 cytokines act in concert to increase susceptibility to MCMV retinitis during MAIDS.
It is noteworthy, however, that more recent findings from other laboratories have suggested that IL-4 can indeed support development of cytotoxic T cells when incubated with highly purified primary T cells collected from lymph nodes of BALB/c mice.44,45 Studies conducted by Bachmann and coworkers46 also demonstrated that IL-4 −/− C57BL/6 mice, when compared with wild-type C57BL/6 mice, failed to exhibit changes in cytotoxic T-cell responses when infected with lymphocytic choriomeningitis virus or vaccinia virus. These animals were also able to clear virus as a result of an effective cytotoxic T-cell response. Additional studies conducted by Mo and colleagues47 noted that CD8+ T cells collected from IL-4 −/− 129/J mice and wild-type 129/J mice exhibited equivalent cytotoxic activity against Sendai virus infection. It is important to recognize, however, that none of these studies investigated IL-4 and cellular immunity in the context of retrovirus-induced immunosuppression.
In summary, we show herein that mice with MAIDS deficient in either IL-4 or IL-10 do not develop resistance to MCMV retinitis as measured by amounts of intraocular infectious virus, frequency of retinitis, and severity of retinitis. Our findings therefore disprove our initial working hypothesis that IL-4 or IL-10 are key Th2 cytokines that promote increased susceptibility to MAIDS-related MCMV retinitis by dampening cellular immunity, possibly because they favor the Fas/FasL cytotoxic pathway over the perforin cytotoxic pathway. In hindsight, this was probably a naïve hypothesis since IL-4, IL-10, and many other host factors probably work in concert during MAIDS to create the unique intraocular environment that allows for pathogenesis of MCMV retinitis. Our present studies are oriented toward precise identification of these unique factors.
Footnotes
Funding
NIH Grant EY10568, NIH Core Grant P30 EY06360, and Fight for Sight, Inc.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Author Contributions
Conceived and designed the experiments: ELB, HC, RDD. Analyzed the data: ELB, HC, RDD. Wrote the first draft of the manuscript: ELB, HC, RDD. Contributed to the writing of the manuscript: ELB, HC, RDD. Agree with manuscript results and conclusions: ELB, HC, RDD. Jointly developed the structure and arguments for the paper: ELB, HC, RDD. Made critical revisions and approved final version: ELB, HC, RDD. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–57. [PubMed] [Google Scholar]
- 2.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 3.Paul WE. Fundamental Immunology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 4.Wan YY. Multi-tasking of helper T cells. Immunology. 2010 Jun;130(2):166–71. doi: 10.1111/j.1365-2567.2010.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14(3):107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 6.Dix RD, Cousins SW. AIDS-related cytomegalovirus retinitis: lessons from the laboratory. Curr Eye Res. 2004;29(2–3):91–101. doi: 10.1080/02713680490504641. [DOI] [PubMed] [Google Scholar]
- 7.Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philidelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 8.Arvin A, Campadelli-Fiume G, Mocarski ES Jr, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. [PubMed] [Google Scholar]
- 9.Heiden D, Ford N, Wilson D, et al. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med. 2007;4(12):e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabs DA. Cytomegalovirus retinitis and the acquired immunodeficiency syndrome—bench to bedside: LXVII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;151(2):198–216. e1. doi: 10.1016/j.ajo.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart MW. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol. 2010;4:285–99. doi: 10.2147/opth.s6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17(1):1–32. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 13.Keegan AD, Zamorano J. Regulation of gene expression, growth, and cell survival by IL-4: contribution of multiple signaling pathways. Cell Res. 1998;8(1):1–13. doi: 10.1038/cr.1998.1. [DOI] [PubMed] [Google Scholar]
- 14.Chomarat P, Banchereau J. An update on interleukin-4 and its receptor. Eur Cytokine Netw. 1997;8(4):333–44. [PubMed] [Google Scholar]
- 15.Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol. 1998;17(1–4):1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 17.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 18.Erard F, Dunbar PR, Le Gros G. The IL4-induced switch of CD8+ T cells to a TH2 phenotype and its possible relationship to the onset of AIDS. Res Immunol. 1994;145(8–9):643–6. doi: 10.1016/s0923-2494(05)80047-1. [DOI] [PubMed] [Google Scholar]
- 19.Erard F, Wild MT, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD8-CD4-cells that make TH2 cytokines and help B cells. Science. 1993;260(5115):1802–5. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 20.Elrefaei M, Ventura FL, Baker CA, Clark R, Bangsberg DR, Cao H. HIV-specific IL-10-positive CD8+ T cells suppress cytolysis and IL-2 production by CD8+ T cells. J Immunol. 2007;178(5):3265–71. doi: 10.4049/jimmunol.178.5.3265. [DOI] [PubMed] [Google Scholar]
- 21.Aung S, Graham BS. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity In vivo. J Immunol. 2000;164(7):3487–93. doi: 10.4049/jimmunol.164.7.3487. [DOI] [PubMed] [Google Scholar]
- 22.Dace DS, Khan AA, Stark JL, Kelly J, Cross AH, Apte RS. Interleukin-10over-expression promotes Fas-ligand-dependent chronic macrophage-mediated demyelinating polyneuropathy. PLoS One. 2009;4(9):e7121. doi: 10.1371/journal.pone.0007121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furukawa H, Oshima K, Tung T, Cui G, Laks H, Sen L. Overexpressed exogenous IL-4 And IL-10 paradoxically regulate allogenic T-cell and cardiac myocytes apoptosis through FAS/FASL pathway. Transplantation. 2008;85(3):437–46. doi: 10.1097/TP.0b013e31816026e7. [DOI] [PubMed] [Google Scholar]
- 24.Dix RD, Podack ER, Cousins SW. Loss of the perforin cytotoxic pathway predisposes mice to experimental cytomegalovirus retinitis. J Virol. 2003;77(6):3402–8. doi: 10.1128/JVI.77.6.3402-3408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dix RD, Giedlin M, Cousins SW. Systemic cytokine immunotherapy for experimental cytomegalovirus retinitis in mice with retrovirus-induced immunodeficiency. Invest Ophthalmol Vis Sci. 1997;38(7):1411–7. [PubMed] [Google Scholar]
- 26.Dix R, Cousins S. Murine cytomegalovirus retinitis during MAIDS: Susceptibility correlates with elevated intraocular levels of interleukin-4 mRNA. Curr Eye Res. 2003;26(3–4):211–7. doi: 10.1076/ceyr.26.3.211.14902. [DOI] [PubMed] [Google Scholar]
- 27.Kienzle N, Buttigieg K, Groves P, Kawula T, Kelso A. A clonal culture system demonstrates that IL-4 induces a subpopulation of noncytolytic T cells with low CD8, perforin, and granzyme expression. J Immunol. 2002;168(4):1672–81. doi: 10.4049/jimmunol.168.4.1672. [DOI] [PubMed] [Google Scholar]
- 28.Kienzle N, Olver S, Buttigieg K, et al. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8 low phenotype in the presence of IL-4. J Immunol. 2005;174(4):2021–9. doi: 10.4049/jimmunol.174.4.2021. [DOI] [PubMed] [Google Scholar]
- 29.Oshima K, Cui G, Tung T, Okotie O, Laks H, Sen L. Exogenous IL-10 over-expression reduces perforin production by activated allogenic CD8+ cells and prolongs cardiac allograft survival. Am J Physiol Heart Circ Physiol. 2007;292(1):H277–84. doi: 10.1152/ajpheart.00441.2006. [DOI] [PubMed] [Google Scholar]
- 30.Saito I, Haruta K, Shimuta M, et al. Fas ligand-mediated exocrinopathy resembling Sjogren’s syndrome in mice transgenic for IL-10. J Immunol. 1999;162(5):2488–94. [PubMed] [Google Scholar]
- 31.Baschuk N, Utermöhlen O, Gugel R, et al. Interleukin-4 impairs granzyme-mediated cytotoxicity of Simian virus 40 large tumor antigen-specific CTL in BALB/c mice. Cancer Immunol Immunother. 2007;56(10):1625–36. doi: 10.1007/s00262-007-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dix RD, Cray C, Cousins SW. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr Eye Res. 1994;13(8):587–95. doi: 10.3109/02713689408999892. [DOI] [PubMed] [Google Scholar]
- 33.Morse HC, 3rd, Giese N, Morawetz R, et al. Cells and cytokines in the pathogenesis of MAIDS, a retrovirus-induced immunodeficiency syndrome of mice. Springer Semin Immunopathol. 1995;17(2–3):231–45. doi: 10.1007/BF00196167. [DOI] [PubMed] [Google Scholar]
- 34.Gazzinelli RT, Makino M, Chattopadhyay SK, et al. CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J Immunol. 1992;148(1):182–8. [PubMed] [Google Scholar]
- 35.Kühn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254(5032):707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 36.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75(2):263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 37.Green KA, Okazaki T, Honjo T, Cook WJ, Green WR. The programmed death-1 and interleukin-10 pathways play a down-modulatory role in LP-BM5 retrovirus-induced murine immunodeficiency syndrome. J Virol. 2008;82(5):2456–69. doi: 10.1128/JVI.01665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland GN, Tufail A, Jordan MC. Cytomegalovirus diseases. In: Prepose JS, Holland GN, Wilhelmus KR, editors. Ocular Infection and Immunity. St. Louis: Mosby; 1996. [Google Scholar]
- 39.Loh J, Chu DT, O’Guin AK, Yokoyama WM, Virgin HW., 4th Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79(1):661–7. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 41.Elrefaei M, Barugahare B, Ssali F, Mugyenyi P, Cao H. HIV-specific IL-10-positive CD8+ T cells are increased in advanced disease and are associated with decreased HIV-specific cytolysis. J Immunol. 2006;176(2):1274–80. doi: 10.4049/jimmunol.176.2.1274. [DOI] [PubMed] [Google Scholar]
- 42.Jackson RJ, Ramsay AJ, Christensen CD, Beaton S, Hall DF, Ramshaw IA. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J Virol. 2001;75(3):1205–10. doi: 10.1128/JVI.75.3.1205-1210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartley JW, Fredrickson TN, Yetter RA, Makino M, Morse HC., 3rd Retro-virus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63(3):1223–31. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller CL, Hooton JW, Gillis S, Paetkau V. IL-4 potentiates the IL-2-dependent proliferation of mouse cytotoxic T cells. J Immunol. 1990;144(4):1331–7. [PubMed] [Google Scholar]
- 45.Trenn G, Takayama H, Hu-Li J, Paul WE, Sitkovsky MV. B cell stimulatory factor 1 (IL-4) enhances the development of cytotoxic T cells from Lyt-2+ resting murine T lymphocytes. J Immunol. 1988;140(4):1101–6. [PubMed] [Google Scholar]
- 46.Bachmann MF, Schorle H, Kühn R, et al. Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J Virol. 1995;69(8):4842–6. doi: 10.1128/jvi.69.8.4842-4846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mo XY, Sangster MY, Tripp RA, Doherty PC. Modification of the Sendai virus-specific antibody and CD8+ T-cell responses in mice homozygous for disruption of the interleukin-4 gene. J Virol. 1997;71(3):2518–21. doi: 10.1128/jvi.71.3.2518-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]