Abstract
Emerging evidence is revealing the different roles of steady laminar flow (s-flow) and disturbed flow (d-flow) in the regulation of the vascular endothelium. s-flow is atheroprotective while d-flow creates an atheroprone environment. Most recently, we found unique atheroprone signals, which involve protein kinase C (PKC)ζ activation, elicited by d-flow. We and others have defined a novel role for PKCζ as a shared mediator for tumor necrosis factor alpha (TNF alpha) and d-flow, which cause pro-inflammatory and pro-apoptotic events in endothelial cells (ECs) in the atheroprone environment. Under such conditions, ONOO− formation is increased in a d-flow-mediated PKCζ-dependent manner. Here, we propose a new signaling pathway involving d-flow-induced EC inflammation via PKCζ – ERK5 interaction-mediated downregulation of KLF2/eNOS stability, which leads to PKCζ-mediated p53-SUMOylation and EC apoptosis. In addition, we highlight several mechanisms contributing to endothelial dysfunction, focusing on the relations between flow patterns and activation of reactive oxygen species generating enzymes.
Keywords: Atherosclerosis, Blood flow, Endothelial dysfunction, Oxidative stress
Blood flow is known to regulate a variety of signaling pathways and molecules in endothelial cells (EC) that lead to changes in cellular functions such as proliferation, apoptosis, migration, gene and protein expression, secretion, interaction with other cells, and morphological alignment.1-3 In addition, blood flow is recognized as a major factor in the focal development of atherosclerosis, which is characterized by highly localized endothelial dysfunction, including altered vasoregulation, activation of inflammatory processes, and compromised barrier function.4,5 Thus, ECs may be an important predictor of cardiovascular outcomes and an independent predictor of future events in patients with cardiovascular risk factors.6,7
Manifestations of dysfunctional ECs are readily observed in certain areas of the arterial branches and curvatures, where disturbed flow (eg, non-uniform, irregular oscillation, and recirculation; termed d-flow) occurs.8,9 ECs in these regions have an activated, pro-inflammatory phenotype that is characterized by poor alignment, high turnover, and being under oxidative stress.3 ECs in the straight part of the arterial tree are exposed to steady laminar flow (10–20 dyn/cm2; termed s-flow) which promotes release of factors from ECs that inhibit coagulation, leukocyte diapedesis, and smooth muscle cell proliferation while simultaneously promoting EC survival.10,11 Thus, understanding how the various signaling pathways are affected by different patterns of blood flow such as s- and d-flow in ECs dysfunction is critically important. This review will focus on the emerging evidence that s- and d-flow differentially regulate ROS production in ECs, which is the critical early step in atherosclerosis.
Different Roles of s-Flow and d-Flow on EC Function
ECs in vivo are constantly exposed to the hemodynamic forces, such as shear stress and cyclic strain, of flowing blood and there is a strong correlation between localized atherosclerotic plaque and regions exposed to the d-flow found at vessel curvatures, bifurcations and branches. In contrast, atherosclerosis is rare in areas exposed to s-flow and in fact, ECs in such areas are thought to be more resistant to the development of atherosclerosis (Figure 1).12 For example, the human coronary artery exposed to complex and d-flow patterns has an extremely high susceptibility to atherosclerosis whereas in the internal mammary artery where there are less discontinuities in the flow patterns, the atherosclerotic incidence is low.13 In genetic modification models, such as the low-density lipoprotein receptor knockout mouse (LDLR−/−), atherosclerotic plaques are reliably and rapidly formed and are morphologically similar to those observed in human, particularly when the mice are fed a high-fat diet. Plaques tend to form at the aortic sinus, the lesser curvature of the aortic arch, on the upstream edge of the brachiocephalic and left common carotid arteries proximal to branching from the (Figure 1). All those areas are d-flow regions, where are exposed to relatively low levels of wall shear stress when compared with nearby sections of artery. Therefore, the importance of shear stress in vascular biology and pathophysiology is highlighted by the fact that s-flow is protective against atherosclerosis while d-flow is a strong risk factor of the disease.
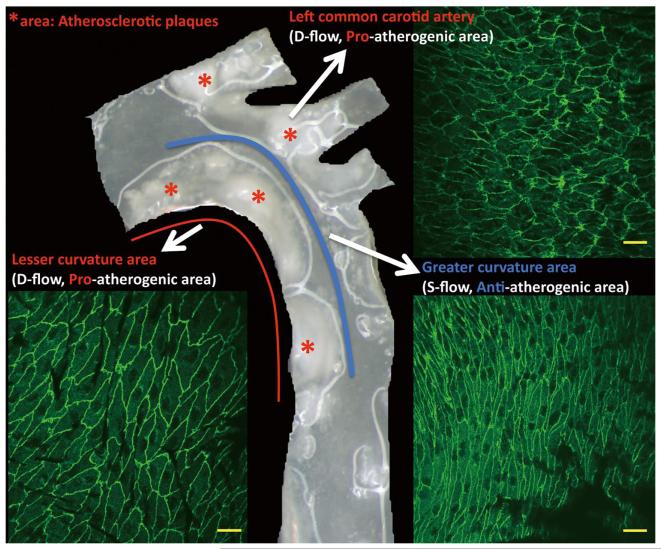
Figure 1.
Morphology of endothelial cells and atherosclerotic plaques in the arch region of the mouse aorta. The greater curvature of the aortic arch and the straight region of the aorta indicated by the blue line are exposed to s-flow and are protected from atherosclerosis. Regions of curvature indicated by the red line and side branches are exposed to d-flow and are atheroprone areas. The aorta was prepared from a LDLR−/− mouse fed with high cholesterol diet for 16 weeks; red stars indicate atherosclerotic plaques. En face endothelial cell morphology illustrated by anti-VE-cadherin staining was prepared from the aortic arch of a wild-type mouse. Scale bars, 20μm. d-flow, disturbed flow; s-flow, steady laminar flow; LDLR−/−; low-density lipoprotein receptor knockout.
Induction of mechanosensitive genes is likely to be mediated by transcription factors (eg, nuclear factor κ B (NF-κB), AP-1, early growth response-1, c-Jun, c-fos, and c-myc)14-16 that are regulated by upstream signaling molecules, such as the family of mitogen-activated protein kinases (MAPKs), including extracellular signal regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 MAP kinase (p38 MAPK), and ERK5. The anti-inflammatory and anti-atherosclerotic effects of s-flow on ECs have been attributed to its effects on MAPK signaling.1,17,18 Our group have reported the atheroprotective role of s-flow, which upregulates MAPK, especially ERK5, activation and downregulates JNK.1,17,18 In another study, we have shown that s-flow-induced ERK5 activation has a critical role in regulating peroxisome proliferator-activated receptor (PPAR) γ and Kruppel-like factor (KLF) 2 (a recently identified transcriptional inhibitor of EC inflammation) and inhibiting tumor necrosis factor (TNF) α-mediated adhesion molecule expression,19 demonstrating an anti-inflammatory role of ERK5 activation in ECs (Figure 2).
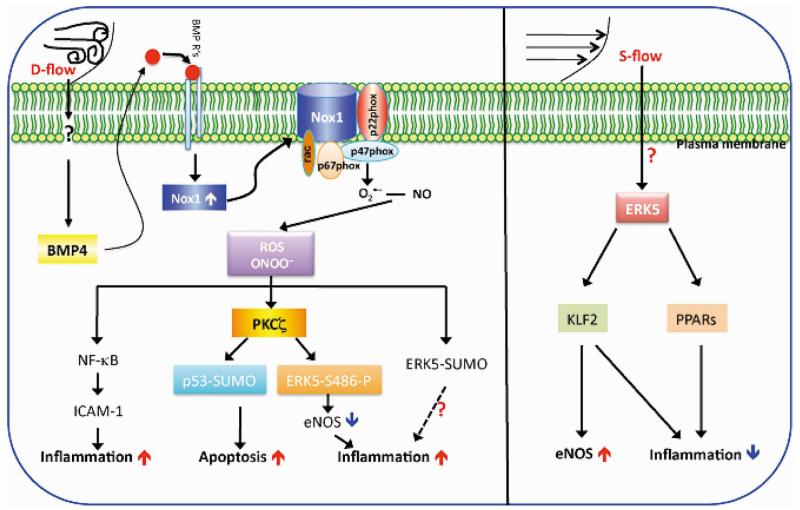
Figure 2.
Schematic of recently identified endothelial signaling modules that can be activated by s- or d-flow. d-flow induces production of ROS such as .O2− and ONOO− via activation of NADPH oxidase and causes endothelial dysfunction. BMP-4 is generated by d-flow and activates NADPH oxidase. ROS can then activate NF-κB and induce ICAM-1 expression and inflammation. d-flow can also induce PKCζ activation, leading to EC apoptosis via p53-SUMOylation and to inflammation via ERK5 phosphorylation, which downregulates eNOS expression. On the other hand, s-flow, through activation of ERK5, increases the activity of PPARs and KLF2, which are responsible for inducing anti-inflammatory responses. (See text for details.)
Recently, we found the involvement of PKCζ and the MEK5/ERK5 pathway in the TNFα-induced downregulation of endothelial nitric oxide synthase (eNOS) expression in ECs and in the initiation of atherosclerosis (Figure 2). d-flow increases endothelial apoptosis and proliferation, resulting in a high turnover rate of ECs, which then creates highly localized hot spots of increased endothelial permeability, inflammation, and atherosclerosis.20 d-flow also increases apoptosis by promoting the production of reactive oxygen species (ROS), which react with nitric oxide (NO) to form peroxynitrite (ONOO−) and induce proatherogenic responses in ECs.2,21 Our more recent study has shown that PKCζ activation by d-flow induces EC apoptosis by regulating p53 (Figure 2).2
EC Function and Signaling Pathways Activated by Different Flow Types
Flow-Mediated ROS Production
ROS function as signaling molecules that mediate various biological responses, such as gene expression, cell proliferation, migration, inflammation and apoptosis, in ECs but also cause dysfunction of these cells, leading to atherosclerosis.22-24 Atherogenic events, such as the loss of bioavailable NO production and increased levels of ROS, including superoxide (.O2−), hydrogen peroxide (H2O2), and ONOO−, are highly linked to atherosclerosis formation and are caused by d-flow.25,26 Several vascular ROS-producing enzymes have been identified and include NADPH oxidase, uncoupled eNOS, mitochondrial electron transport enzymes, xanthine oxidase, cyclooxygenase, lipoxygenase, myeloperoxidase, and cytochrome P450 enzymes.27 Among these, NADPH oxidase (Nox) has been identified as the major ROS-producing enzyme in blood vessels in response to d-flow.28
Five NADPH oxidase proteins have been found (Nox 1, 2, 3, 4, and 5).29 Specific NADPH oxidase complexes have been identified as major .O2− generators in various cell types in the vessel wall. In ECs, NADPH oxidase similar to the complex in granulocytes was initially reported to be the main .O2− producer.28 A later study has shown that accelerated endothelial ROS formation after application of d-flow involves 47phoxcontaining NADPH oxidase complexes.30 d-flow also significantly upregulates Nox4 expression accompanied by an increase in ROS production in aortic ECs, whereas s-flow upregulates eNOS expression and NO production.30
NADPH Oxidase Increases ROS and ONOO− Production
The type and duration of (blood) flow has an important impact on endothelial ROS formation, because continuous d-flow can induce ROS production.25 ROS arise from several sources in the endothelium, including NADPH oxidase, xanthine oxidase, mitochondrial oxidase, cytochrome P450, and uncoupled eNOS.31 However, NADPH oxidase and xanthine oxidase are likely to be the main oxidases responsible for the increased oxidative state of ECs.25,32 The balance between the NO and ROS levels is shifted by the accumulation of .O2− and H2O2 produced by these oxidases in ECs exposed to d-flow, and this is the key step in the initial development of atherosclerosis.14,33 In this area, the increased activity of .O2− generating NADPH oxidase and expression of redox-sensitive genes, such as c-fos and heme oxygenase-1 (HO-1), has been noted.25
Because ROS and NO can interact and generate ONOO− and increase nitrative stress, higher levels of 3-nitrotyrosine, dityrosine, and o-hydroxyphenylalanine under d-flow than s-flow have been observed.24 We and others also found that nitrosylation was increased in d-flow areas compared with s-flow areas in the aortic arch of the mouse.2,34 Inhibition of ONOO− formation significantly reduced d-flow-mediated EC apoptosis, suggesting that ONOO− formation induced by d-flow is one of the initial events that lead to EC dysfunction.2
d-Flow Increases Bone Morphogenetic Protein (BMP) 4 Expression and Activates Nox1(2)
BMPs were originally identified as proteins that induced bone formation at extraskeletal sites.35 They are members of the transforming growth factor-β super family.36 More than 30 BMPs have been identified, and among them BMP-4 is expressed in both arterial ECs and smooth muscle cells in human coronary arteries, as well as in cultured human and mouse aortic ECs.37,38 Recent studies suggest that BMPs are upregulated in d-flow areas in blood vessels and may contribute to vascular calcification and development of atherosclerotic plaques.38,39 BMP-4 and BMP-2 reportedly induce endothelial dysfunction and enhanced monocyte adhesion to the endothelium via increasing ROS.38,39 Sorescu et al found that d-flow stimulated monocyte adhesion by inducing intracellular adhesion molecule-1 (ICAM-1) without affecting vascular cell adhesion molecule-1 (VCAM-1) and E-selectin levels, and that treatment of ECs with BMP-4 also increased ICAM-1 expression and monocyte adhesion. The d-flow-induced ICAM-1 expression and monocyte adhesion were completely prevented by inhibiting BMP-4 production in ECs by noggin (the BMP antagonist) or BMP-4 siRNA,38 suggesting the essential role of BMP-4 in d-flow-induced inflammatory responses in ECs (Figure 2). They have also shown that exposure of ECs to d-flow stimulates ROS production via increased Nox1 and Nox2 expression and significantly reduces Nox4 levels. While BMP-4 upregulates Nox1 mRNA levels, it inhibits Nox2 mRNA expression,30 suggesting that d-flow-mediated Nox1 induction can be explained by BMP-4 induction. Of note, the importance of p47phox-dependent enzymes such as Nox1 and 2 in BMP-4-induced ROS production, ICAM-1 induction, and monocyte adhesion has been demonstrated using p47phox knockout mice.40 These finding suggest the importance of the regulation of p47phox-dependent enzymes in d-flow-mediated ROS production and subsequent endothelial inflammation and dysfunction.
s-Flow Does Not Increase BMP-4 Expression
Recent studies suggest that s-flow inhibits endothelial BMP-4 expression,38,41 and in particular, the significance of flow-mediated BMP-4 regulation in vivo is supported by studies on porcine aortic valves. BMP-4 expression in calcification-susceptible valvular regions was significantly increased compared with that in the calcification-protected, high-shear regions.38 It has been shown that s-flow-mediated activation of the cAMP/PKA pathway transcriptionally regulated BMP-4 (Figure 3).42 Cyclic AMP (cAMP) is known to bind to the regulatory subunit of protein kinase A (PKA), activating the enzyme, and PKA has been shown to be activated by s-flow.43 In parallel with these in vitro findings, increasing the shear stress from 2.6 to 15.6 dyn/cm2 on human coronary artery ECs elicited translocation of the catalytic subunit of PKA into the nucleus.44 The central role of PKA signaling in BMP-4 regulation is supported by the finding that inhibition of PKA using a specific inhibitor, H89, attenuates cAMP-dependent regulation of BMP-4. s-flow-mediated activation of the cAMP/PKA pathway increases the phosphorylation and activation of cAMP response element binding protein (CREB), and CREB phosphorylation is significantly inhibited by H89.42,44 Although that study did not show the effect of cAMP/PKA pathway-mediated activation of CREB on the regulation of BMP-4, the rVISTA (http://www.gsd.lbl.gov/vista) analysis, designed to confirm the presence of putative cAMP-responsive elements (CRE), found a highly conserved proximal part of the 5′-flanking region of the human BMP-4 gene, suggesting a putative relationship between CREB and BMP-4.
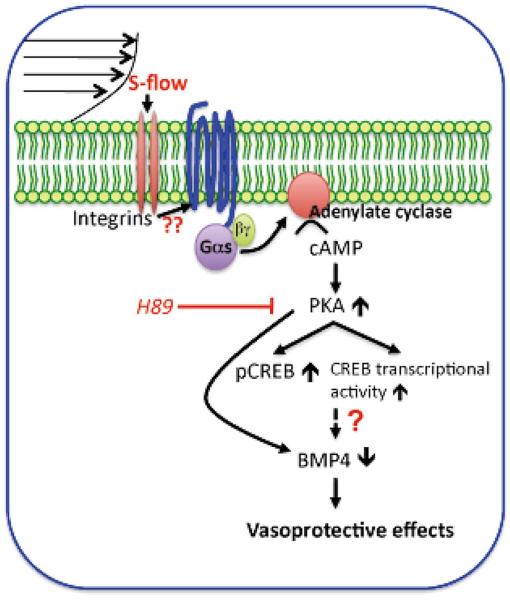
Figure 3.
A model for the cAMP/PKA pathway that regulates the expression of BMP-4 in the vascular endothelium. s-flow activates the cAMP/PKA pathway via Gα activation. This increases the phosphorylation and transcriptional activity of CREB and inhibits BMP-4 activation. Because BMP-4 elicits endothelial activation and vascular calcification, the model predicts that cAMP/PKA-mediated inhibition of BMP-4 expression contributes to the anti-atherogenic and vasculoprotective effects of s-flow. (See text for details.)
Role of d-Flow-Mediated ROS Production on EC Dysfunction
Nox1(2)-Mediated ROS Production Increases the NF-κB and Subsequent ICAM-1 Expression (EC Inflammation)
One of the first visible markers of endothelial dysfunction in the d-flow area is upregulation of inflammatory adhesion molecules such as E-selectin, VCAM-1, and ICAM-1.45,46 These endothelial adhesion molecules play essential roles in the adhesion and recruitment of monocytes to the subendothelial layer.6,47 Oscillatory flow (OS) stimulates monocyte adhesion by specifically inducing ICAM-1 without affecting VCAM-1 and E-selectin levels.38 For example, OS-induced ICAM-1 expression is completely abolished by noggin (the BMP antagonist) or BMP-4 siRNA38 and monocyte adhesion is completely inhibited by an ICAM1 blocking antibody (YN1 Ab). The critical role of NF-κB activation in this process was supported by experiments in which the d-flow and BMP-4-mediated ICAM-1 expression was inhibited by NF-κB inhibitors (Figure 2).38 Because both antioxidants and genetic depletion of p47phox prevent d-flow-induced NF-κB activation,30,48 d-flow-mediated ROS production via Nox1 is crucial for NF-κB activation and subsequent ICAM-1 expression in ECs.
ONOO− Increases PKCζActivation
PKCζ-Induced p53-SUMOylation and Subsequent EC Apoptosis
It has been reported that d-flow increases EC apoptosis and proliferation, causing high EC turnover, which creates hot spots of increased endothelial permeability, inflammation, and dysfunction.49 Despite intense studies, however, the mechanisms by which d-flow regulates endothelial turnover are unclear. PKC isozymes are serine – threonine kinases that phosphorylate multiple proteins, which in turn regulate intracellular signaling.50 Among the PKC family members, atypical PKCζ has recently emerged as an important isoform in ECs.34, 51 Magid and Davies reported that PKCζ was highly expressed in ECs in d-flow areas of the porcine aorta.51 Frey et al demonstrated involvement of PKCζ in oxidant generation in ECs via NADPH oxidase activation.34 Consistent with those findings, we found that endothelial PKCζ activation was elevated in atherosclerotic lesions.1,2
It has been shown that SUMO (small ubiquitin-like modifier) influences many different biological processes by altering transcriptional activity, protein stability, or localization change.52 The SUMOylation pathway is analogous to that of ubiquitination, but SUMO conjugation involves a different set of enzymes. SUMO is activated in an ATP-dependent manner by an E1-activating enzyme that consists of a SAE1(AOS1)-SAE2(UBA2) heterodimer. Activated SUMO is transferred to Ubc9, the E2-conjugating enzyme, and is subsequently attached to the ε amino group of specific residues in target proteins.53 Finally, E3 SUMO ligase-like PIASy conjugates activated SUMO to the target protein.53 Recently, we reported that d-flow and ONOO− induced EC apoptosis and that this was mediated by p53 SUMOylation via PKCζ binding to the E3 SUMO ligase protein inhibitor of activated STATy (PIASy).2 This binding was found to occur between the C-terminus of the kinase domain of PKCζ and the RING domain of PIASy, which contains its catalytic site and may alter the structure and enzymatic activity of PIASy. Interestingly, d-flow and ONOO− did not upregulate the p53 level, instead they actually inhibited p53 transcription.2 Notably, p53-SUMOylation was a key event for translocation of p53 from the nucleus to the cytosol and for the apoptosis induced by d-flow. All these observations suggest that PKCζ activity is an important factor in d-flow-mediated EC apoptosis by regulating p53-SUMOylation (Figure 4).
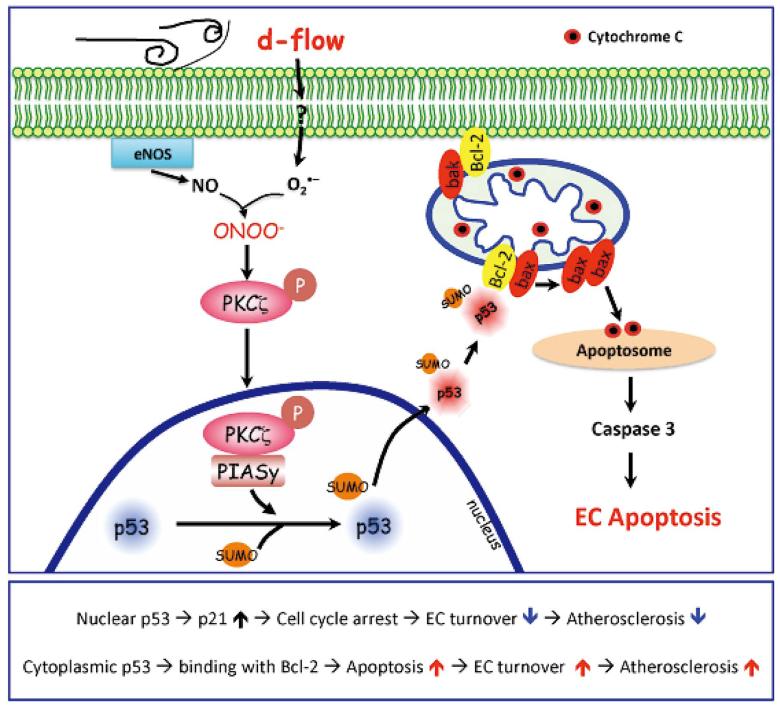
Figure 4.
d-flow-activated PKCζ effects on EC apoptosis. (Upper) d-flow activates PKCζ, which induces activation of E3 SUMO ligase PIASy, SUMOylation of p53, and translocation of p53 to the cytosol where it binds to Bcl-2. Binding between SUMOylated p53 and Bcl-2 results in bax release, which stimulates cytochrome c release from mitochondria, leading to apoptosome formation, caspase activation, and subsequent apoptosis. (Lower) A scheme of the role of localization-dependent p53 on atherosclerosis. (See text for details.)
Lin et al have reported that s-flow induces EC cycle arrest by a mechanism involving increased p53 expression and phosphorylation,54 but this growth arrest was via increased GADD45 and p21cip1 expression not by p53-mediated apoptosis. It is important to emphasize that p53 can induce growth arrest by inhibiting apoptosis. Depletion of p21 maintains DNA synthesis without undergoing mitosis. This process duplicates DNA abnormally in the cell and causes subsequent cell death.55 Because p21 expression is directly increased by p53, p53 can be cell-protective by increasing p21 expression. The interplay between the cell cycle arrest and apoptosis mechanisms is possibly critical in maintaining endothelial function, because p53 may be able to reduce DNA damage from apoptosis by preventing entry into the S phase.56 However, it remains unclear what determines the pro- and anti-apoptotic activities of p53. Of note, most of the anti-apoptotic effects of p53 have been explained by its nuclear localization. Nuclear localization of p53 in ECs under s-flow may indeed inhibit cell proliferation as Lin et al have reported,54 but p53 can also inhibit apoptosis, as we show in our recent study.2 In contrast, cytosolic p53 (ie, nuclear export) may accelerate apoptosis, which occurs under d-flow conditions.2 Our data suggest that d-flow-mediated PKCζ activation and subsequent p53 nuclear export promotes EC apoptosis. Taken together, we suggest that the evidence shows that s-flow maintains nuclear p53 localization, leading to endothelial growth arrest and inhibition of EC apoptosis,54 whereas d-flow promotes p53 nuclear export, leading to increased apoptosis (Figure 4). Further investigations are necessary to clarify the role of p53-mediated growth arrest and apoptosis in ECs, especially under s-flow, both in vitro and in vivo.
PKCζ Phosphorylates ERK5 S486 and Decreases eNOS Stability
eNOS is a key enzyme involved in the regulation of vascular function, and altered activity and expression of this enzyme has been linked to atherosclerosis.57,58 TNFα inhibits eNOS expression by downregulating both transcriptional and posttranscriptional processes.59 TNFα, in addition to regulating eNOS expression, is a mediator of inflammation, and PKCζ is a key enzyme involved in TNFα-mediated inflammation.1 When ECs are stimulated by TNFα, PKCζ is activated and monocyte adhesion because increased NF-κB-dependent ICAM-1 expression is promoted.60 In addition, our group has found that exposure of ECs to s-flow inhibits the JNK-caspase-3-dependent CATζ generation, reducing apoptosis and pro-inflammatory endothelial adhesion protein expression.11
Recently, eNOS expression was shown to be positively regulated in ECs by the MEK/ERK5/KLF2 pathway.61 We have reported that PKCζ binds directly to ERK5 via its catalytic domain and phosphorylates ERK5.1 The study demonstrates that PKCζ binds and phosphorylates ERK5 at S486 and that these events are required to increase eNOS protein degradation. Furthermore, PKCζ activity is upregulated in d-flow regions of the ApoE−/− mouse aorta. Collectively, these results suggest the importance of PKCζ activity in determining the atherogenesis susceptibility of ECs by regulation of inflammatory pathways.
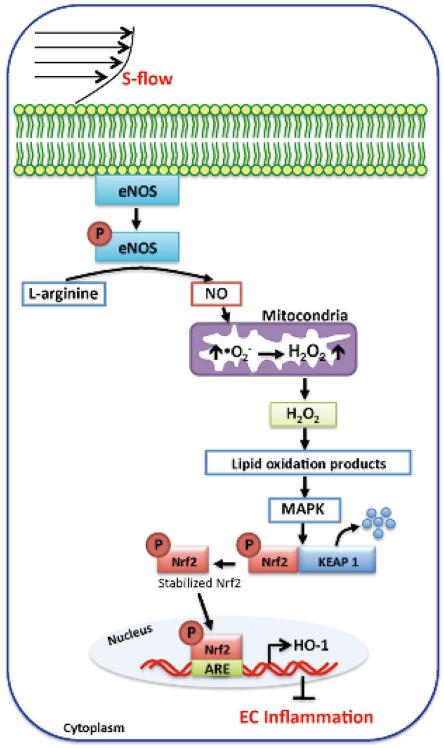
s-Flow Activates HO-1 by Increasing Mitochondrial H2O2 Production
Unlike d-flow, s-flow induces only transient activation of NADPH oxidase in cultured ECs,62,63 but both Nox2 and Nox4 mRNA expression are generally downregulated with an accompanying reduction in .O2− synthesis.64 One of the targets of s-flow mediated ROS production, heme oxygenase (HO)-1, is activated by s-flow at the transcriptional level in human aortic ECs.65 Exposure of cultured ECs to s-flow causes a sustained increase in NO and a transient increase in .O2− production, and ROS such as these act as the second messenger for the induction of gene expression.62,66 Recently, Han et al showed that mitochondria-derived H2O2 plays an important role in intracellular signaling.62 H2O2, at least partially of mitochondrial origin, and NO play an important role in flow-induced HO-1 overexpression (Figure 5).63 For example, HO-1 induction by s-flow is inhibited by the NOS inhibitor, NG-nitro-L-arginine methyl ester, and by catalase, which catalyzes the transformation of H2O2 into H2O. Mitochondrial electron transport chain inhibitors, antimycin A and rotenone, also markedly reduce the s-flow-induced increase in HO-1 expression.63,67 Phosphatidylinositol 3-kinase (PI3K) or MAPK cascade inhibitors block HO-1 induction, resulting in Nrf2 phosphorylation and its dissociation from Keap1.67 NO can directly contribute to Nrf2 activation by modifying the critical cysteine residues of Keap1, possibly via S-nitrosylation.68 Because there is less bioavailable NO in the d-flow areas, because of downregulated eNOS expression and increased .O2− generation by NADPH oxidase,24 less NO may, at least in part, be responsible for decreased Nrf2 nuclear translocation and suppressed HO-1 expression in areas of the vasculature that are exposed to d-flow (Figure 5).
Figure 5.
ROS-dependent signaling pathway activated by s-flow, which induces eNOS-mediated NO production and subsequently increases mitochondria-derived H2O2. H2O2 then activates MAPK such as PI3K/Akt and ERK1/2, which induces Nrf2 translocation into the nucleus, leading to up-regulation of the cytoprotective HO-1. (See text for details.)
Unique Role of ERK5 Activation Under s-Flow
Our study has revealed that s-flow-mediated ERK5 activation, leading to activation of PPARγ and KLF2, contributes to the anti-inflammatory and atheroprotective effects of s-flow.19
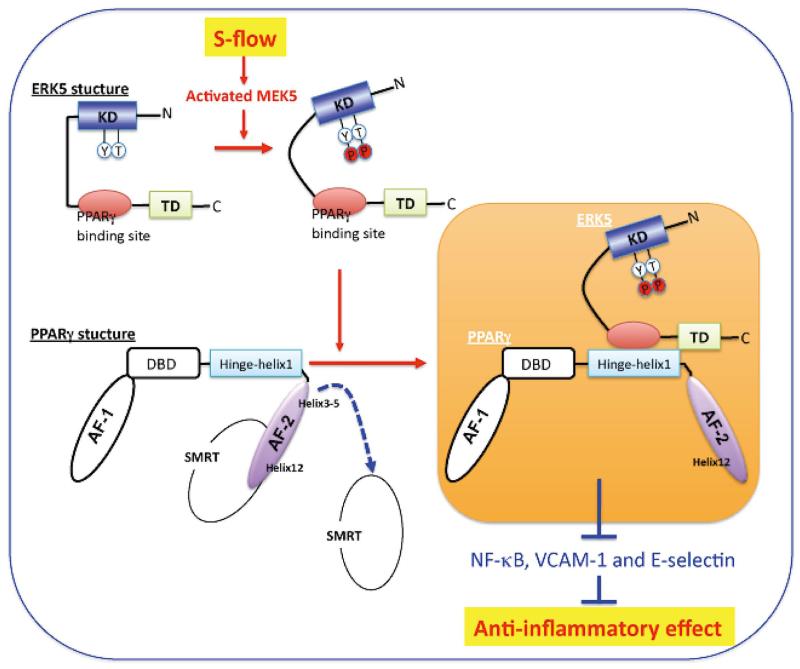
The upstream kinase that phosphorylates ERK5 has been identified as MEK5.69 Activation of ERK5 is documented to have an anti-apoptotic effect in cardiac, neuronal, and ECs.70,71 ERK5 is not only a kinase enzyme but also possesses transcriptional activity. Although phosphorylation of substrates represents the defining function of kinases, there are also numerous cell physiology events that are regulated through the interaction of kinases with other molecules outside of phosphorylation.19,72 ERK5 is serine – threonine kinase that can function like a typical kinase by phosphorylating its substrates and altering their function through this post-translational modification.73 However, ERK5 is able to regulate other substrates outside of its kinase activity. For example, the s-flow induced association of activated ERK5 with PPARγ to regulate PPARγ’s function does not involve phosphorylation of PPARγ by ERK5 (Figure 6).19 In contrast, the dominant negative MEK5 prevents s-flow-mediated inhibition of TNFα-mediated NF-κB activation and adhesion molecule expression, including VCAM-1 and E-selectin, indicating a physiological role for ERK5 and PPARγ activation in s-flow-mediated anti-inflammatory effects. Here, ERK5 kinase activation is required, likely by inducing a conformational change in the NH2-terminal region of ERK5 that prevents the association of ERK5 and PPARγ. Together, it suggests that ERK5 mediates s-flow- and ligand-induced PPARγ activation via its interaction with the hinge-helix 1 region of PPARγ.
Figure 6.
PPARγ transactivation by ERK5 When the ligand binds to PPARγ, Helix 12 folds back to form a part of the co-activator binding surface and inhibits co-repressor (such as SMRT) binding to PPARγ. The co-repressor interaction surface requires Helix 3-5 region. The inactive N-terminal kinase domain of ERK5 inhibits its own transactivation and PPARγ binding. After ERK5 activation by s-flow stimulation, the inhibitory effect of N-terminal domain of ERK5 decreases, and subsequently the middle region of ERK5 can fully interact with the hinge-helix 1 region of PPARγ. The association of ERK5 with the hinge-helix 1 region of PPARγ releases co-repressor of SMRT and induces full activation of PPARγ. KD, kinase domain; TD, transactivation domain; AF-1/2, activating function (AF)-1/2 transactivation domain, DBD, DNA binding domain. (Modified from Akaike et al,19 Copyright © 2004, American Society for Microbiology, MCB.24.19.8691-8704. 2004, DOI: 10.1128) (See text for details.)
The KLF family is a recently highlighted group of zinc finger transcription factors with important biological roles, including in the vasculature.74 Dekker et al first identified KLF2 as a gene regulated by s-flow in the endothelium.75 One of the major endothelial functions regulated transcriptionally by KLF2 is the control of vessel tone. Overexpression of KLF2 induces eNOS expression, which concomitantly downregulates caveolin-1, a negative regulator of eNOS activity, as well as inhibiting cytokine-mediated adhesion molecule expression.61,75,76 Recently, Parmar et al reported that KLF2 is selectively induced in ECs exposed to s-flow and that this flow-mediated increase in expression occurs via the MEK5/ERK5/MEF2 signaling pathway.61 In addition, KLF2 induction results in the orchestrated regulation of EC transcriptional programs controlling inflammation, thrombosis/hemostasis, vascular tone, and blood vessel development.61 Both proinflammatory and anti-inflammatory stimuli likely modulate PPARγ/KLF2 and adhesion molecule (activity or expression) via the transcriptional activity of ERK5. Consistent with such key roles of ERK5 in EC physiology, endothelial-specific ERK5 knockout mice shows a cardiovascular defect attributable to increased EC apoptosis.71,77
ROS-Mediated ERK5-SUMOylation
It is clear that SUMO influences many different biological processes, but particularly important in the present context is the regulation of transcription.52 Our studies have shown that s-flow has an anti-inflammatory effect via ERK5-mediated KLF2 and eNOS expression.1,18 ERK5 is an s-flow reactive protein that has SUMOylation motifs (K6 and K22) at the divergent N-terminal region close to the ATP-binding site and it contains a cytoplasmic targeting region.18 In our recent study, we found that H2O2 significantly inhibited ERK5/MEF2 transcriptional activity, as well as the subsequent s-flow-mediated KLF2 promoter activity and the KLF2 and eNOS expression mediated by ERK5 SUMOylation. ERK5 SUMOylation can also inhibit both ERK5/MEF2 and PPARγ, and promote strong pro-inflammatory and atherogenic responses in ECs.18,78 At present, it is not known how d-flow conditions affect ERK5-SUMOylation or if d-flow-induced EC dysfunction is medicated by ERK5 SUMOylation. However, the experimental results discussed here appear to suggest that inhibition of ERK5 SUMOylation may be a potential therapeutic target for shear stress-mediated EC dysfunction and inflammation.
Future Direction of Flow Research
Although the possible involvement of PECAM-1, integrins, p130Cas (Crk-associated substrate), ion channels, and certain EC surface structures has been proposed as the mechanosensor of s-flow, the sensing mechanism and its downstream signaling mechanism are still elusive. As we have discussed in this review, we believe that d-flow has its own unique sensing or signaling mechanism, because the signaling pathways of s- and d-flow seem distinctly different. Further investigations are necessary to clarify these issues.
Conclusions
Studies described in this review highlight the current status regarding the role of d-flow-mediated ROS production in the pathogenesis of atherosclerosis. Accumulated evidence indicates that s-flow is atheroprotective, whereas d-flow, which occurs near arterial bifurcations and curvatures, is a proatherogenic factor. Thus, understanding the mechanisms of endothelial activation and development of endothelial dysfunction may provide critical information for finding therapeutic targets of cardiovascular disease, especially atherosclerosis.
Acknowledgments
This work is supported by grants from the America Heart Association to Dr Fujiwara (Grant-in-Aid 11GRNT5850001) and from the National Institute of Health to Dr Abe (HL-108551, HL-088637, HL-064839, and HL-102746). Dr Abe is a recipient of Established Investigator Awards of the American Heart Association (0740013N).
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Nigro P, Abe J, Woo CH, McClain C, O’Dell MR, Lee H, et al. PKCzeta decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood. 2010;116:1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, et al. PKCzeta mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J Cell Biol. 2011;193:867–884. doi: 10.1083/jcb.201010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ando J, Yamamoto K. Vascular mechanobiology: Endothelial cell responses to fluid shear stress. Circ J. 2009;73:1983–1992. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 4.Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem. 2008;283:12446–12455. doi: 10.1074/jbc.M708208200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Hu YL, Li S, Shyy JY, Chien S. Sustained JNK activation induces endothelial apoptosis: Studies with colchicine and shear stress. Am J Physiol. 1999;277:H1593–H1599. doi: 10.1152/ajpheart.1999.277.4.H1593. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. Atherosclerosis: An inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 7.Rossig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol. 2001;96:11–22. doi: 10.1007/s003950170073. [DOI] [PubMed] [Google Scholar]
- 8.Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 9.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, et al. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. 2007;171:1691–1704. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhart-King CA, Fujiwara K, Berk BC. Physiologic stress-mediated signaling in the endothelium. Methods Enzymol. 2008;443:25–44. doi: 10.1016/S0076-6879(08)02002-8. [DOI] [PubMed] [Google Scholar]
- 11.Garin G, Abe J, Mohan A, Lu W, Yan C, Newby AC, et al. Flow antagonizes TNF-alpha signaling in endothelial cells by inhibiting caspase-dependent PKC zeta processing. Circ Res. 2007;101:97–105. doi: 10.1161/CIRCRESAHA.107.148270. [DOI] [PubMed] [Google Scholar]
- 12.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann NY Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239 – 240. [DOI] [PubMed] [Google Scholar]
- 13.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh HJ, Li NQ, Frangos JA. Pulsatile and steady flow induces c-fos expression in human endothelial cells. J Cell Physiol. 1993;154:143–151. doi: 10.1002/jcp.1041540118. [DOI] [PubMed] [Google Scholar]
- 15.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- 17.Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann NY Acad Sci. 2001;947:93–109. doi: 10.1111/j.1749-6632.2001.tb03932.x. discussion 109 - 111. [DOI] [PubMed] [Google Scholar]
- 18.Woo CH, Shishido T, McClain C, Lim JH, Li JD, Yang J, et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ Res. 2008;102:538–545. doi: 10.1161/CIRCRESAHA.107.156877. [DOI] [PubMed] [Google Scholar]
- 19.Akaike M, Che W, Marmarosh NL, Ohta S, Osawa M, Ding B, et al. The hinge-helix 1 region of peroxisome proliferator-activated receptor gamma1 (PPARgamma1) mediates interaction with extracellular signal-regulated kinase 5 and PPARgamma1 transcriptional activation: Involvement in flow-induced PPARgamma activation in endothelial cells. Mol Cell Biol. 2004;24:8691–8704. doi: 10.1128/MCB.24.19.8691-8704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, et al. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–e73. doi: 10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Bräsen JH, Skatchkov M, et al. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: Evidence for involvement of the renin- angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 23.Frey RS, Ushio-Fukai M, Malik A. NADPH oxidase-dependent signaling in endothelial cells: Role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiai TK, Hwang J, Barr ML, Correa A, Hamilton R, Alavi M, et al. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radic Biol Med. 2007;42:519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox-state: Role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 26.Go YM, Patel RP, Maland MC, Park H, Beckman JS, Darley-Usmar VM, et al. Evidence for peroxynitrite as a signaling molecule in flow-dependent activation of c-Jun NH2-terminal kinase. Am J Physiol. 1999;277:H1647–H1653. doi: 10.1152/ajpheart.1999.277.4.H1647. [DOI] [PubMed] [Google Scholar]
- 27.Ago T, Kuroda J, Kamouchi M, Sadoshima J, Kitazono T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system: Review and perspective. Circ J. 2011;75:1791–1800. doi: 10.1253/circj.cj-11-0388. [DOI] [PubMed] [Google Scholar]
- 28.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 29.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 30.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, et al. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 31.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 32.Masai N, Tatebe J, Yoshino G, Morita T. Indoxyl sulfate stimulates monocyte chemoattractant protein-1 expression in human umbilical vein endothelial cells by inducing oxidative stress through activation of the NADPH oxidase-nuclear factor-κB pathway. Circ J. 2010;74:2216–2224. doi: 10.1253/circj.cj-10-0117. [DOI] [PubMed] [Google Scholar]
- 33.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: Role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 34.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKCzeta regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 35.Li RH, Wozney JM. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol. 2001;19:255–265. doi: 10.1016/s0167-7799(01)01665-1. [DOI] [PubMed] [Google Scholar]
- 36.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 37.Resnick N, Gimbrone MA., Jr. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 38.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 39.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, et al. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol. 2006;168:629–638. doi: 10.2353/ajpath.2006.050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation. 1997;96:2361–2367. doi: 10.1161/01.cir.96.7.2361. [DOI] [PubMed] [Google Scholar]
- 41.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 42.Csiszar A, Labinskyy N, Smith KE, Rivera A, Bakker EN, Jo H, et al. Downregulation of bone morphogenetic protein 4 expression in coronary arterial endothelial cells: Role of shear stress and the cAMP/protein kinase A pathway. Arterioscler Thromb Vasc Biol. 2007;27:776–782. doi: 10.1161/01.ATV.0000259355.77388.13. [DOI] [PubMed] [Google Scholar]
- 43.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: Role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 44.Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol. 2000;2:666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- 45.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Endres M, Laufs U, Merz H, Kaps M. Focal expression of intercellular adhesion molecule-1 in the human carotid bifurcation. Stroke. 1997;28:77–82. doi: 10.1161/01.str.28.1.77. [DOI] [PubMed] [Google Scholar]
- 47.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 48.Mohan S, Mohan N, Valente AJ, Sprague EA. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am J Physiol. 1999;276:C1100–C1107. doi: 10.1152/ajpcell.1999.276.5.C1100. [DOI] [PubMed] [Google Scholar]
- 49.Chiu YJ, Kusano K, Thomas TN, Fujiwara K. Endothelial cell-cell adhesion and mechanosignal transduction. Endothelium. 2004;11:59–73. doi: 10.1080/10623320490432489. [DOI] [PubMed] [Google Scholar]
- 50.Dekker LV, Parker PJ. Protein kinase C: A question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 51.Magid R, Davies PF. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ Res. 2005;97:443–449. doi: 10.1161/01.RES.0000179767.37838.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 53.Melchior F. SUMO: Nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 54.Lin K, Hsu PP, Chen BP, Yuan S, Usami S, Shyy JY, et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci USA. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian H, Wittmack EK, Jorgensen TJ. p21 WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679–684. [PubMed] [Google Scholar]
- 56.Garner E, Raj K. Protective mechanisms of p53-p21-pRb proteins against DNA damage-induced cell death. Cell Cycle. 2008;7:277–282. doi: 10.4161/cc.7.3.5328. [DOI] [PubMed] [Google Scholar]
- 57.Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, Maeda N. Enhanced atherosclerosis and kidney dysfunction in eNOS−/− ApoE−/− mice are ameliorated by enalapril treatment. J Clin Invest. 2000;105:451–458. doi: 10.1172/JCI8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhlencordt PJ, Gyurko R, Han F, Scherrer-Crosbie M, Aretz TH, Hajjar R, et al. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 59.Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- 60.Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circ Res. 2003;92:1089–1097. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- 61.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther. 2009;329:94–101. doi: 10.1124/jpet.108.145557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 64.Hwang J, Ing MH, Salazar A, Lassèque B, Friendling K, Navab M, et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: Implication for native LDL oxidation. Circ Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen YR, Chen CL, Yeh A, Liu X, Zweier JL. Direct and indirect roles of cytochrome b in the mediation of superoxide generation and NO catabolism by mitochondrial succinate-cytochrome c reductase. J Biol Chem. 2006;281:13159–13168. doi: 10.1074/jbc.M513627200. [DOI] [PubMed] [Google Scholar]
- 66.Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, et al. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol. 1999;276:C838–C847. doi: 10.1152/ajpcell.1999.276.4.C838. [DOI] [PubMed] [Google Scholar]
- 67.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells: A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 68.Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem Biophys Res Commun. 2003;307:973–979. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- 69.Zhou G, Bao ZQ, Dixon JE. Components of a new human protein kinase signal transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 70.Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: A new mammalian MAP kinase. Biochem Biophys Res Commun. 1995;213:715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, et al. Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest. 2004;113:1138–1148. doi: 10.1172/JCI19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 73.Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, Nakajima K. Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. Mol Cell. 2006;24:63–75. doi: 10.1016/j.molcel.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- 75.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 76.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sohn SJ, Sarvis BK, Cado D, Winoto A. ERK5 MAPK Regulates Embryonic Angiogenesis and Acts as a Hypoxia- sensitive Repressor of Vascular Endothelial Growth Factor Expression. J Biol Chem. 2002;277:43344–43351. doi: 10.1074/jbc.M207573200. [DOI] [PubMed] [Google Scholar]
- 78.Woo CH, Massett MP, Shishido T, Itoh S, Ding B, McClain C, et al. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor delta (PPARdelta) stimulation. J Biol Chem. 2006;281:32164–32174. doi: 10.1074/jbc.M602369200. [DOI] [PubMed] [Google Scholar]