Abstract
Although numerous experimental investigations have evaluated the neurobehavioral effects of either short periods of total sleep deprivation or selective rapid eye movement sleep deprivation, few studies have examined the effects of chronic sleep restriction (CSR). Long-Evans rats were deprived of sleep by the automated movement of activity wheels for 18 h/day for 5 consecutive days from 16:00 to 10:00 h, and were allowed 6 h/day of sleep opportunity (10:00–16:00 h; lights on from 10:00 to 22:00 h). Activity wheels were intermittently activated on a 3 s on : 12 s off schedule for the CSR condition, whereas a schedule of 36 min of continuous wheel movement in every 3 h was used for a cage movement control condition. A cross-over design was used with rats serving in both the CSR and the movement control conditions with 2 days of rest between conditions. Water maze acquisition training occurred at 16:00 h immediately after the 6-h sleep opportunity on each of the first 4 days, followed by a probe trial on day 5 to assess spatial memory recall. Although the rate of learning/acquisition was not affected by the daily 18 h of CSR, the day 5 recall of the platform location was impaired on three different probe trial measures. Thus, CSR impaired spatial memory, but did not affect the rate of learning/acquisition in the water maze.
Keywords: animal model, behavior, cognition, hippocampus, learning, memory, sleep, sleep deprivation, sleep loss, water maze
Introduction
Many people in modern society sleep less than the recommended amount of time each day for vocational or lifestyle reasons. Significant impairments in cardiovascular, immune, endocrine, and cognitive functions have been reported in humans who sleep 2–3 h less than their normal sleep time, even for only a few consecutive days (for a review [1,2]). In fact, the impairments in humans produced by 2 weeks of sleep restriction (e.g. 6 h of sleep/night) on psychomotor vigilance response times are comparable with the deficits caused by two nights of total sleep deprivation (SD) [3]. Epidemiological studies suggest that habitual short sleep duration is associated with increased obesity [4], heart disease [5], and mortality [6].
Although rodent models of chronic sleep restriction (CSR) have recently been developed [7–10], experimental research with models of CSR lags far behind the considerable body of work published on the neurobehavioral effects of total SD or on selective rapid eye movement (REM) SD (for a review, see McCoy and Strecker [11]). To our knowledge, only two studies have assessed the effect of CSR on spatial learning and memory in the water maze [12,13]. Although both studies used small amounts of daily SD, behavioral impairments were observed. Hairston et al. [12] found that mild daily SD (6 h/day for 4 days) impaired spatial learning, whereas performance on a nonspatial task was actually improved. Probe trials were not conducted in this study and thus, memory for the hidden platform was not assessed. More recently, Yang et al. [13] reported evidence for deficits in spatial learning as well as memory with 4 h/day of SD for 7 days. However, these behavioral deficits were observed only in adolescent rats and not in adult rats. In both studies, the mild degree of SD allowed plenty of time for rats to gain daily recovery sleep. As memory for the hidden platform was either not assessed [12] or was unaffected in adult rats [13], the present study evaluated the effects of a more severe schedule of CSR; SD in the present study lasted 18 h/day, followed by a 6-h sleep opportunity (SO) each day for 5 consecutive days.
As water maze training occurred after the daily 6 h of SO, it was predicted that CSR would not interfere with the learning/acquisition of the platform location as animals were ‘rested’ during this training. However, as the animals experienced the daily SD immediately after the acquisition training, it was predicted that the CSR manipulation would interfere with spatial memory for the hidden platform location, possibly by interfering with memory consolidation.
Materials and methods
Animals
Twenty-two adult male Long-Evans rats (280–350 g; Charles River, Wilmington, Massachusetts, USA) were housed under constant temperature (23°C) and a 12 : 12 light : dark cycle (lights on at 10:00 h). In pilot studies, untreated Sprague–Dawley rats showed poor initial learning of the platform location (rendering evaluating memory for the platform location problematic); in contrast, Long-Evans control rats learned the water maze to asymptotic levels in less than 4 days with four acquisition trials/day. Hence, Long-Evans rats were used in this study. Rat strain differences in cognitive performance are well known [14,15]. All animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care policy on the care and use of laboratory animals. All procedures were approved by the Institutional Animal Care and Use Committee of the Boston VA Healthcare System. Food and water were available ad libitum. Experimental animals were housed in activity wheels and habituated to wheel movement for 2 days before the experiment. Day 1 of habituation consisted of 2 h in the activity wheels with no movement. Day 2 of habituation consisted of 2 h in the activity wheels with movement (i.e. 3 s on : 12 s off).
Chronic sleep restriction
CSR consisted of 18 h/day of total SD from 16:00 to 10:00 h for 5 days. CSR began the day before the first day of water maze training and ended 5 days later when spatial memory recall was assessed with the probe trial. On days 1–4 of CSR (water maze acquisition/learning days), rats were placed in activity wheels immediately after training. The CSR condition consisted of an activity wheel schedule of 3 s on : 12 s off for the 18 h. This procedure reduces the rats’ total daily sleep time to under 6 h/day [7], whereas rats normally sleep 10–12 h/day. The movement control (MC) condition consisted of an activity wheel schedule of 36 min of continuous wheel movement every 3 h, allowing opportunities for consolidated sleep while controlling for the nonspecific effects of cage movement and potential exercise. After 18 h in the activity wheels, rats were placed in their home cages for 6 h SO from 10:00 to 16:00 h.
To minimize the number of animals required for the study, a cross-over design was utilized with rats serving in both the CSR condition and the MC condition during separate 5-day periods; two groups of rats (N = 11/group) were run in this manner, referred to as part 1 and 2 in the statistical analysis. Thus, the study was carried out over a 4-week period with rats that were exposed to the CSR condition during week 1 (i.e. the first 5-day period) assigned to the MC condition during week 2 (i.e. the second 5-day period). Conversely, animals in the MC condition during week 1 were exposed to the CSR protocol during week 2. The two identical 5-day periods were separated by 2 days of uninterrupted SO in the rats’ home cages.
Water maze: acquisition training and memory testing
All animals were trained in a pool 2.0 m in diameter and 0.4 m in depth, containing water made opaque by nontoxic, water-soluble paint at room temperature (23°C). The pool was in a 3 × 5 m room with several distinctive spatial cues (e.g. signs, laboratory furniture). A 10 cm diameter platform was submerged ~1 cm below the surface of the pool to allow the animals to escape the water. During data analysis, a 20 cm target-platform zone was used to assess behavior to capture near-miss behavior. Rodent performance was tracked using a video tracking system (EzVideo Multi Track System; AccuScan, Columbus, Ohio, USA).
The investigator training the rats in the water maze was kept blind to the treatment condition (CSR or MC) of each rat. In the water maze protocol, each rat underwent four trials/day of water maze acquisition from 16:00 to 18:00 h for 4 consecutive days with an intertrial interval of ~20 min. All animals were observed in between trials to ensure that they were awake, and gentle handling (noise, tapping on the cage, light tactile stimulation) was used to maintain wakefulness when necessary. In each trial, rats were placed in the water maze facing the wall in one of three quadrants that did not contain the hidden platform. The starting position was in a semirandom order so that no start point was immediately repeated. The location of the hidden platform remained constant. If the rat did not find the hidden platform within 60 s, the rat was guided manually to the platform by the experimenter and allowed to remain on it for ~10 s before being placed in a holding cage for an additional 60 s.
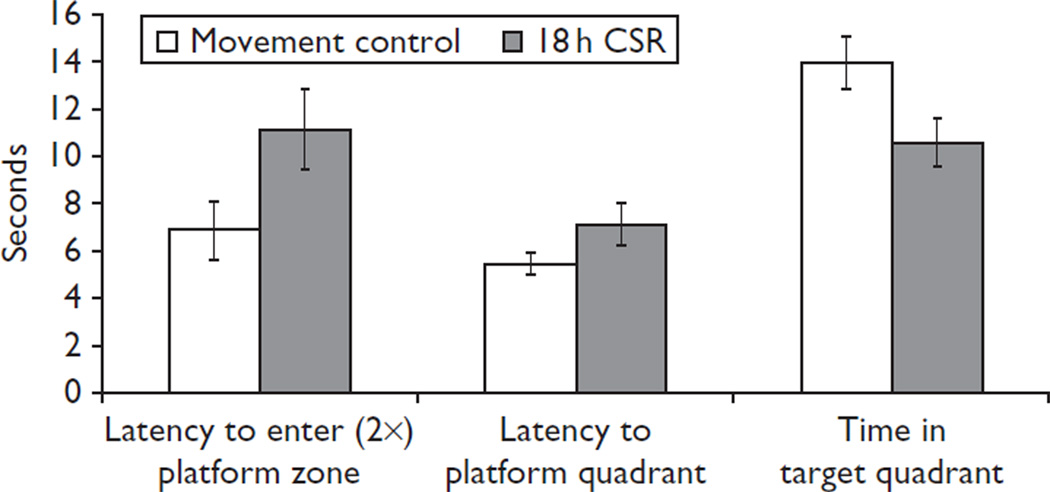
On the fifth day, each rat was subjected to a single water maze probe trial to test for retention (i.e. memory) for the location of the hidden platform. The hidden platform was removed on probe trials. A probe trial consisted of a 30 s free swim time, after which the rodent was removed from the pool. Probe trials occurred at 10:00 h. The primary measures of performance during probe trials were as follows: (a) latency to enter the (2×) platform zone, (b) latency to enter the platform quadrant, and (c) time in the target quadrant.
Statistical analysis
On acquisition trials, a four-way mixed-model analysis of variance (ANOVA) was carried out on the latency to enter the target-platform zone. Specifically, the design called for a 2 (part) × 4 (days) × 4 (swims) × 2 (sleep status) ANOVA with repeated measures on the last three variables. ‘Part’ refers to the fact that this study was carried out in two parts with a cross-over design (described above), with half (n = 11) going through the 2-week experimental procedure first, followed by a second set of animals (n = 11) during the subsequent 2-week period. On probe trials, paired samples t-tests were carried out on (a) latency to enter the (2×) platform zone, (b) latency to enter the platform quadrant, and (c) time in the target quadrant.
Results
Acquisition trials to assess spatial learning
Learning the platform location over the 4 days of acquisition training was unaffected by CSR. The 2 × 4 × 4 × 2 ANOVA on the acquisition data showed the following: the main effect of sleep status was not significant, indicating that CSR and MC rats acquired the platform location at the same rate [F(1,20) = 0.043, P = 0.838]. The main effect of days was significant; thus, rats (irrespective of sleep status) entered the target-platform zone faster from days 1 to 4 of acquisition (collapsed across swims) [F(1,20) = 54.67, P < 0.0001]. The main effect of swims was significant [F(1,20) = 69.96, P < 0.0001], indicating that rats (irrespective of sleep status) entered the target-platform zone faster across the four swims that occurred each day (collapsed across days). The interaction effect of days × swims was significant because the latency to reach the target-platform zone decreased over the four swims to a greater degree on day 1 than it did on day 4 [F(1,20) = 17.25, P < 0.0001]. All other overall interaction effects based on analysis of the acquisition data were not significant.
To assess learning across the acquisition days, the latency to reach the target quadrant on the first trial of each day was analyzed using a 2 × 2 ANOVA with repeated measures on the day factor. No significant effects were indicated by this analysis. However, t-tests (one tailed) showed a trend toward longer latencies to reach the target quadrant in CSR rats on the first swim trial of day 3 (P = 0.069) and on the first swim trial of day 4 (P = 0.073). This trend is consistent with the day 5 probe trial data assessing memory of platform location described next.
Probe trials to assess spatial memory
CSR rats had impaired recall of the platform location on the probe trial, which was significant on three measures of probe trial performance (Fig. 1). There was a significant difference in the latency to enter the (2×) platform zone [t(21) = 2.432, P = 0.012], indicating that CSR rats took longer to reach the platform zone, compared with the MC rats. CSR rats also took significantly longer to enter the quadrant that contained the platform [t(21) = 2.31, P = 0.016]. Finally, rats in the CSR condition spent significantly less time in the target quadrant than the animals in the MC condition [t(21) = 2.334, P = 0.015]. No other measures were significant.
Fig. 1.
CSR impairs spatial memory of the water maze platform location. Memory was assessed on CSR day 5 with a probe trial. Latency to enter the (2×) platform zone was significantly longer in animals in the CSR condition compared with animals in the movement control condition (P = 0.012). Latency to reach the quadrant containing the platform was significantly longer in animals in the CSR condition compared with animals in the movement control condition (P = 0.016). Animals in the CSR condition spent significantly less time in the target quadrant than animals in the movement control condition (P = 0.015). CSR, chronic sleep restriction.
Discussion
As predicted, rats exposed to CSR for 4 days learned the water maze at the same rate as MC rats, but their memory (recall) of the platform location trial was impaired on the fifth day compared with MC rats. The findings are interpreted to indicate that CSR produced a spatial memory deficit. The use of a cross-over design showed that differences in latency to the platform on probe trials were not simply a function of practice over time, nor were they a function of a particular group of rats being consistently slower. Rather, the longer latencies on probe trials were specifically a function of the manipulation; the group receiving CSR on a given week was the group that showed the longer latencies that week.
Until recently, there have been few experimental investigations (either in animals or in humans) that have studied the physiological and neurobehavioral effects of CSR. The importance of identifying the effects of CSR is underscored by the increased prevalence of CSR in modern societies because of lifestyle factors (e.g. shift work, jet lag), specific sleep disorders, and the multitude of medical conditions that are accompanied by a reduction in daily sleep time [1,2].
Previous attempts to develop an animal model of the cognitive effects of CSR have used only mild levels of sleep restriction [12,13]. Hence, it is not surprising that memory was unaffected in one of these studies [13], and only learning (not memory) was assessed in the other [12]. Water maze impairments have been observed following other types of experimental sleep manipulations, such as SD or sleep fragmentation (see McCoy and Strecker [11] for a review). Most of these experiments have evaluated acute periods (e.g. 24–72 h) of sleep disturbance [11], accentuating the need for experimentation using CSR paradigms.
Evidence from both human [16] and animal [11] studies implicates important roles for both non-REM (NREM, also known as slow wave sleep) and REM sleep in learning and memory. Some evidence suggests that NREM may be more important for the consolidation of declarative memories, which require conscious recall. The term ‘consolidation’ here refers to the incorporation of newly learned material into long-term (or reference) memory. Conversely, REM sleep may be more important for the consolidation of procedural memories, which are implicit and hence do not require conscious recall [17]. Reactivation of firing of hippocampal ensembles of neurons during sleep (after a learned experience) has been observed and is postulated to represent the consolidation of labile memory traces into more stable forms of memory [18]. High-frequency oscillations that are interspersed throughout periods of NREM sleep have a relatively short time scale (milliseconds to seconds), suggesting a role in the initial encoding of a memory [18]. In contrast, neuronal replay of hippocampal ensemble activity during REM sleep lasts much longer (i.e. tens of seconds to a minute) and may reflect later stages of memory consolidation [19].
Increases in NREM and REM sleep time as well as NREM electroencephalographic (EEG) slow wave activity (SWA) during the sleep opportunities that follow acute SD are widely thought to reflect an increase in the homeostatic sleep drive and to represent an increase in sleep depth. However, when rats were exposed to 5 days of CSR, Kim et al. [7,8] only found a significant increase in SWA during the SO on day 1 (analogous to acute SD), but not on days 2–5 of CSR. In human studies of CSR, it is not clear whether EEG delta power is homeostatically regulated. One recent study found only a slight increase in EEG SWA during the first SO that followed 20 h of SD [20] and another study found no increase [21]. The absence of a robust SWA rebound after the first day of CSR makes it difficult to interpret the similarity in the levels of SWA across days 1–5.
Even mild exposure to CSR (6 h/day of SD for 4 consecutive days) has been shown to selectively impair spatial learning in rats [12]. As both improvements (in the case of the nonspatial task) and impairments in performance accompanied this schedule of sleep restriction, the present study evaluated rats under a more severe sleep restriction schedule (i.e. 18 h/day of CSR). Another recent experiment compared the cognitive performance of adolescent rats to adult rats using 4 h of SD immediately after daily water maze training. Impaired spatial learning was observed, but only in the adolescent rats [13]. The adolescent rats showed both REM and NREM sleep rebound during the SO that followed, and yet, still, a behavioral deficit was apparent. These authors concluded that there may be a critical time period after training during which sleep is necessary for optimal memory consolidation. However, the adult rats showed no such behavioral changes, nor did the adult rats show the same increase in the intensity or the quality of sleep observed in the adolescents [13]. Here, we report an impairment in spatial memory in adult rats, but our animals were subjected to a much more severe daily schedule of SD (18 h/day of SD). Taken together, these findings suggest that multiple days of partial SD can impair memory consolidation in adult rats, although adult rats may be less sensitive to short deprivation periods, in comparison with adolescent rats. Although a spatial memory deficit was detected with the CSR regimen used herein, further research needs to be carried out to identify the minimal conditions required to impair performance on probe trials and to determine whether the memory impairment is permanent. Moreover, the question of brain regions and mechanism(s) mediating the amnestic effects of CSR remains to be addressed.
One possible neurochemical mediator of the behavioral effects observed is adenosine, an inhibitory neuromodulator and putative endogenous hypnogen. For example, findings suggest that adenosine mediates the deficits in attention that accompany acute SD. Specifically, 300 µM adenosine infused into the local basal forebrain slowed response latency and increased lapses on the rat psychomotor vigilance test (PVT), an animal analogue of the human PVT [22]. This effect mimicked the effect of 24 h of SD on performance in the rat PVT. Extracellular levels of adenosine have been shown to increase in the basal forebrain when the duration of wakefulness is extended experimentally in rats [23]. Moreover, adenosine acts to inhibit acetylcholine-containing neurons (reviewed in Brown et al. [24]) that project from the basal forebrain to the frontal cortex (as well as other cortices). These cholinergic neurons are well known to play important roles not only in attention but also in higher cognitive processes, such as memory. Hence, it is plausible that changes in adenosine tone may mediate the amnestic effect observed following CSR in the present study, and recent findings reported by Kim et al. [7] support this possibility. In addition to adenosine, various other neurotransmitters known to be modulated during sleep (e.g. serotonin, norepinephrine, and dopamine) are being investigated for their role in sleep-dependent changes in learning, memory, and cognition. Other factors may contribute toward the cognitive deficit as well. Recently, it has been reported that 1 month of CSR (20 h SD/day) resulted in a significant decrease in hippocampal volume in adolescent rats, an alteration that was independent of changes in glucocorticoid levels or in rates of hippocampal neurogenesis [25]. Finally, the possibility that the behavioral impairment observed is related to the nonspecific stress effects associated with the sleep manipulation requires further investigation. Whether these or other mechanisms mediate the effect of CSR on cognitive performance remains an open question.
Conclusion
CSR (18 h/day for 4 consecutive days) in rats impaired spatial memory in the water maze, but did not affect the rate of learning/acquisition. This model may be utilized in future studies aimed at elucidation of the biological mechanism(s) mediating the cognitive consequences of CSR.
Acknowledgements
This research was supported by the Department of Veterans Affairs Medical Research Service Award (R.E.S.), T32 HL07901 (Y.K.), R37 MH039683 (R.W.M.), P50 HL060292 (R.E.S. and R.W.M.), HL095491 (R.W.M. and R.E.S.), Stonehill College (J.G.M., D.L.P., R.B.).
This work was performed at VA Boston Healthcare System, Research Service and Harvard Medical School, Department of Psychiatry, Brockton, Massachusetts, USA.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- 2.Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–734. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose–response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 5.Gangwisch JE, Heymsfield SB, Boden Albala B, Buijs RM, Kreier F, Pickering TG, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 6.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of sleepiness from sleep time and intensity during chronic sleep restriction: evidence for a role of the adenosine system. Sleep. 2012;35:861–869. doi: 10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci USA. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everson CA, Szabo A. Recurrent restriction of sleep and inadequate recuperation induce both adaptive changes and pathological outcomes. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1430–R1440. doi: 10.1152/ajpregu.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci USA. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 13.Yang SR, Sun H, Huang ZL, Yao MH, Qu WM. Repeated sleep restriction in adolescent rats altered sleep patterns and impaired spatial learning/memory ability. Sleep. 2012;35:849–859. doi: 10.5665/sleep.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews JS. Possible confounding influence of strain, age and gender on cognitive performance in rats. Brain Res Cogn Brain Res. 1996;3:251–267. doi: 10.1016/0926-6410(96)00011-0. [DOI] [PubMed] [Google Scholar]
- 15.Harker KT, Whishaw IQ. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague-Dawley) but not domestication (wild rat vs. Long-Evans, Fischer-Norway) Behav Brain Res. 2002;134:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 16.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 18.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 19.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 20.Goel N, Banks S, Mignot E, Dinges DF. PER3 polymorphism predicts cumulative sleep homeostatic but not neurobehavioral changes to chronic partial sleep deprivation. PLoS One. 2009;4:e5874. doi: 10.1371/journal.pone.0005874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep. 2009;32:217–222. doi: 10.1093/sleep/32.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christie MA, Bolortuya Y, Chen LC, McKenna JT, McCarley RW, Strecker RE. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep. 2008;31:1393–1398. [PMC free article] [PubMed] [Google Scholar]
- 23.McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1473. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novati A, Hulshof HJ, Koolhaas JM, Lucassen PJ, Meerlo P. Chronic sleep restriction causes a decrease in hippocampal volume in adolescent rats, which is not explained by changes in glucocorticoid levels or neurogenesis. Neuroscience. 2011;190:145–155. doi: 10.1016/j.neuroscience.2011.06.027. [DOI] [PubMed] [Google Scholar]