Abstract
Dietary carbohydrates regulate hepatic lipogenesis by controlling the expression of critical enzymes in glycolytic and lipogenic pathways. Here we report that the transcription factor XBP1, a key regulator of the Unfolded Protein Response (UPR), is required for the unrelated function of normal fatty acid synthesis in the liver. XBP1 protein expression is induced in the liver by a high carbohydrate diet and directly controls the induction of critical genes involved in fatty acid synthesis. Inducible, selective deletion of XBP1 in liver resulted in marked hypocholesterolemia and hypotriglyceridemia, secondary to a decreased production of lipids from the liver. This phenotype was not accompanied by hepatic steatosis or significant compromise in protein secretory function. The discovery of XBP1 as a regulator of lipogenesis has important implications for human dyslipidemias.
Hepatic lipid synthesis increases upon ingestion of carbohydrates, which may be converted into triglyceride (TG) in the liver and transported to adipose tissue for energy storage. Dysregulation of hepatic lipid metabolism is closely related to the development of metabolic syndrome, a condition characterized by the constellation of central obesity, dyslipidemia, elevated blood glucose and hypertension (1). In mammals, hepatic lipid metabolism is controlled by transcription factors, such as liver X receptor (LXR), sterol regulatory element-binding proteins (SREBPs) and ChREBP that regulate the expression of critical enzymes involved in glycolytic and lipogenic pathways (2).
XBP1 is a key regulator of the mammalian unfolded protein response (UPR) or endoplasmic reticulum (ER) stress response (3). Upon ER stress, the proximal sensor and endoribonuclease IRE1α induces unconventional splicing of XBP1 mRNA to generate a mature mRNA encoding an active transcription factor, XBP1s, which directly binds to the promoter region of ER chaperone genes (4–6). Mice lacking XBP1 display severe abnormalities in the development and function of professional secretory cells, such as plasma B cells and pancreatic acinar cells resulting in greatly reduced secretion of immunoglobulin and zymogens (7, 8). XBP1 is also required for embryonic liver development (9). To investigate the role of XBP1 in postnatal hepatic function, we examined Xbp1Δ mice bearing an inducible, conditional disruption of the Xbp1 gene in the liver (Fig. S1).
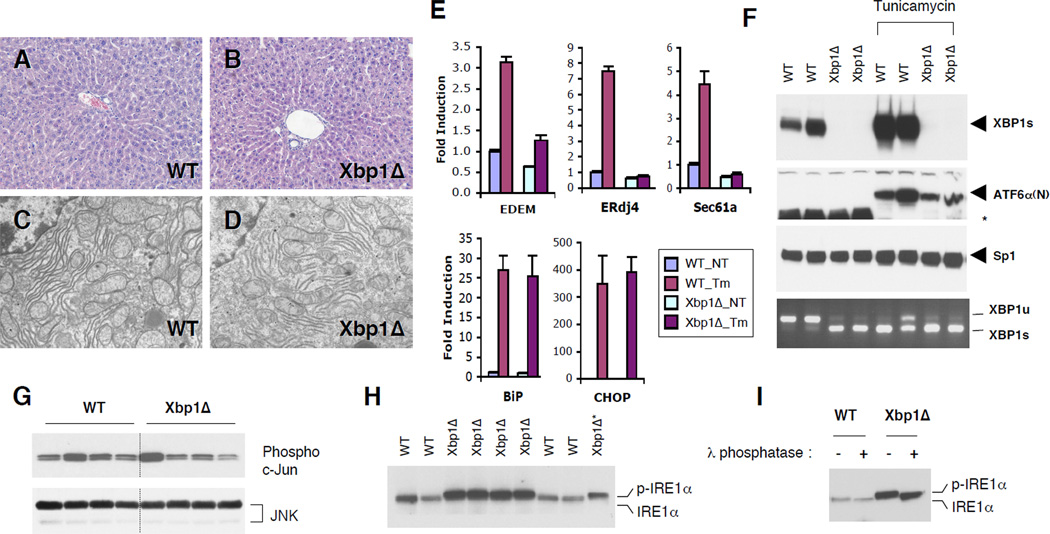
Xbp1Δ mice that lacked XBP1 in the liver postnatally did not show any noticeable gross abnormalities, had normal body weight and liver mass (Table S1) and no evidence of liver damage as determined by serum ALT levels and histological analysis (Table S1 and Fig. 1a, b). XBP1Δ hepatocytes had normal ER ultrastructure, although the ER was less abundant (Fig. 1c, d). Serum albumin and total protein levels were slightly decreased in Xbp1Δ mice, suggesting minor compromise in hepatic protein secretory function (Table S1). XBP1 dependent UPR target genes EDEM, ERdj4 and Sec61α were only modestly down regulated in Xbp1Δ liver in the basal state (Fig. 1e). Further, deletion of XBP1 in liver did not itself evoke ER stress as evidenced by the lack of ATF6α processing, JNK activation, and induction of the PERK regulated BiP and CHOP mRNAs. (Fig. 1e–g). Microarray analysis also confirmed normal expression of XBP1-independent stress markers in Xbp1Δ liver (Table S2). The pharmacological ER stress inducer tunicamycin (Tm) normally activated ATF6α processing and PERK-dependent gene expression in Xbp1Δ liver, excluding the possibility that the hepatocytes had adapted to a putative stress milieu. As expected, XBP1-dependent UPR target genes such as EDEM, ERdj4 and Sec61α were not induced by Tm in Xbp1Δ liver (Fig. 1e and f). However, XBP1 deficiency did lead to constitutive activation of its upstream activator IRE1α, made evident by robust splicing of the mutant XBP1 mRNA and induction of both IRE1α protein and its phosphorylation, measured by a band shift sensitive to phosphatase treatment (Fig. 1f, h and i). Hence, IRE1α, but not PERK or ATF6, is activated in XBP1 deficient liver, suggesting feedback regulation of IRE1α by its downstream target XBP1 in an ER stress independent manner.
Figure 1. ER stress response in XBP1 deficient mouse liver.
(A and B) Hematoxylin and eosin staining of liver sections. (C and D) Transmission electron micrographs of WT and Xbp1Δ liver. (E) Total RNAs were prepared from the liver of WT and Xbp1Δ mice 8 hours after tunicamycin injection. Expression of representative UPR target genes were tested by quantitative real-time PCR analysis. Fold induction represents relative expression level ± SEM compared to that of untreated WT mice. N=4 per group (F) Western blot of nuclear XBP1s and the processed ATF6α(N) proteins. Sp1 expression is a loading control. Levels of IRE1α-mediated XBP1 mRNA (both WT and mutant) splicing were measured by RT-PCR. *non-specific band. G) Total and active JNK protein levels were determined by western blot and c–Jun kinase assay, respectively. (H) IRE1α was detected by immunoprecipitation followed by western blot with IRE1α antibody. One third of the Xbp1Δ immunoprecipitation product was loaded on the last lane for a better comparison of band migration. Phosphorylated IRE1α displayed slowed migration on the gel. (I) IRE1α immunoprecipitation products were treated with λ phosphatase. Western blot analysis shows a band shift upon phosphatase treatment.
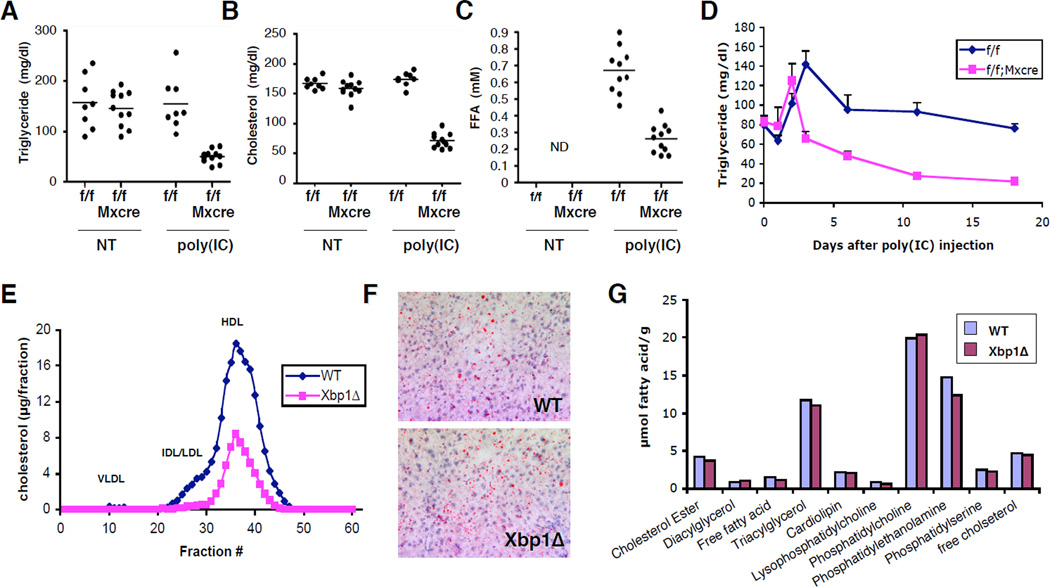
Since XBP1 plays an important role in membrane lipid synthesis in the ER (10), we asked whether it regulate fatty acid synthesis in the liver. Injection of poly(I:C) into Xbp1f/f;Mx1cre mice resulted in dramatic decreases of plasma TG, cholesterol and free fatty acids (Fig. 2a–c). In a time course experiment, the plasma TG level was lower compared to WT as early as three days after a single injection of poly(I:C) into Xbp1f/f;Mx1cre mice (Fig. 2d) and further decreased over time. XBP1 deletion also caused remarkable changes in the distribution of cholesterol, resulting in an almost complete absence of low density lipoprotein (LDL) associated cholesterol (Fig. 2e). In contrast, the content and composition of hepatic lipids were not significantly changed in Xbp1Δ mice (Fig. 2f, g), indicating that lipid retention in liver was not responsible for low plasma lipid levels.
Figure 2. Plasma and hepatic lipid profiles of XBP1 deficient mice.
Xbp1f/f and Xbp1f/f;Mx1-cre mice were injected 3X with poly(I:C). Three weeks after the last injection, plasma TG (A), cholesterol (B), and serum free fatty acid levels (C) were measured. (D) Plasma TG levels were measured over time in mice that received a single injection of 250 µg of poly(I:C). Error bars represent SEM. N=6–7 (E) Distribution of plasma cholesterol was determined by FPLC separation of lipoprotein particles. (F) Fat content in the liver was determined by oil red O staining. (G) Lipid composition in the liver was determined by Lipomics analysis. N=4/group.
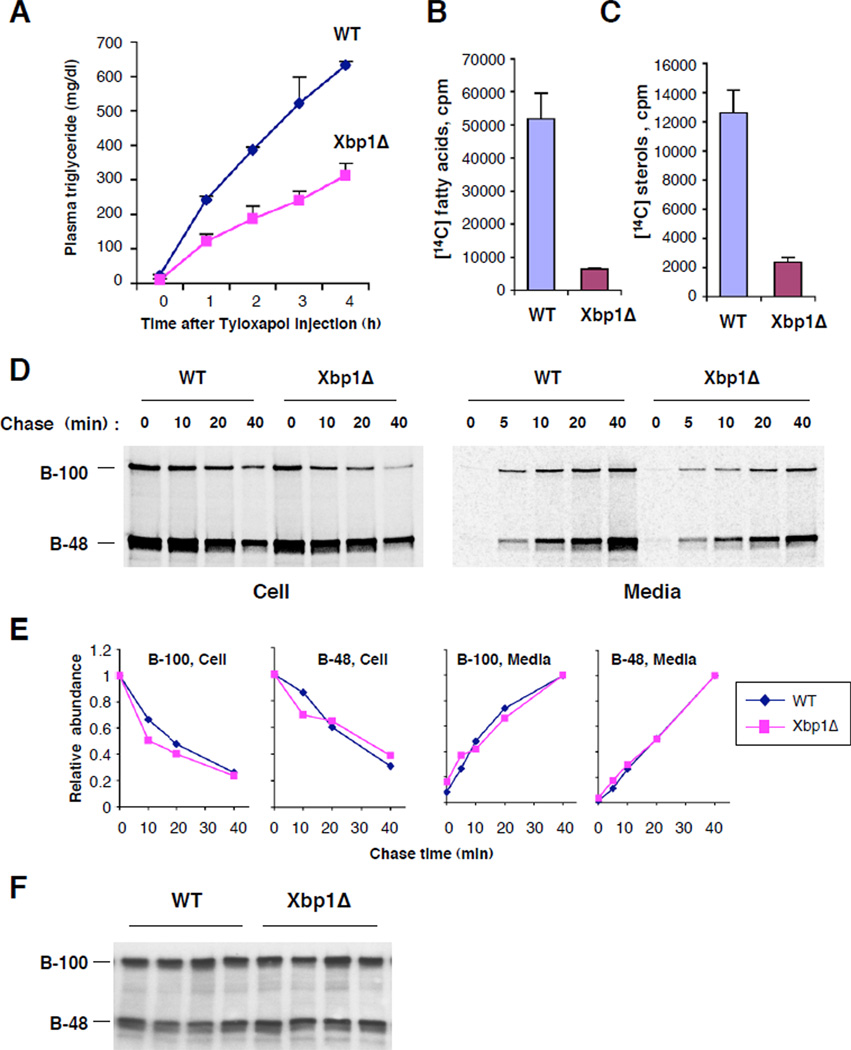
The liver secretes very low density lipoprotein (VLDL) particles that transport fatty acids in the form of triglycerides together with cholesterol to peripheral tissues (11, 12). To test whether VLDL-associated TG secretion was impaired in XBP1’s absence, we injected mice with Tyloxapol (13), a compound that inhibits the breakdown of VLDL lipids (Fig. 3a). Xbp1Δ mice displayed a significantly decreased rate of plasma TG accumulation, indicating impaired TG secretion from liver. We next examined whether the impaired TG secretion was due to compromised lipid synthesis. Incorporation of [14C]-acetate into fatty acids and sterols was dramatically decreased in primary hepatocytes lacking XBP1 (Fig. 3b, c), indicating that XBP1 is required for de novo lipid synthesis in the liver.
Figure 3. Diminished hepatic TG secretion and lipid synthesis, but normal turnover of apoB in the absence of XBP1.
(A) Mice were injected with Tyloxapol after a four-hour fast and plasma triglyceride levels measured over time. N=4/group. Primary hepatocytes were labeled with [14C]-acetate. Radiolabeled fatty acids (B) and sterols (C) extracted from equal numbers of cells were measured by scintillation counting. (D) Primary hepatocytes from WT and Xbp1Δ mice were labeled with 35S-methionine/cysteine for 30 min and then chased for indicated times. Radiolabeled apoB protein species were immunoprecipitated from cells and media and revealed by SDS-PAGE followed by fluorography. (E) The disappearance from cells and the accumulation in media of radiolabeled apoB protein species were determined by plotting relative radioactivity of each band (normalized to initial level in cells or final level in media) versus chase time. (F) Western blot analysis of plasma apoB protein.
Interestingly, neither the steady state level of plasma apolipoprotein B (apoB) proteins, a major protein component of VLDL, nor the stability and secretion from primary hepatocytes of newly synthesized apoB proteins were altered by loss of XBP1 (Fig. 3d–f). Therefore, VLDL particles produced from XBP1 deficient liver displayed low lipid, but normal ApoB protein content, as demonstrated by FPLC analysis (Fig. S2).
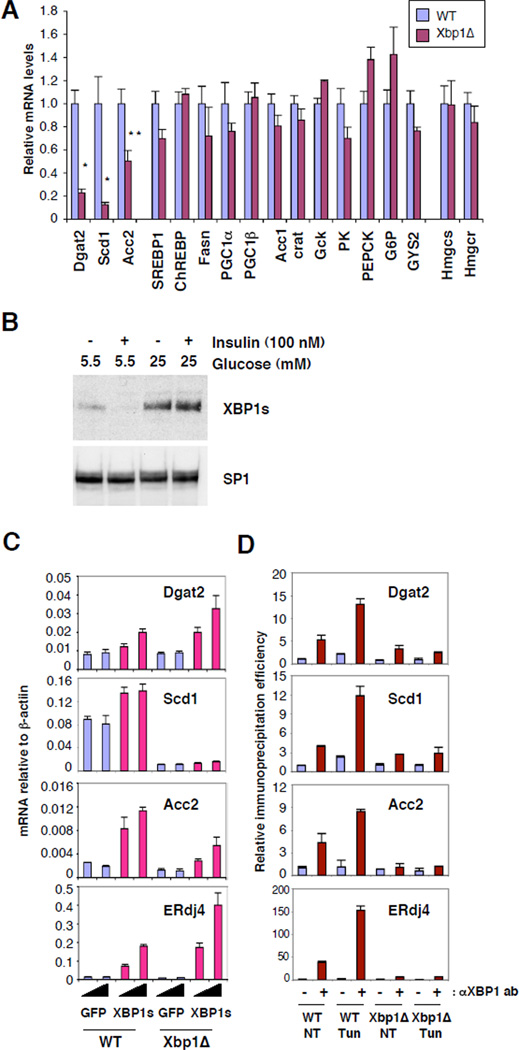
Given the compromised de novo lipid synthesis in Xbp1Δ liver we asked whether XBP1 regulates the expression of genes involved in glycolysis and lipid synthesis pathways. Critical lipogenic genes such as stearyl coA desaturase 1 (Scd1), diacyl glycerol acetyltransferase 2 (Dgat2), and acetyl coA carboxylase 2 (Acc2) were significantly 6 downregulated in Xbp1Δ liver (Fig. 4a and S3). Genetic ablation or inhibition using antisense oligonucleotides of these genes has profound effects on hepatic lipid metabolism (14–18). In contrast, expression of SREBP family regulated genes, fatty acid synthase (Fasn), HMG-CoA synthase (Hmgcs) and HMG-CoA reductase (Hmgcr) (19), was not altered in Xbp1Δ mice, consistent with normal SREBP-1 and SREBP-2 transcripts and processed nuclear protein species (Fig. 4a and S3). ChREBP expression was also normal in Xbp1Δ liver. Hence XBP1 regulates the expression of a subset of lipogenic genes in an SREBP and ChREBP independent manner.
Figure 4. XBP1 directly regulates lipogenic genes in the liver.
(A) Expression of lipogenic genes in the liver of mice fed a standard chow diet was measured by quantitative real time PCR with actin mRNA as control. Values represent relative amounts of each mRNA compared to WT. Statistical significance of differences between WT and Xbp1Δ were determined. *, p<0.005; **, p<0.0001. (B) Primary hepatocytes were cultured in media containing indicated concentrations of glucose and insulin for 24 h. Nuclear extracts were subjected to western blot analysis. (C) Primary hepatocytes were infected with adenoviruses expressing XBP-1s or GFP control at 2 or 10 pfu per cell. mRNA levels were measured 24 hours after infection by real-time PCR. (D) CHIP assays were performed with liver nuclei of mice untreated or injected with Tm 6 hrs prior to sacrifice. Values represent fold increases of real-time PCR signals compared to the signal for the untreated WT CHIP with control serum.
Prolonged feeding of high carbohydrate diets such as fructose increases de novo lipid synthesis in the liver through induction of genes encoding lipogenic enzymes (20–22). Hepatic XBP1s protein was dramatically induced in mice fed a 60% fructose diet, along with a modest increase of its mRNA reflecting increased splicing by IRE1α (Fig.S4). ER stress markers, BiP and CHOP were not induced under the same conditions, indicating an absence of ER stress. XBP1s was also induced in primary hepatocytes cultured in medium containing high glucose (Fig. 4b), suggesting that increased glucose availability upon carbohydrate ingestion induces XBP1s in the liver. As expected, the high fructose diet significantly increased mRNA levels of lipogenic genes such as Fasn, Scd1, Acc1 and Acc2 in WT but not Xbp1Δ liver (Fig. S4), suggesting that XBP1 is required for the expression of a subset of critical lipogenic genes in the setting of high carbohydrate intake. Interestingly, SREBP-1c expression was modestly reduced in Xbp1Δ compared to WT liver upon high fructose diet, perhaps contributing to the decreased expression of lipogenic genes such as Fasn in Xbp1Δ liver. The relationship of XBP1 to high fructose feeding remains to be clarified, since fructose-induced lipogenesis leads to complex metabolic changes in liver (22).
We asked whether the compromised expression of lipogenic genes was due directly to the lack of XBP1 or indirectly to constitutively active IRE1α by over-expressing a recombinant XBP1s adenovirus in primary mouse hepatocytes. Forced XBP1s expression significantly increased mRNAs encoding Dgat2 and Acc2, as well as the known XBP1 target gene, ERdj4 both in WT and XBP1Δ hepatocytes (Fig. 4c). XBP1s did not increase Scd1 transcripts suggesting the requirement for additional transcription factor(s). Further, chromatin immunoprecipitation (CHIP) assays using liver nuclear extracts from mice fed a high fructose diet demonstrated direct XBP1 binding to promoter regions of the Dgat2, Scd1 and Acc2 genes that was further increased by Tm treatment, which increased levels of nuclear XBP1s (Fig. 4d).
Here we establish XBP1 as a novel transcription factor governing hepatic lipogenesis. XBP1 deficiency resulted in profound compromise of de novo hepatic lipid synthesis leading to concomitant decreases in serum TG, cholesterol and free fatty acids without causing hepatic steatosis. XBP1 was induced upon high carbohydrate diet feeding and directly activated the transcription of key lipogenic genes in the liver. Our data reveal an unexpected and biologically critical function of XBP1 in hepatic lipogenesis, quite separate from its function as a mediator of the ER stress response (3). Hence XBP1 has at least two distinct roles: in some organs and cells, it is required for protein secretion (plasma cells, pancreatic exocrine cells) and in others, such as adult liver, it does not substantially affect protein secretory function but rather controls select transcriptional programs such as lipogenesis. Preservation of the normal hepatic lipid profile suggests that compounds that inhibit XBP1 activation in the liver may reduce serum lipids without causing hepatic steatosis in patients with dyslipidemias.
Given XBP1’s known function as a key mediator of the UPR, it was surprising that its function in regulating lipogenesis was unrelated to the ER stress response. Indeed, apoB-100 folding and secretion, and the overall hepatocyte protein secretory function were minimally compromised by loss of XBP1, likely because XBP1 independent basal chaperone gene expression is sufficient to accommodate moderate secretory loads. Interestingly, IRE1α, the upstream activator of XBP1, was constitutively active in the Xbp1Δ liver, suggesting the presence of a negative feedback loop that precisely maintains XBP1s protein levels even in the absence of ER stress. The nature of this signal and its relationship to the ER stress response and the activation of XBP-1 in the liver by carbohydrate feeding require further investigation.
Supplementary Material
Acknowledgments
Supported by the National Institute of Health,AI32412 and P01 AI56296 (L.H.G.), DK48873 and DK56626 (D.E.C.); and the Ellison Medical Foundation (L.H.G.). We thank Drs. K. Rajewsky for providing Mx1-cre mice, J. Goldstein and M. Brown for SREBP antibodies, R. Milne for ApoB antibody, K. Mori for ATF6a antibody, E. Fisher for advice on pulse-chase experiments, M. Wu and J. Wei for help with FPLC analyses, and D. Hu for histologic analyses. We thank K. Heidtman for excellent technical assistance and Drs. M. Wein and W. Garrett for critical reading of the manuscript.
Footnotes
Supporting Online Material
Materials and Methods
Tables. S1, S2, S3, S4
Figs. S1, S2, S3, S4
References
- 1.Ginsberg HN, Zhang YL, Hernandez-Ono A. Obesity. 2006;14(Suppl 1):41S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- 2.Foufelle F, Ferre P. Biochem. J. 2002;366:377. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Nat. Rev. Mol. Cell Biol. 2007;8:519. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer AL, et al. Immunity. 2004;21:81. [Google Scholar]
- 5.Lee AH, Iwakoshi NN, Glimcher LH. Mol. Cell. Biol. 2003;23:7448. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acosta-Alvear D, et al. Mol. Cell. 2007;27:53. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Reimold AM, et al. Nature. 2001;412:300. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 8.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. Embo J. 2005;24:4368. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reimold AM, et al. Genes Dev. 2000;14:152. [PMC free article] [PubMed] [Google Scholar]
- 10.Sriburi R, Jackowski S, Mori K, Brewer JW. J. Cell Biol. 2004;167:35. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson NO, Shelness GS. Annu. Rev. Nutr. 2000;20:169. doi: 10.1146/annurev.nutr.20.1.169. [DOI] [PubMed] [Google Scholar]
- 12.Hussain MM, Iqbal J, Anwar K, Rava P, Dai K. Front. Biosci. 2003;8:s500. doi: 10.2741/1071. [DOI] [PubMed] [Google Scholar]
- 13.Schotz MC, Scanu A, Page IH. Am. J. Physiol. 1957;188:399. doi: 10.1152/ajplegacy.1957.188.2.399. [DOI] [PubMed] [Google Scholar]
- 14.Stone SJ, et al. J. Biol. Chem. 2004;279:11767. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 15.Yu XX, et al. Hepatology. 2005;42:362. [Google Scholar]
- 16.Ntambi JM, et al. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11482. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen P, et al. Science. 2002;297:240. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10207. doi: 10.1073/pnas.1733877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horton JD, Goldstein JL, Brown MS. J. Clin. Invest. 2002;109:1125. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towle HC, Kaytor EN, Shih HM. Annu. Rev. Nutr. 1997;17:405. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, et al. J. Biol. Chem. 2004;279:25164. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 22.Basciano H, Federico L, Adeli K. Nutr. Metab. 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.