Abstract
TNFAIP8 is a NF-κB-inducible, oncogenic molecule. Previous “promoter array” studies have identified differential methylation and regulation of TNFAIP8 in prostate epithelial and cancer cell lines. Here we demonstrate that TNFAIP8 expression is induced by androgen in hormone-responsive LNCaP prostate cancer cells. In athymic mice bearing hormone-refractory PC-3 prostate tumor xenografts, intravenous treatment with a liposomal formulation of TNFAIP8 antisense oligonucleotide (LE-AS5) caused reduced expression of TNFAIP8 in tumor tissues, and a combination of LE-AS5 and radiation or docetaxel treatment resulted in significant inhibition of PC-3 tumor growth as compared to single agents. The immunohistochemical evaluation of TNFAIP8 expression revealed correlation of both cytoplasmic and nuclear TNFAIP8 overexpression with high grade prostatic adenocarcinomas, while nuclear overexpression was found to be an independent predictor of disease recurrence controlling for tumor grade. Increased nuclear TNFAIP8 expression was statistically significantly associated with a 2.44 fold (95 % confidence interval: 1.01–5.91) higher risk of prostate cancer recurrence. Mechanistically, TNFAIP8 seems to function as a scaffold (or adaptor) protein. In the antibody microarray analysis of proteins associated with the TNFAIP8 immune-complex, we have identified Karyopherin alpha2 as a novel binding partner of nuclear TNFAIP8 in PC-3 cells. The Ingenuity Pathway Analysis of the TNFAIP8 interacting proteins suggested that TNFAIP8 influences cancer progression pathways and networks involving integrins and matrix metalloproteinases. Taken together, present studies demonstrate that TNFAIP8 is a novel therapeutic target in prostate cancer, and indicate a potential relationship of the nuclear trafficking of TNFAIP8 with adverse outcomes in a subset of prostate cancer patients.
Keywords: prostate cancer, therapy response, prognosis, TNFAIP8, KPNA2
Introduction
Prostate cancer is the second leading cause of cancer-related deaths in men.1 Approximately 38–51% of patients present with locally advanced disease, and 10–50% of this group of patients rapidly progress to a hormone-refractory state. Five year survival rate of metastasized tumor is only 31%. Currently, there are no biological benchmarks for stratification of clinically localized prostate cancer that has a high likelihood of recurrence.2 The elucidation of molecules and signaling pathways that drive recurrent and metastatic prostate cancer is likely to offer new avenues for an early identification and therapeutic intervention of aggressive prostate cancer.
TNFAIP8 (TNF-α-inducible protein 8) is a transcription factor NF-κB-inducible, anti-apoptotic and oncogenic molecule.3–7 TNFAIP8 gene also reported as SCC-S2/GG2-1/NDED was discovered by comparison of the expression profile of a primary human head and neck squamous cell carcinoma-derived cell line with its matched metastatic cell line established from a recurrent lymph node following surgery and radiation therapy.8 We first showed that TNFAIP8 is a 21 kDa cytosolic TNF-α-inducible molecule and a member of the FLIP family of cell death inhibitory proteins.3 TNF-α-mediated induction of TNFAIP8 mRNA is reversible by IκBα, an inhibitor of NF-κB, and expression of TNFAIP8 in NF-κB-null cells causes inhibition of TNF-α-induced apoptosis.5 Positive correlations of TNFAIP8 expression with tumor growth, in vitro invasion, and experimental metastasis have been demonstrated.6, 7 The “promoter array” studies have identified differential methylation and regulation of TNFAIP8 in prostate epithelial and cancer cell lines.9 In a recent study, polymorphism of TNFAIP8 has been associated with non-Hodgkin’s lymphoma.10
The therapeutic significance of TNFAIP8 is unknown. Mechanistically, TNFAIP8 has been suggested to function as a cytosolic scaffold (or adaptor) protein. However, the binding partners and signaling pathways of TNFAIP8 are also not known. This is the first study to demonstrate that TNFAIP8 is an androgen-inducible molecule with a dual role in therapy response in vivo and poor prognosis of prostate cancer. For the first time, we have revealed the nuclear TNFAIP8 and its link with recurrent prostatic adenocarcinomas (PACs). Karyopherin alpha 2 (KPNA2/importin-α1) is a cytosolic receptor known to play a pivotal role in the nuclear protein import pathway in multiple cancers.11 Interestingly, nuclear KPNA2 expression has been reported as an independent prognostic marker of disease recurrence after radical prostatectomy.12 It remains unclear how KPNA2 impacts prostate cancer progression. Using the combined siRNA, immunoprecipitation, and antibody microarray approaches, we have identified KPNA2 as a novel binding partner of nuclear TNFAIP8. In addition, we have identified potential protein-protein interactions involving TNFAIP8, and developed a working hypothesis of integrative pathways signifying TNFAIP8 function in prostate cancer progression. These studies demonstrate that TNFAIP8 is a therapeutic and prognostic target in prostate cancer and indicate potentially adverse consequences of the trafficking of TNFAIP8 to the nucleus.
Materials and Methods
Cell cultures
LNCaP prostate cancer cells were cultured in Improved Minimum Essential Medium (IMEM) supplemented with heat inactivated 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen Life Technologies, Inc., Carlsbad, CA). PC-3 and DU-145 prostate cancer cells were cultured in IMEM or Dulbecco’s minimum essential medium (DMEM) (Invitrogen) supplemented as above. All cultures were maintained at 37°C in a humidified incubator containing 5% CO2 and 95% air.
Androgen and TNFAIP8 siRNA treatments
Logarithmically growing LNCaP cells were cultured in IMEM with 5% FBS for three days and medium was then switched to IMEM containing 5% charcoal stripped fetal calf serum for overnight followed by treatment with synthetic androgen as described in Supporting Information Materials and Methods. For TNFAIP8 siRNA treatment, logarithmically growing PC-3 cells were seeded into 100 mm dishes (1.5 × 106 cells/dish) in complete IMEM medium containing 10% FBS. Next day, medium was switched to 5 ml Opti-MEMI medium (GIBCO Invitrogen) containing indicated concentration of stealth TNFAIP8 siRNA (Invitrogen) and Lipofectamine 2000 complex prepared according to supplier’s instructions (Invitrogen) as described in Supporting Information Materials and Methods.
Animals
Male athymic nude mice 6–8 weeks old were purchased from the Animal Production Area of the National Cancer Institute (Frederick, MD) and used for subcutaneous tumor xenograft experiments. The mice were maintained at the AAALAC-accredited Research Resource Facility of the Division of Comparative Medicine, Georgetown University Medical Center. All animal experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee for humane treatment and care of animals in research and education.
Design and synthesis of oligonucleotides
A 14-mer antisense TNFAIP8 oligodeoxyribonucleotide sequence (AS5) with only one base at each end phosphorothioated (5′-GsTGGCCATCGGAGsG-3′) was designed against the translation initiation region of the TNFAIP8 mRNA. The fully phosphorothioated oligonucleotides containing the CpG motifs are immunostimulatory; however, no such activity has been seen with short phosphodiester oligos (< 44 nucleotides) containing CpG motifs.13,14 In addition, we have demonstrated that short phosphodiester oligos with single end base modifications did not contribute to changes in complement activity.15 The AS5 sequence does not contain the human CpG motif sequence and is not likely to generate the immune response. The pre-clinical grade HPLC purified oligos were custom-synthesized (AS5: Genset Oligos, Paris and TriLink Biotechnologies, Inc., San Diego, CA). A control terminal base-modified mismatch oligo (MM: 5′-GsTGTTCGACCTATGsC-3′) was custom synthesized at Hybridon Specialty Products (Milford, MA). All oligos were reconstituted in normal saline.
Formulation of liposome-entrapped TNFAIP8 antisense oligonucleotide, and liposome-entrapped TNFAIP8 antisense oligonucleotide and radiation or docetaxel treatments in vivo
Cationic liposome-entrapped terminal base-modified TNFAIP8 antisense oligo (LE-AS5) and control mismatch oligo (LE-MM) (particle size approximately 450 nm, entrapment efficiency > 85%) were formulated as we reported earlier and detailed in Supporting Information Materials and Methods.15, 16 PC-3 tumor cells were injected subcutaneously in the left flank regions of 6-to 8- week old male athymic nude mice. Tumor-bearing animals were randomized into various treatment groups and treated as detailed in Supporting Information Materials and Methods.
Tissue micro-array, clinical specimens, immunohistochemistry and statistical analysis
A prostate cancer tissue micro-array (TMA) representing 64 evaluable cases of histologically confirmed PACs was commercially obtained (PR8010, US Biomax, Inc., Rockville, MD). Of these 64 cases, 26 cases were low grade (Gleason score ≤ 6), 18 cases were intermediate grade (Gleason score = 7), and the remaining 20 cases were high grade (Gleason score = 8–10). In addition, formalin-fixed, paraffin-embedded tissue sections from a total of 135 biopsy-proven PACs were available from the Albany Medical Center Hospital. Cases in which neoadjuvant hormone therapy was administered were excluded. Hematoxylin and eosin-stained slides from each radical prostatectomy case were reviewed, and a Gleason grade and pathologic stage were assigned as described earlier.17–19 Multiple blocks were reviewed to verify the presence of adequate tumor and the nature of the overall grade as well as the presence of adjacent benign epithelium. Of the 135 PACs, there were 26 high/intermediate grade (Gleason score ≥ 7) non-recurrent cases, 29 high/intermediate grade, recurrent cases, 51 low grade (Gleason score ≤ 6) non-recurrent cases, and 29 low grade recurrent cases. A post-surgical elevation of the serum prostate-specific antigen (PSA) level from a baseline level of 0 ng/ml to greater than 0.4 ng/ml on 2 consecutive occasions was considered as biochemical evidence of disease recurrence. In accordance with the institutional review board guidelines and policies, all existing pathological specimens, including the commercial TMA, were distributed and used in this study in a de-identified manner and could not be linked to individual subjects.
The prostate tumor TMA and the standard histological slides with paraffin-embedded tissue sections were immunostained by an automated and standardized method on the Ventana ES (Ventana Medical Systems, Inc., Tucson, AZ) using a custom-made and validated polyclonal anti-peptide TNFAIP8 antibody (Covance Research Products Inc.).7 Immunohistochemical (IHC) evaluation included staining intensity subjectively graded as weak, 1; moderate, 2: or intense, 3; and percentage of positive cells graded as focal (<10%), 1; regional (10–50%), 2; or diffuse distribution (>50%), 3. Intensity and distribution scores were given equal weight and a total score was obtained by multiplying the intensity score by the distribution score. In tumor versus adjacent benign specimens, both the malignant and adjacent benign prostatic epithelia were separately scored for cytoplasmic and nuclear TNFAIP8, and each component (cytoplasmic or nuclear) was assigned into 1 of 3 categories as follows: cases in which the score for the tumor was equal to benign, less than benign, and greater than benign in the same case.
Statistical comparisons were carried out with the STATA software (Stata Corporation, College Station, TX). The χ2 test was used to determine the statistical significance of the associations between cytoplasmic or nuclear TNFAIP8 expression and prognostic variables. Multivariable analysis to evaluate the associations between time to recurrence and predictors of interest, including clinicopathological parameters and expressions of nuclear or cytoplasmic TNFAIP8, was performed using the Cox proportional hazards model. P values < 0.05 were considered statistically significant.
Antibody microarray and bioinformatics for analysis of TNFAIP8 interacting proteins
PC-3 cells were treated with TNFAIP8 siRNA (Si2siRNA/siRNA) or a scrambled siRNA (ScrsiRNA/MM). The knockdown of TNFAIP8 in siRNA treated cells versus ScrsiRNA treated cells was verified by immunoprecipitation and immunoblotting with anti-TNFAIP8 antibody. The TNFAIP8 immune-complexes were analyzed for differences in levels of proteins co-immunoprecipitating with TNFAIP8 in siRNA versus ScrsiRNA- treated samples using antibody microarray (Antibody Microarray 507, Clontech) according to the manufacturer’s instructions as we have detailed earlier and in Supporting Information Materials and Methods.20, 21
Results
Characterization of TNFAIP8 in prostate cancer cells
The schematic maps of the genomic organization and the promoter region of human TNFAIP8 are shown in Supporting Information Figs. 1a and 1b. TNFAIP8 gene promoter is TATAless. Genomatix analysis (http://www.genomatix.de/en/index.htm) of the TNFAIP8 promoter region showed androgen response elements (ARE) within 1500 bp upstream of the 5′-UTR. In the Northern blot hybridization and immunoblotting assays, our data demonstrate that TNFAIP8 expression is induced by synthetic androgen in hormone-responsive LNCaP prostate cancer cells (R1881, 1nM, 48 hr: TNFAIP8 mRNA, 2–3 fold; TNFAIP8 protein, 3–4 fold) (Figs. 1a and1b, and data not shown). In the publicly available datasets (https://www.oncomine.org/resource/login.html and http://www.ncbi.nlm.nih.gov/geoprofiles?term=TNFAIP8) (Supporting Information Figs. 2a and 2b) and from our Northern blot analysis of TNFAIP8 mRNA in renal cell carcinoma and matched benign specimens (data not shown), increased TNFAIP8 mRNA expression seems to correlate with tumor initiation and/or progression in diverse organs. In addition, several reports indicate a role of the vascular endothelial growth factor axis in prostate cancer lymphatic metastasis via its receptor VEGFR-2. To begin to understand the role of TNFAIP8 in prostate cancer progression, we determined the effects of TNFAIP8 silencing on expression of key invasion and angiogenic molecules (MMPs and VEGFR-2) in prostate cancer cells. As shown in Supporting Information Figs. 3a–3c, siRNA knockdown of TNFAIP8 protein in hormone refractory PC-3 prostate cancer cells was found to be associated with a concomitant inhibition of MMP-1, MMP-2, MMP-9, MT1-MMP, and VEGFR-2. The siRNA knockdown of TNFAIP8 was also found to be associated with decreased expression of several of these molecules in pancreatic (Aspc-1, Colo-357), melanoma (SK-Mel-5) and colon carcinoma cells (HT-29 and SW620) (protein expression,% of ScrSi: Aspc-1, TNFAIP8, 0.1%, MMP-1, 52.6%; Colo-357, TNFAIP8, 30.2%, MMP-2, 81.4%; SK-Mel-5, TNFAIP8, 60.7%, MMP-1, 66.2%, MMP-9 (proform), 62.9%, VEGFR-2, 46.4%; HT-29, TNFAIP8, 18.3%, VEGFR-2, 79.1%; SW620, TNFAIP8, 0.1%, MMP-9, 67.3%). These data demonstrate that TNFAIP8 is an androgen-inducible molecule, and a regulator of members of the matrix metalloproteinase (MMPs) family and VEGFR-2 in prostate and other highly aggressive cancer cells.
Figure 1.
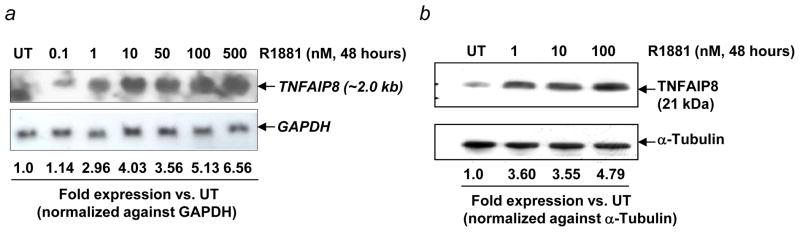
Androgen induces TNFAIP8 mRNA and protein expression in prostate cancer cells. (a) LNCaP cells were grown in IMEM supplemented with 5% FBS. Logarithmically growing cells were switched to medium containing 5% charcoal-stripped fetal calf serum for 24 hr, followed by treatment with indicated concentrations of synthetic androgen R1881 for 48 hr. Total RNA was analyzed by Northern blotting using radiolabeled TNFAIP8 cDNA and GAPDH cDNA probes as described in Supporting Information Materials and Methods. TNFAIP8 mRNA expression was normalized to the GAPDH mRNA signal in the corresponding lanes. (b) LNCaP cells were treated with R1881 as in panel (a). Whole cell extracts were subjected to 4–12% NuPAGE (50 μg protein/lane), followed by sequential immunoblotting with anti-TNFAIP8 (1:1000 dilution) and anti-α-Tubulin antibodies (1:1000 dilution). TNFAIP8 expression was normalized to the α-Tubulin signal in the corresponding lanes. UT, untreated.
Enhanced antitumor effect of a combination of systemically-delivered liposome-entrapped TNFAIP8 antisense oligonucleotide and ionizing radiation or docetaxel in PC-3 tumor-bearing athymic mice
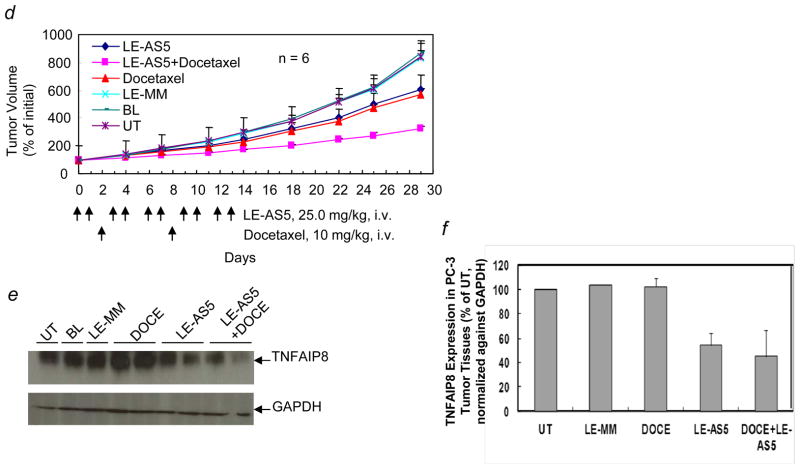
Next, to determine the therapeutic benefit of targeting TNFAIP8 in a hormone refractory model of prostate cancer, we used a systemically-delivered liposomal formulation of TNFAIP8 antisense oligonucleotide (LE-AS5) to downregulate TNFAIP8 expression in PC-3 tumor xenografts grown in athymic mice. TNFAIP8 antisense oligonucleotide AS5 is a 14-mer sequence with one base at each end phosphorothioated. The experiments were performed to determine the TNFAIP8 knockdown efficiencies of AS5 in in vitro and in vivo tumor cell models (Supporting Information Figs. 4a–4c). Interestingly, AS5 exhibited superior efficiency in vitro compared to its fully phosphorothioated counterpart sequence (AS1) (Supporting Information Fig. 4a). In initial pilot in vivo experiments, systemically delivered liposome-entrapped AS5 (LE-AS5) was found to inhibit TNFAIP8 expression in PC-3 tumor xenografts grown in athymic mice (Supporting Information Fig. 4b). Similar results were obtained in MDA-MB-435 tumor xenograft model (data not shown). We asked whether a combination of LE-AS5 and ionizing radiation (IR) or docetaxel (DOCE) treatments will result in an enhanced antitumor efficacy as compared to IR or DOCE alone. Systemically delivered liposome-entrapped mismatch oligonucleotide (LE-MM) at the same dosing and schedules as LE-AS5, systemic blank liposomes without oligonucleotide (BL), and untreated tumor-bearing mice (UT) were included as controls. As shown in Fig. 2a, LE-AS5 or IR treatment led to inhibition of PC-3 tumor growth, and a combination of LE-AS5 and IR resulted in significant decrease in tumor growth as compared to IR treatment alone. The mean tumor volumes ± S.E. (% initial) in various treatment groups on day 29, 16 days after last dosing, were: LE-AS5 + IR, 69 ± 4.6; IR, 162.2 ± 27.9; LE-AS5, 246.6 ± 78.2; LE-MM, 427.8 ± 135.3; BL, 504.2 ± 194.9; UT, 368.5 ± 53.8 (IR versus UT, p < 0.01, IR versus LE-AS5, p > 0.05; IR versus LE-AS5 + IR, p < 0.01, n = 3–6). Within 6–12 hr after the last LE-AS5 treatment, representative mice in various treatment groups were sacrificed and tumors were excised for expression analysis (Fig. 2b). In PC-3 tumor tissues from mice treated with LE-AS5 or a combination of LE-AS5 and IR, TNFAIP8 expression was significantly decreased as compared to LE-MM or IR alone treated mice (TNFAIP8 expression, % of UT: LE-MM, 80.6 ± 7.1%, IR, 78.4 ± 2.3%; LE-AS5, 28 ± 15.3 %, LE-AS5 + IR, 31.8 ± 1%; n =2) (Fig. 2c).
Figure 2.
Enhanced antitumor efficacy of a combination of ionizing radiation or docetaxel and systemically administered liposome-entrapped TNFAIP8 antisense oligonucleotide (LE-AS5) in PC-3 tumor xenograft model. (a) LE-AS5-mediated PC-3 tumor radiosensitization in vivo. PC-3 tumor bearing athymic mice were randomized into six groups. LE-AS5 and LE-AS5 + IR treatment groups received 25.0 mg/kg/dose (i.v., ×10) of LE-AS5 over 14 days. IR alone and LE-AS5 + IR groups received 3.8 Gy/day IR daily (day 5 through day 8). BL and LE-MM groups received blank liposomes and liposome-encapsulated mismatch oligo, respectively, at the same dosing schedule as LE-AS5. Additional control group was left untreated (UT). Tumor volumes were monitored and individual tumor volume (% initial) was calculated as the percentage of pre-treatment tumor volume (day 0, the first day of dosing; 100%). The values shown are mean tumor volume (% initial) ± S.E. (b) Inhibitory effect of LE-AS5 on TNFAIP8 expression in PC-3 tumor tissues. Two mice from each treatment group in panel (a) were sacrificed within 6–12 hr after the last dosing, and TNFAIP8 expression in tumor tissue homogenates as analyzed by Western blotting with anti-TNFAIP8 antibody. The blot was reprobed with anti-GAPDH antibody. (c) Quantification of TNFAIP8 expression relative to UT was performed after normalization of the signal against GAPDH signal in corresponding lanes. (d) LE-AS5-mediated PC-3 tumor sensitization to docetaxel in vivo. PC-3 tumor bearing mice were randomized into six groups. LE-AS5 and LE-AS5 + docetaxel treatment groups received 25.0 mg/kg/dose (i.v., ×10) of LE-AS5 over 14 days. Docetaxel alone and LE-AS5 + docetaxel groups received 10 mg/kg docetaxel (i.v.) on day 2 and day 8. BL and LE-MM groups received blank liposomes (BL) and liposome-encapsulated mismatch oligo, respectively, systemically at the same dosing schedule as LE-AS5. Additional control group was left untreated (UT). Tumor volumes were monitored and individual tumor volume (% initial) was calculated as the percentage of pre-treatment tumor volume (day 0, the first day of dosing; 100%). The values shown are mean tumor volume (% initial) ± S.E. (e) Effect of LE-AS5 on TNFAIP8 expression in PC-3 tumor tissues. Representative animals from each treatment group in panel (d) were sacrificed within 6–12 hr after last dosing, and tumor tissues homogenates were analyzed by Western blotting with anti-TNFAIP8 antibody. The blot was reprobed with anti-GAPDH antibody. (f) Quantification of TNFAIP8 expression relative to UT was performed after normalization of the signal against GAPDH signal in corresponding lanes. DOCE, docetaxel.
In independent experiments, a combination of systemically-administered LE-AS5 and DOCE caused significant inhibition of tumor growth as compared to DOCE alone. The mean tumor volumes ± SE (% initial) in various treatment groups on day 29, 16 days after last dosing were: LE-AS5 + DOCE, 321.1 ± 24.5; DOCE, 571.5 ± 39.7; LE-AS5, 604.9 ± 110; LE-MM, 830.2 ± 52.4; BL, 864.7 ± 45.9; UT, 841.6 ± 91.5 (DOCE versus UT, p < 0.01, DOCE versus LE-AS5, p > 0.05, DOCE versus LE-AS5 + DOCE, p < 0.005, n = 4) (Fig. 2d). The experiment was repeated with similar results. In the repeat experiment, one animal out of six in the combination group showed tumor cure. This animal was monitored for up to 70 days and no tumor recurrence was noted. Within 6–12 hr after the last LE-AS5 treatment, representative mice in various treatment groups were sacrificed and tumors were excised for expression analysis (Fig. 2e). In PC-3 tumor tissues from mice treated with LE-AS5 or a combination of LE-AS5 and DOCE, TNFAIP8 expression was significantly decreased as compared to LE-MM or DOCE alone treated mice (TNFAIP8 expression, % of UT: LE-MM, 103.4 ± 0.0%, DOCE, 102.4 ± 7.1%, LE-AS5, 54.4 ± 10.0%, LE-AS5 + DOCE, 44.9 ± 21.5%; n =2) (Fig. 2f).
Blank liposomes have been shown to cause dose-related elevations in liver enzymes (AST and ALT), mild to moderate histopathological changes in liver, and mild to moderate splenic enlargements in mice15. In addition, TNFAIP8 expression seems to vary significantly in different normal tissues of mice tested, with relatively high expression in spleen, kidney and mammary gland, low expression in lung and heart, and an almost undetectable expression in liver (data not shown). In this study, no morbidity or mortality was observed in any of the treatment groups. In addition, no gross pathology changes were noted in any of the treatment groups.
Prognostic significance of TNFAIP8 expression in prostatic adenocarcinomas: nuclear TNFAIP8 immunoreactivity is an independent predictor of disease recurrence controlling for tumor grade
To evaluate the status of TNFAIP8 protein expression in clinical specimens from prostate cancer patients, we first characterized our custom-generated rabbit polyclonal TNFAIP8 antibody for specificity and sensitivity by Western blotting and immunoprecipitation in a variety of human cancer cell lines, human tumor xenografts and clinical specimens (Supporting Information Figs. 5a–5c), and by IHC in standard histological slides of PACs and adjacent benign tissues (Fig. 3a) as well as in a prostate tumor TMA (Supporting Information Fig. 6a). The TNFAIP8 antibody specifically and distinctly detected the expected one or both isoforms of TNFAIP8 (21–23 kDa) in whole cell extracts from various cancer cell lines and clinical tissues (Supporting Information Figs. 5a–5c). The siRNA knockdown of TNFAIP8 expression in prostate and other cancer cells, serial dilutions of the antibody, pre-immune serum, and non-specific IgG were tested as controls (Supporting Information Figs. 3 and 5c). In the cell fractionation experiments, relatively high expression of cytosolic TNFAIP8 was observed in hormone refractory PC-3 and DU-145 cells as compared to hormone responsive LNCaP cells (Supporting Information Fig. 7). Unexpectedly, we also detected nuclear TNFAIP8 expression in PC-3 and DU-145 cells, albeit to a much lesser extent as compared to the cytosolic TNFAIP8 in these cells (Supporting Information Fig. 7). The nuclear TNFAIP8 expression was undetectable in LnCaP cells (Supporting Information Fig. 7). In a prostate tumor TMA with 64 independent, evaluable cases, 42% (11/26) of low grade PACs (Gleason score ≤ 6) revealed moderate to intense cytoplasmic TNFAIP8. In contrast, 89% (34/38) of intermediate (Gleason score = 7) and high grade tumors (Gleason score ≥ 8) on the same TMA had moderate to intense cytoplasmic TNFAIP8 (P < 0.001). In the same TMA, moderate to intense nuclear TNFAIP8 was seen in 4% (1/26) of low grade and 13% (5/38) of intermediate and high grade PACs (P= 0.209) (Supporting Information Fig. 6a).
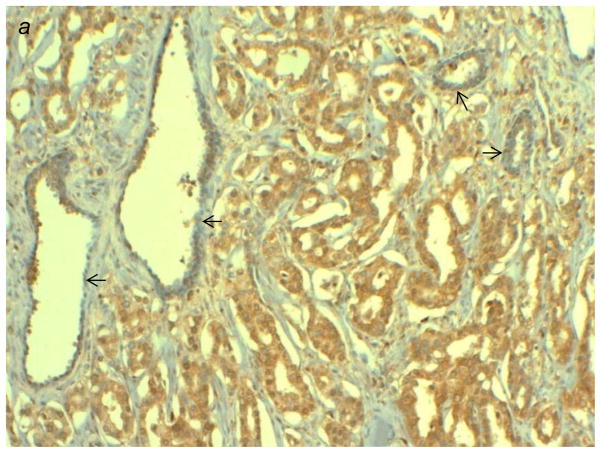
Figure 3.
(a) Immunostaining patterns of TNFAIP8 in high grade prostatic adenocarcinoma (PAC) versus adjacent benign glands (indicated by arrows). High grade PAC is showing intense cytoplasmic staining, whereas adjacent benign prostate shows weak, focal staining of TNFAIP8. (b–d) Expression and localization of TNFAIP8 in representative clinical specimens of PACs. (b) Low grade PAC showing weak to moderate cytoplasmic TNFAIP8, (c) high grade PAC showing intense cytoplasmic and intense nuclear TNFAIP8, and (d) high grade PAC showing intense nuclear TNFAIP8. (e) Kaplan-Meier survival curves estimates by nuclear TNFAIP8 immunoreactivity groups in prostatic adenocarcinoma patients.
Next, we set out to examine the prognostic significance of cytoplasmic and nuclear TNFAIP8 in formalin-fixed, paraffin-embedded sections of human PACs (Figs. 3a–3e, and Supporting Information Figs. 6b and 6c). In brief, variable cytoplasmic and nuclear TNFAIP8 immunoreactivity was observed in both malignant glands and adjacent benign epithelium. The cytoplasmic immunoreactivity in tumor versus benign cells was comparable in 117/135 cases. In the remaining cases, cytoplasmic TNFAIP8 expression correlated with tumor grade with 75% (6/8) high grade tumors overexpressing cytoplasmic TNFAIP8 protein relative to adjacent benign epithelium, while 90% (9/10) low grade tumors showed decreased expression relative to adjacent benign epithelium (P = 0.017) (Supporting Information Fig. 6b). Nuclear TNFAIP8 expression was noted in 42/135 PACs and was either comparable to (26/42) or increased over the adjacent benign epithelium (16/42). Among the tumors that showed increased nuclear expression, 81% (13/16) were high grade (P = 0.072) (Supporting Information Fig. 6b). Prostate cancer recurrence has been associated with advanced stage and/or high grade tumor. Among the 42 cases showing nuclear TNFAIP8 expression, increased nuclear expression correlated with disease recurrence with 52% (11/21) recurrent tumors overexpressing nuclear TNFAIP8 as compared to 24% (5/21) non-recurrent tumors (P = 0.057) (Supporting Information Fig. 6c). When the time until recurrence was considered using a Cox univariate regression analysis, increased nuclear TNFAIP8 predicted earlier disease recurrence (P = 0.021) (Fig. 3e). In a multivariable Cox regression analysis of grade, stage, cytoplasmic and increased nuclear TNFAIP8 expression, only grade (P = 0.011) and increased nuclear TNFAIP8 (P = 0.048) were found to be independent prognostic variables for time to recurrence. Independent of tumor grade, we found that increased nuclear TNFAIP8 expression was statistically significantly associated with a 2.44 fold (95 % confidence interval: 1.01–5.91) higher risk of prostate cancer recurrence. With these results, we conclude that both cytoplasmic and nuclear TNFAIP8 overexpression correlate with high grade PACs, while nuclear TNFAIP8 overexpression is an independent predictor of recurrent prostate cancer controlling for tumor grade.
Identification of KPNA2 (importin-α1) as a novel binding partner of TNFAIP8
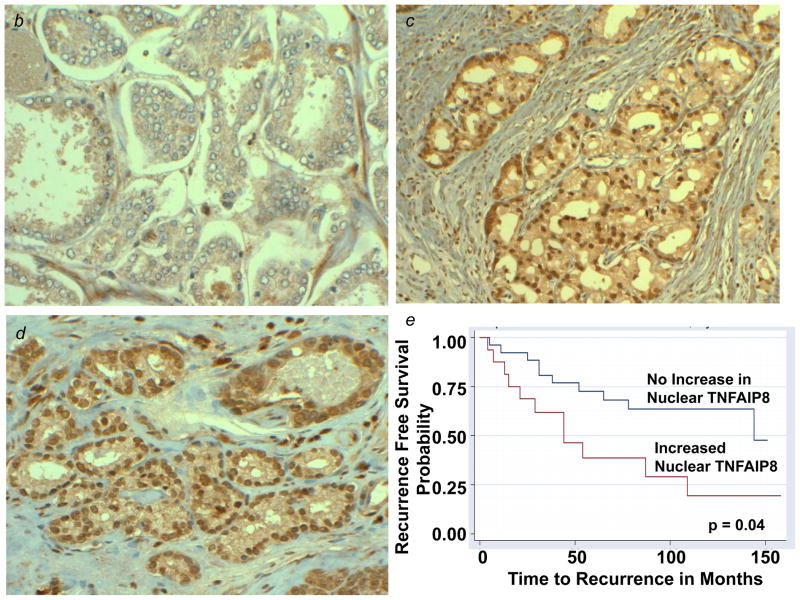
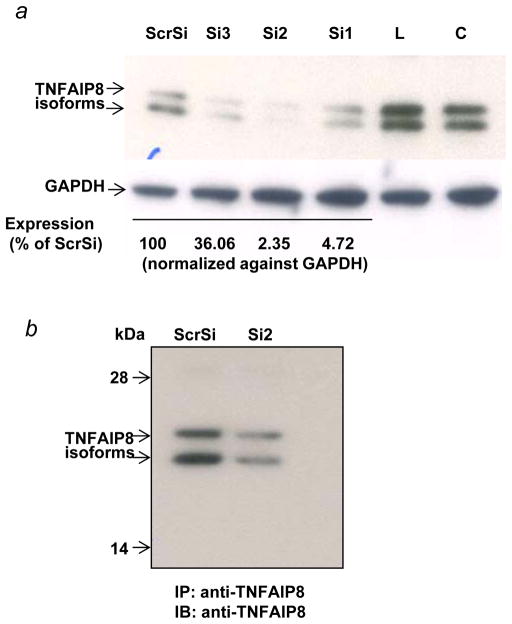
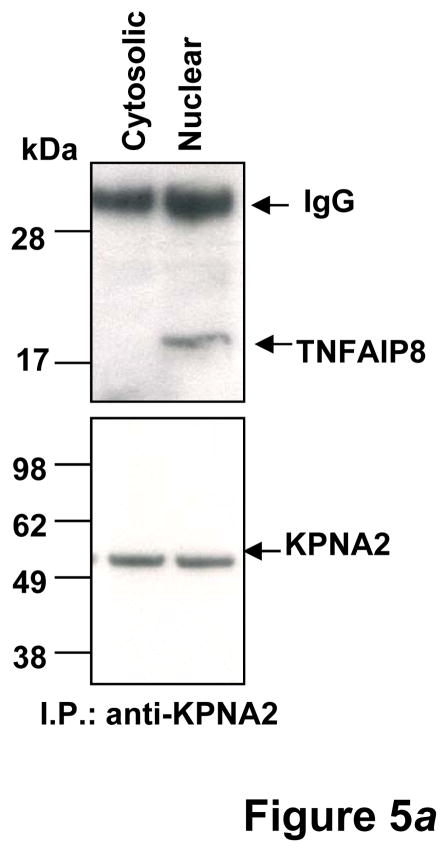
TNFAIP8 is a predominately cytosolic protein and the TNFAIP8 open reading frame has no apparent nuclear localization signal. As a first step toward elucidation of mechanism of nuclear transport of TNFAIP8, we used a combination of the siRNA knockdown, immunoprecipitation and antibody microarray approaches to identify potential binding partners of TNFAIP8. Down regulation of TNFAIP8 in PC-3 cells was verified by the Western blotting and immunoprecipitation assays (Figs. 4a and 4b). A comparison of the proteomic profile of TNFAIP8 immune-complex from TNFAIP8 siRNA treated PC-3 cells with scrambled siRNA treated cells revealed several potential binding partners of TNFAIP8 (Table 1, and Supporting Information Table 1). Of particular interest, we found that a relatively significant level of Karyopherin alpha 2 (KPNA2/importin-α1) was present in the TNFAIP8 immune-complex from scrambled siRNA treated PC-3 cells as compared with TNFAIP8 siRNA treated cells (P= 0.00019) (Table 1). To verify KPNA2 as a novel binding partner of TNFAIP8, we performed the reciprocal immunoprecipitation using anti-Karyopherin α/Rch-1 antibody and the cytosolic and nuclear fractions from PC-3 cells. Interestingly, TNFAIP8 was detectable in the Karyopherin α/Rch-1 immune-complex from the nuclear fraction but not the cytosolic fraction (Fig. 5a).
Figure 4.
(a) Verification of the siRNA silencing of TNFAIP8 in PC-3 cells by Western blotting. PC-3 cells were treated with 100 nM of the stealth TNFAIP8 siRNA (Si1, Si2, or Si3) or a scrambled stealth siRNA (ScrSi) for 4–6 hr, followed by incubation in complete IMEM for 72 hr as explained in Materials and Methods. The whole cell lysates were sequentially immunoblotted with anti-TNFAIP8 antibody (1:1000 dilution) and anti-GAPDH antibody. Data were quantified using NIH ImageJ software (version 1.45). C, untreated control; L, lipofectin-treated control. (b) Analysis of TNFAIP8 expression in TNFAIP8 immune-complexes from PC-3 cells treated with TNFAIP8 siRNA (Si2) versus Scrambled siRNA (ScrSi). PC-3 cells were treated with Si2 siRNA or Scr siRNA (ScrSi) as above and whole cell lysates (1.0 mg protein) were immunoprecipitated (IP) with anti-TNFAIP8, followed by immunoblotting (IB) with anti-TNFAIP8 antibody (1:1000 dilution).
Table 1.
Selected list of TNFAIP8 interacting proteins identified by antibody microarray analysis
| ID | Symbol | Fold Change1 | P-value2 | Entrez Gene Name | Location | Type(s) |
|---|---|---|---|---|---|---|
| P56159 | GFRA1 | 2 | 4.00E-05 | GDNF family receptor alpha 1 | Plasma Membrane | transmembrane receptor |
| P09874 | PARP1 | 1.8 | 4.00E-05 | poly (ADP-ribose) polymerase 1 | Nucleus | enzyme |
| Q01130 | SRSF2 | 1.5 | 4.00E-06 | serine/arginine-rich splicing factor 2 | Nucleus | transcription regulator |
| P52292 | KPNA2 | 1.5 | 1.90E-04 | karyopherin alpha 2 (RAG cohort 1, importin alpha 1) | Nucleus | transporter |
| Q9UHI6 | DDX20 | 1.4 | 1.90E-04 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 20 | Nucleus | transcription regulator |
| P18031 | PTPN1 | 1.3 | 1.50E-04 | protein tyrosine phosphatase, non-receptor type 1 | Cytoplasm | phosphatase |
| P24864 | CCNE1 | 1.2 | 4.00E-05 | cyclin E1 | Nucleus | transcription regulator |
PC-3 cells were treated with TNFAIP8 siRNA (Si2 SiRNA/SiRNA) or a scrambled siRNA (ScrSiRNA/MM) and the proteomic profiles in the TNFAIP8 immune-complexes were identified by the antibody microarray analysis as described in Materials and Methods. Detailed list of all proteins is provided in Supporting Information Table 1. Fold change represents the ratio of largest of MM. Si, divided by smallest.
P-value is the significance of the difference between the two spots for MM and the two spots for SiRNA. All results shown above were found to be statistically significant even under the very stringent Bonferroni criterion to adjust for multiple testing.
Figure 5.
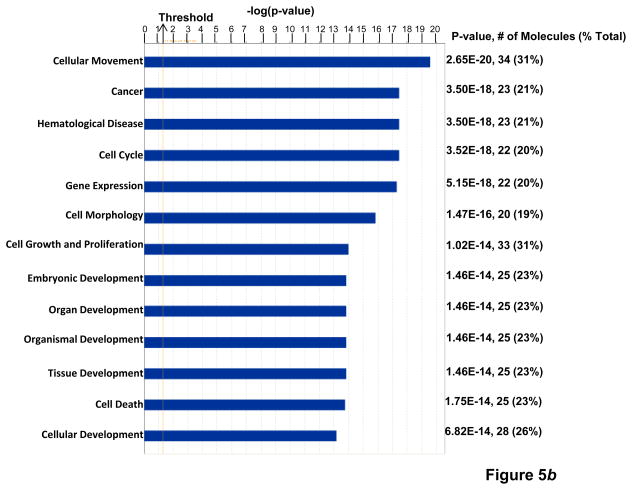
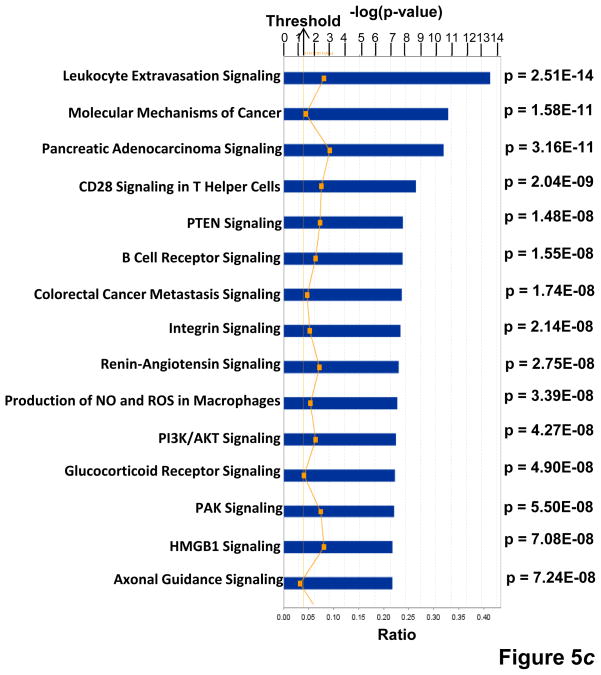
(a) KPNA2 (importin-α1) is a new binding partner of TNFAIP8. PC-3 cells were fractionated into cytosolic and nuclear fractions and the lysates (1 mg protein) were immunoprecipitated (IP) with anti-Karyopherin α/Rch-1 antibody (3μg), followed by immunoblotting with anti-TNFAIP8 antibody (1:1000 dilution). The blot was reprobed with anti-Importin-α1 antibody (0.2 μg/ml). (b) Top functional categories of TNFAIP8-centric proteins identified in PC-3 cells. TNFAIP8-centric proteins refer to the TNFAIP8 interacting proteins identified in this study by the antibody microarray analysis of TNFAIP8 immune-complexes from PC-3 cells (Supporting Information Table 1) and by the expression analysis in PC-3 cells treated with TNFAIP8 siRNA or scrambled siRNA (Supporting Information Fig. 3). (c) Top canonical pathways in which TNFAIP8 interacting proteins are implicated in PC-3 cells.
 = “p-value” = Chance that the selected molecules fit into the given canonical pathway at random.
= “p-value” = Chance that the selected molecules fit into the given canonical pathway at random.
 = “Ratio” = # of selected molecules in canonical pathway / Total # of molecules in canonical pathway. Threshold = 0.04.
= “Ratio” = # of selected molecules in canonical pathway / Total # of molecules in canonical pathway. Threshold = 0.04.
TNFAIP8 appears to function as a scaffold (or adaptor) protein. However, the functional modules and binding partners of TNFAIP8 are not known. The antibody microarray analysis of the TNFAIP8 immune-complex revealed a number of proteins with diverse functions (Table 1, and Supporting Information Table 1). For example, TNFAIP8 interactions with GDNF family receptor α1, PARP, SRSF2, DDX20, PTPN1, and cyclin E1 were found to be relatively high in scrambled siRNA relative to TNFAIP8 siRNA-treated PC-3 cells (Table 1). In order to begin to understand the systems biology of TNFAIP8, we performed the Ingenuity Pathway Analysis of various significantly affected proteins identified in the TNFAIP8 immune-complex (Supporting Information Table 1) and/or downregulated in TNFAIP8 knockdown prostate cancer cells (Supporting Information Fig. 3). Our data suggest that the cellular movement, cancer, cell cycle, and cell growth and proliferation are among the top-ranked functional categories (Fig. 5b), and leukocyte extravasation signaling, molecular mechanisms of cancer, colorectal cancer metastasis signaling and integrin signaling are among the top-ranked canonical pathways in which TNFAIP8 and associated proteins are implicated (Fig. 5c). Consistent with a role of TNFAIP8 in cancer progression, the connectivity map revealed that TNFAIP8 may significantly impact signaling pathways involving integrins (α6β1, α3β1, αvβ3) and MMPs (MMP-1 and MT1-MMP/MMP-14) (Supporting Information Figs. 8 and 9).
Discussion
This is the first report to simultaneously investigate the therapeutic, prognostic and mechanistic significance of TNFAIP8. Our data shows that TNFAIP8 is an androgen-inducible molecule in hormone-responsive prostate cancer cells (Figs. 1a and 1b). Given that androgen receptor signaling is intact in castration resistant prostate cancer (CRPC) patients,22–24 we reasoned that aberrant expression and/or functioning of TNFAIP8 may be a logical route to activation of metastatic signals in prostate cancer cells. Indeed, modulation of TNFAIP8 expression was found to influence many of the known signals of cancer invasion and metastasis. For example, prostate cancer cells in skeletal metastases have been shown to express high levels of MT1-MMP, facilitating degradation of collagen I and intraosseous expansion of tumor mass.25,26 Additional components of the bone metastasis signature include MMP-1 (collagenase I) and MMP-9 (Gelatinase B).27–29 In addition, increased expression of VEGFR-2 in prostate cancer cells may provide an autocrine mechanism of VEGF-dependent tumor growth.30,31 In this study, we have demonstrated that siRNA down regulation of TNFAIP8 expression correlates with decreased expression of the key components of the extracellular matrix (MMP-1, MMP-2, MMP-9, MT1-MMP) and VEGFR-2 in PC-3 cells (Supporting Information Figs. 3a–3c). These observations imply that TNFAIP8 could be an important functional link between the MMPs and VEGFR-2 signaling pathways and mechanisms of prostate cancer progression.
The treatment of metastatic CRPC remains a clinical challenge.32–34 The Food and Drug Administration approved docetaxel in 2004 for patients with hormone-refractory prostate cancer based on two phase III trials; however, patients with metastatic hormone-refractory prostate cancer ultimately succumb to their disease. Therefore, it is not surprising that numerous clinical trials of drugs with distinct targets including androgen biosynthesis, androgen receptor, angiogenesis, microtubules, and bone are currently underway in an effort to improve the clinical outcomes in metastatic CRPC patients.34 While most CRPC express androgen receptor (AR), a subset of CRPC do not express AR and cells derived from their bone metastases can grow in castrated male mice.35,36 PC-3 cells, originally derived from bone metastasis, contain a truncated form of the AR and may represent this subtype of CRPC. The antisense oligonucleotide and RNA interference strategies remain to be the most effective sequence-specific gene silencing approaches for improved therapy response.37,38 In present study, we used a systemically delivered liposomal formulation of TNFAIP8 antisense oligo (LE-AS5) to knockdown TNFAIP8 in androgen-independent PC-3 prostate tumor tissues. Our data show significant antitumor efficacy of a combination of LE-AS5 and radiation or docetaxel versus single agents in PC-3 tumor xenograft model (Figs. 2a–2f). Thus TNFAIP8 is a promising target for therapeutic intervention of hormone refractory prostate cancer in combination protocols including conventional radiation therapy or chemotherapy drugs.
Prognostic markers that could definitively predict prostate cancer recurrence will be of enormous clinical utility for the accurate counseling of the patients and developing personalized therapies matching the disease profile. We have observed correlations of both the cytoplasmic and nuclear TNFAIP8 overexpression with high grade PACs. Of the most clinical value is present observation of the nuclear TNFAIP8 as an independent predictor of disease recurrence controlling for tumor grade (Figs. 3a–3e). These results provide a rationale for the prospective clinical translational studies of nuclear TNFAIP8 as a potential biological benchmark for identification of high risk patients during the non-life threatening stages of prostate cancer. In the absence of a classical nuclear localization signal (NLS) in the sequence of TNFAIP8, it is unclear how might TNFAIP8 translocate to the nucleus. In the antibody microarray screen of proteins interacting with TNFAIP8, we have identified a potential mechanism of nuclear translocation of TNFAIP8 whereby KPNA2 (importin −α1), a cytosolic receptor that plays a pivotal role in the nuclear protein import pathways in multiple cancers,11 binds to TNFAIP8 in PC-3 prostate cancer cells (Table 1). In this same context, nuclear translocation of thioredoxin-binding protein-2 (TBP-2) is mediated by KPNA2 and there is no NLS in the TBP-2 sequence.39 Remarkably, nuclear KPNA2 expression has been reported as an independent prognostic marker of disease recurrence after radical prostatectomy.12 Accordingly, we propose a feed forward loop whereby high KPNA2 facilitates an inherent increase in nuclear TNFAIP8 which, in turn, may function to drive prostate cancer recurrence. The present observation of the KPNA2-TNFAIP8 complex in the nucleus (Fig. 5a) is a first step in understanding the biological consequences of the trafficking of the TNFAIP8 to the nucleus.
The protein-protein interaction data presented here offer a starting point for understanding the scaffold (or adaptor) nature of TNFAIP8 and identification of the functional protein complexes and pathways regulated by TNFAIP8. While further studies are necessary to validate physical and functional interactions with TNFAIP8, the potential interaction of TNFAIP8 with GDNF family receptor α1 (GFRA1) (Table 1) is noteworthy as it could be central to the mechanisms underlying the role of TNFAIP8 in cancer invasion and metastasis. Neural invasion by cancer is found in 80% of pancreatic and 30% of prostate, head and neck, breast, and gastrointestinal cancer patients.40 Nerves secrete Glial cell-derived neurotrophic factor (GDNF), a potent chemoattractant which activates its receptor tyrosine kinase RET and co-receptor GFRA1, inducing neural invasion by cancer cells.41 Furthermore, the GDNF-GFRA1-RET-signaling pathway of invasion and metastasis involves activation of integrins and MMPs.40 Given a link between TNFAIP8 and the integrins and MMP pathways observed in this study (Fig. 5c, and Supporting Information Figs. 3a–3c, 8 and 9), and the known roles of TNFAIP8 in cell invasion and experimental metastasis,7 TNFAIP8 may promote metastasis, in part, by facilitating engagement of the GFRA1 and RET receptors. The TNFAIP8 expression may also regulate a distinct set of genes in prostate epithelial cells. Indeed, in the RNA array studies, TNFAIP8 knockdown seems to be associated with increased expression of LPHN2 (GDS2443 / 9810 / Lphn2 / Mus musculus), previously shown to be high in pre-malignant Prostatic Intraepithelial Neoplasia versus invasive prostate tumor,42 and EPHA3(GDS1439 / 206071_s_at / EPHA3 / Homo sapiens), a tumor suppressor shown to be high in benign prostate versus metastatic prostate43 (T. Day and U. Kasid, unpublished data). Understanding of the molecular profiles of TNFAIP8 should advance the actionable omics of not just recurrent and metastatic prostate cancer but many other cancers with poor prognosis.
In summary, this study demonstrates that TNFAIP8 is a therapeutic target in prostate cancer, and underscores the value of nuclear TNFAIP8 as a potential biomarker of recurrent prostate cancer. In addition, present data suggest that TNFAIP8 functions in oncogenesis are likely to involve activation of the integrin, MMP and VEGFR-2 signaling pathways. Current investigations in our laboratory are aimed to identify the GFRA1 and KPNA2 binding sites in TNFAIP8, understand the functionality of these interactions, and determine the role of nuclear TNFAIP8 in prostate carcinogenesis. Future investigations of TNFAIP8 and its key effectors and binding partners revealed in this study will be necessary in large cohorts of the premalignant, clinically localized and malignant prostate cancer specimens to then recognize the “TNFAIP8 signature” representing a subset of high risk prostate cancer patients.
Supplementary Material
A brief description of the novelty and impact of the work.
TNFAIP8 is an emerging NF-κB-inducible, oncogenic molecule. In this study, the first to investigate the therapeutic significance of TNFAIP8, in vivo TNFAIP8 knockdown was found to enhance the efficacies of radiation and docetaxel in a hormone-refractory prostate tumor xenograft model. For the first time, nuclear TNFAIP8 overexpression was found to be an independent predictor of recurrent prostate cancer. The findings reveal the nuclear trafficking of TNFAIP8 and its relationship with adverse outcomes in prostate cancer patients.
Acknowledgments
All cell lines were obtained from the Tissues Culture Shared Resource (TCSR) of the Lombardi Cancer Center and authentication of all cell lines was established at the TCSR by fingerprinting. This work was supported by grants from the National Institutes of Health (CA68322, CA74175), Department of Defense Prostate Cancer Research Program (PC074171) and NeoPharm, Inc.
Abbreviations
- TNFAIP8
TNF-α-inducible protein 8
- oligo
terminal base-modified oligodeoxyribonucleotide
- LE-AS5
liposome-entrapped TNFAIP8 antisense oligo
- PACs
prostatic adenocarcinomas
- TMA
tissue micro-array
- MMPs
matrix metalloproteinases
- KPNA2
Karyopherin alpha 2
- GFRA1
GDNF family receptor α1
Footnotes
Conflicts of Interest
None.
Additional supporting information: Supporting Materials and Methods, Figure Legends, 9 Figures, and 1 Table
References
- 1.Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Smith MR. Effective treatment for early-stage prostate cancer--possible, necessary, or both? N Engl J Med. 2011;364:1770–72. doi: 10.1056/NEJMe1100787. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D, Whiteside TL, Kasid U. Identification of a novel tumor necrosis factor-α-inducible gene, SCC-S2, containing the consensus sequence of a death effector domain of fas-associated death domain-like interleukin-1β-converting enzyme-inhibitory protein. J Biol Chem. 2000;275:2973–78. doi: 10.1074/jbc.275.4.2973. [DOI] [PubMed] [Google Scholar]
- 4.Horrevotes AJG, Fontijn RD, Van Zonneveld AJ, De Vries CJM, Ten Cate JW, Pannekoek H. Vascular endothelial genes that are responsive to tumor necrosis factor-α in vitro are expressed in atherosclerotic lesions, including inhibitor of apoptosis protein-1, stannin, and two novel genes. Blood. 1999;93:3418–31. [PubMed] [Google Scholar]
- 5.You Z, Ouyang H, Lopatin D, Polver PJ, Wang CY. Nuclear factor-κB-inducible death effector domain-containing protein suppresses tumor necrosis factor-mediated apoptosis by inhibiting caspase-8 activity. J Biol Chem. 2001;276:26398–404. doi: 10.1074/jbc.M102464200. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Gokhale P, Broustas C, Chakravarty D, Ahmad I, Kasid U. Expression of SCC-S2, an antiapoptotic molecule, correlates with enhanced proliferation and tumorgenicity of MDA-MB435 cells. Oncogene. 2004;23:612–16. doi: 10.1038/sj.onc.1207123. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Chakravarty D, Sakabe I, Mewani RR, Boudreau HE, Kumar D, Ahmad I, Kasid U. Role of SCC-S2 in experimental metastasis and modulation of VEGFR-2, MMP-1 and MMP-9 expression. Molecular Therapy. 2006;13:947–55. doi: 10.1016/j.ymthe.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, Wang FH, Whiteside TL, Kasid U. Identification of seven differentially displayed transcripts in human primary and matched metastatic head and neck squamous cell carcinoma cell lines: implications in metastasis and/or radiation response. Oral Oncol. 1997;33:197–203. doi: 10.1016/s0964-1955(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J, Adamson E, Mercola D, McClelland M. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia. 2005;7:748–60. doi: 10.1593/neo.05289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang MY, He J, Wang JC, Yang YJ, Jin L, Chen ZY, Ma XJ, Sun MH, Xia KQ, Hong XN, Wei QY, Zhou XY. Tumor Necrosis Factor-α Induced Protein 8 Polymorphism and Risk of Non-Hodgkin’s Lymphoma in a Chinese Population: A Case-Control Study. PLoS One. 2012 May 29;7:e37846. doi: 10.1371/journal.pone.0037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutress ML, Whitaker HC, Mills IG, Stewart M, Neal DE. Structural basis for the nuclear import of the human androgen receptor. J Cell Sci. 2008;121:957–68. doi: 10.1242/jcs.022103. [DOI] [PubMed] [Google Scholar]
- 12.Mortezavi A, Hermanns T, Seifert HH, Baumgartner MK, Provenzano M, Sulser T, Burger M, Montani M, Ikenberg K, Hofstädter F, Hartmann A, Jaggi R, Moch H, Kristiansen G, Wild PJ. KPNA2 expression is an independent adverse predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. 2011;17:1111–21. doi: 10.1158/1078-0432.CCR-10-0081. [DOI] [PubMed] [Google Scholar]
- 13.Roberts TL, Sweet MJ, Hume DA, Stacey KJ. Cutting edge: Species-specific TLR9-mediated recognition of CpG and non-CpG phosphorothioate-modified oligonucleotides. J Immunology. 2005;174:605–608. doi: 10.4049/jimmunol.174.2.605. [DOI] [PubMed] [Google Scholar]
- 14.Roberts TL, Dunn JA, Terry TD, Jennings MP, Hume DA, Sweet MJ, Stacey KJ. Differences in macrophage activation by bacterial DNA and CpG-containing oligonucleotides. J Immunology. 2005;175:3569–76. doi: 10.4049/jimmunol.175.6.3569. [DOI] [PubMed] [Google Scholar]
- 15.Gokhale PC, Zhang C, Newsome J, Pei J, Ahmad I, Rahman A, Dritschilo A, Kasid U. Pharmacokinetics, toxicity, and efficacy of ends-modified raf antisense oligodeoxyribonucleotide encapsulated in a novel cationic liposome (LErafAON) Clinical Cancer Research. 2002;8:3611–21. [PubMed] [Google Scholar]
- 16.Zhang C, Newsome JT, Mewani R, Pei J, Gokhale PC, Kasid UN. Systemic delivery and pre-clinical evaluation of nanoparticles containing antisense oligonucleotides and siRNAs. Methods Mol Biol. 2009;480:65–83. doi: 10.1007/978-1-59745-429-2_5. [DOI] [PubMed] [Google Scholar]
- 17.Ross JS, Sheehan CE, Fisher HA, Kaufman RP, Jr, Kaur P, Gray K, Webb I, Gray GS, Mosher R, Kallakury BV. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–62. [PubMed] [Google Scholar]
- 18.Ross JR, Kallakury BVS, Sheehan CE, Fisher HAG, Kaufman Rp, Kaur P, Gray K, Stringer B. Expression of nuclear factor-κB and IκBα proteins in proteins in prostatic adenocarcinomas: Correlation of nuclear factor-κB immunoreactivity with disease recurrence. Clinical Cancer Research. 2004;10:2466–72. doi: 10.1158/1078-0432.ccr-0543-3. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr, Ross JS. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum Pathol. 2007;38:564–69. doi: 10.1016/j.humpath.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava M, Eidelman O, Jozwik C, Paweletz C, Huang W, Zeitlin PL, Pollard HB. Serum proteomic signature for cystic fibrosis using an antibody microarray platform. Mol Genet Metab. 2006;87:303–10. doi: 10.1016/j.ymgme.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Jozwik CE, Pollard HB, Srivastava M, Eidelman O, Fan Q, Darling TN, Zeitlin PL. Antibody microarrays: analysis of cystic fibrosis. Methods Mol Biol. 2012;23:179–200. doi: 10.1007/978-1-60327-216-2_12. [DOI] [PubMed] [Google Scholar]
- 22.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–55. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 23.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendiratta P, Mostaghel E, Guinney J, Tewari AK, Porrello A, Barry WT, Nelson PS, Febbo PG. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J Clin Oncol. 2009;27:2022–29. doi: 10.1200/JCO.2008.17.2882. [DOI] [PubMed] [Google Scholar]
- 25.Bonfil RD, Dong Z, Trindade Filho JC, Sabbota A, Osenkowski P, Nabha S, Yamamoto H, Chinni SR, Zhao H, Mobashery S, Vessella RL, Fridman R, Cher ML. Prostate cancer-associated membrane type 1-matrix metalloproteinase. A pivotal role in bone response and intraosseous tumor growth. Am J Pathology. 2007;170:1–12. doi: 10.2353/ajpath.2007.060720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Z, Bonfil RD, Chinni S, Deng X, Filho JCT, Bernardo M, Vaishampayan U, Che M, Sloane BF, Sheng S, Fridman R, Cher ML. Matrix metalloproteinase activity and osteoclasts in experimental prostate cancer bone metastasis tissue. Am J Pathology. 2005;166:1173–86. doi: 10.1016/S0002-9440(10)62337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 28.Chinni SR, Sivalogan S, Dong Z, Trindade Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- 29.Upadyay J, Shekarriz B, Nemeth JA, Dong Z, Cummings GD, Fridman R, Sakr W, Grignon DJ, Cher ML. Membrane type 1-matrix metalloproteinase (MT1-MMP) and MMP-2 immunolocalization in human prostate: Change in cellular localization associated with high-grade prostate intraepithelial neoplasis. Clinical Cancer Research. 1999;5:4105–10. [PubMed] [Google Scholar]
- 30.Zeng Y, Openskin K, Goad J, Williams ED. Tumor-induced activation of lymphatic endothelial cells via vascular endothelial growth factor receptor-2 is critical for prostate cancer lymphatic metastasis. Cancer Research. 2006;66:9566–75. doi: 10.1158/0008-5472.CAN-06-1488. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney P, Karashima T, Kim SJ, Kedar D, Mian B, Huang S, Baker C, Fan Z, Hicklin DJ, Pettaway CA, Dinney CP. Anti-vascular endothelial growth factor receptor 2 antibody reduces tumorigenicity and metastasis in orthotopic prostate cancer xenografts via induction of endothelial cell apoptosis and reduction of endothelial cell matrix metalloproteinase type 9 production. Clin Cancer Res. 2002;8:2714–24. [PubMed] [Google Scholar]
- 32.Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8:357–68. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 33.George DJ, Kantoff PW, Lin DW. Clinical roundtable monograph: new and emerging treatments for advanced prostate cancer. Clin Adv Hematol Oncol. 2011;9:1–15. [PubMed] [Google Scholar]
- 34.George D, Moul JW. Emerging treatment options for patients with castration-resistant prostate cancer. Prostate. 2012;72:338–49. doi: 10.1002/pros.21435. [DOI] [PubMed] [Google Scholar]
- 35.Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J, et al. Androgen receptor-negative prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest. 2008;118:2697–710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan X, Liu J, Lu J-F, Tzelepi V, Yang J, Starbuck MW, Diao L, Wang J, Efstathiou E, Vazquez ES, Troncoso P, Maity SN, Navone NM. Activation of β-catenin signaling in androgen receptor-negative prostate cancer cells. Clin Cancer Res. 2012;18:726–36. doi: 10.1158/1078-0432.CCR-11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodchild J. Therapeutic oligonucleotides. Methods Mol Biol. 2011;764:1–15. doi: 10.1007/978-1-61779-188-8_1. [DOI] [PubMed] [Google Scholar]
- 38.Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nature Rev Genet. 2011;12:329–40. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishinaka Y, Masutani H, Oka S, Matsuo Y, Yamaguchi Y, Nishio K, Ishii Y, Yodoi J. Importin alpha1 (Rch1) mediates nuclear translocation of thioredoxin-binding protein-2/vitamin D(3)-up-regulated protein 1. J Biol Chem. 2004;279:37559–65. doi: 10.1074/jbc.M405473200. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Li X, Xu Q, Lv S, Li J, Ma Q. Role of glial cell line-derived neurotrophic factor in perineural invasion of pancreatic cancer. Biochim Biophys Acta. 2012;1826:112–20. doi: 10.1016/j.bbcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the Ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell. 1996;85:1113–24. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 42.Bacac M, Provero P, Mayran N, Stehle JC, Fusco C, Stamenkovic I. A mouse stromal response to tumor invasion predicts prostate and breast cancer patient survival. PLoS One. 2006;1:e32. doi: 10.1371/journal.pone.0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.