Abstract
Background
Defining living donor (LD)-related risk factors affecting kidney transplant outcome will allow better donor selection and more educated informed consent when there is more than one potential donor. We studied risk factors in a large cohort at a single institution.
Methods
We reviewed 1632 recipients who underwent LD kidney transplantation at the University of Minnesota between January 1, 1990, and October 1, 2009. Using Cox regression, we studied the effect of donor and recipient risk factors on patient and graft survival. We specifically examined the effect of donor age and human leukocyte antigen (HLA) matching because these are variables that may help clinical decision making when multiple potential donors exist.
Results
Mean donor age was 40.6 years for all transplants; 180 (11%) donors were 55 years or older, and 24 (1.5%) donors were older than 65 years. Mean number of HLA mismatches (per transplant) was 2.9 (29.2% of recipients had one to two HLA mismatches, 39.8% had three to four HLA mismatches, and 25% had five to six HLA mismatches). Donor age more than 65 years, five to six HLA mismatches, delayed graft function, and acute rejection were independent predictors of decreased patient and graft survival. When controlling for recipient age, donor age more than 65 years remained a risk factor for worse outcome.
Conclusions
Our data suggest that advanced donor age (>65 years) and degree of HLA mismatch (≥5) are independent donor-related risk factors associated with worse outcome. When multiple potential LDs exist, it may be ideal to attempt to use a donor younger than 65 years and with less than five HLA mismatches.
Keywords: Living donor, Kidney transplant, Age
Living donor (LD) kidney transplants are associated with superior short- and long-term results compared with deceased donor transplants. However, within living donation, certain risk factors impact patient and graft survival. Clearly defining such risk factors will improve informed consent and help optimize donor selection when more than one potential willing donor exists. The recent emergence of paired exchange transplantation may provide an additional setting in which improved understanding of risk factors will allow more informed decision making. To better characterize the effect of selected donor and recipient factors, we studied a large cohort from a recent era (i.e., 1990–2009) at a single institution.
RESULTS
Of 1768 LD transplant recipients, 1632 met study entry criteria. Mean follow-up time (±SD) was 91 (±62) months for the study group. Donor and recipient characteristics are shown in Table 1. Mean donor age was 40.6 years; 180 (11%) donors were 55 years or older, and 24 (1.5%) donors were older than 65 years. Of the 1632 donors, 1287 (78.9%) were from related donors and 345 (21.1%) from unrelated donors. Mean number of human leukocyte antigen (HLA) mismatches was 2.9, with 29.2% of LD transplants having one to two HLA mismatches, 39.8% having three to four HLA mismatches, and 25% having five to six HLA mismatches. Mean recipient age was 39.3 years, with 36.7% being 50 years or older. Mean panel reactive antibody (PRA) level was 6.6%, with 6.1% of recipients having PRA more than 50.
TABLE 1.
Summary of demographic data
| N (%) | |
|---|---|
| Total LD transplants | 1632 (100) |
| Donor | |
| Age (yr) | |
| 45–55 | 456 (27.9) |
| 56–65 | 156 (9.6) |
| >65 | 24 (1.5) |
| Male | 743 (42.1) |
| Donor-to-recipient gender match | 849 (48.1) |
| African American | 61 (3.5) |
| Donor BMI ≥30 kg/m2 | 395 (22.5) |
| Living related | 1287 (78.9) |
| Recipient | |
| Age ≥50 yr | 599 (36.7) |
| Male gender | 1117 (68.4) |
| African American | 63 (3.9) |
| Preoperative diabetes | 249 (15.3) |
| Retransplant | 209 (12.3) |
| Immunologic matching | |
| HLA mismatches | |
| 0 | 99 (6.1) |
| 1–2 | 476 (29.1) |
| 3–4 | 649 (39.8) |
| 5–6 | 408 (25.0) |
| PRA level >50 | 100 (6.1) |
| Clinical course | |
| Steroid-free immunosuppression | 689 (42.2) |
| DGF | 45 (2.8) |
| AR | 535 (32.8) |
| Both DGF/AR | 27 (1.7) |
AR, acute rejection; BMI, body mass index; DGF, delayed graft function; HLA, human leukocyte antigen; LD, living donor; PRA, panel reactive antibody.
Postoperatively, 45 (2.8%) recipients experienced delayed graft function (DGF), 535 (32.8%) patients had a biopsy-proven acute rejection (AR) episode, whereas 27 (1.7%) recipients developed both DGF and AR during their clinical course (Table 1).
Multivariate Analyses
By multivariate analysis, risk factors found to significantly impact actuarial posttransplant recipient survival included (Table 2) donor age more than 65 years, five to six HLA mismatches, recipient age more than or equal to 50 years, pretransplant diabetes, and incidence of DGF, AR, and both DGF and AR. Hazard ratio (HR) for the combination of DGF and AR (3.43) was approximately 50% higher than the HR for DGF alone (2.50) and more than two times that for AR alone (1.57).
TABLE 2.
Summary of results from multivariate analysis of selected risk factors and their utility in predicting recipient survival, graft survival, and death-censored graft survival
| Transplant outcome, hazard ratio (P value) |
|||
|---|---|---|---|
| Risk Factor | Recipient survival | Graft survival | Death-censored graft survival |
| Donor | |||
| Age (yr) | |||
| 45–55 | NS | NS | NS |
| 56–65 | NS | NS | 1.66 (0.0106) |
| >65 | 2.24 (0.0059) | 1.93 (0.0192) | NS |
| Living related | NS | 1.28 (0.0187) | NS |
| Recipient | |||
| Age ≥50 yr | 2.53 (<0.0001) | 1.61 (<0.0001) | NS |
| Male gender | 1.24 (0.0291) | NS | NS |
| African American | NS | NS | NS |
| Preoperative diabetes | 2.63 (<0.0001) | 2.20 (<0.0001) | 1.55 (0.0092) |
| Retransplant | NS | 1.43 (0.0096) | 1.65 (0.0030) |
| Immunologic matching | |||
| HLA mismatches | |||
| 1–2 | NS | NS | NS |
| 3–4 | NS | 1.43 (0.0278) | NS |
| 5–6 | 1.71 (0.0209) | 2.02 (0.0002) | 1.81 (0.0211) |
| PRA level >50 | NS | NS | NS |
| Clinical course | |||
| Steroid-free IS | NS | NS | NS |
| DGF | 2.50 (0.0068) | 2.61 (0.0008) | 4.54 (<0.0001) |
| AR | 1.57 (0.0004) | 2.64 (<0.0001) | 5.32 (<0.0001) |
| Both DGF/AR | 3.43 (0.0010) | 5.10 (<0.0001) | 10.55 (<0.0001) |
AR, acute rejection; DGF, delayed graft function; HLA, human leukocyte antigen; IS, immunosuppression; NS, not statistically significant; PRA, panel-reactive antibody.
Risk factors found to significantly impact actuarial graft survival included (Table 2) donor age more than 65 years, living-related donation, three to four HLA mismatches, five to six HLA mismatches, recipient age more than or equal to 50 years, male recipient, pretransplant diabetes, retransplant, and incidence of DGF, AR, and both DGF and AR. HR for the combination of DGF and AR (5.10) was approximately two times that of the HR for DGF (2.61) or AR (2.64) alone.
Risk factors found to significantly impact death-censored graft survival included (Table 2) donor age 56 to 65 years, five to six HLA mismatches, pretransplant diabetes, retransplant, and incidence of DGF, AR, and both DGF and AR. HR for the combination of DGF and AR (10.55) was approximately two times that for DGF (4.54) or AR (5.32) alone. There also seemed to be a trend toward significance in predicting death-censored graft survival for donor age more than 65 years (HR=1.68, P=NS).
A separate multivariate analysis was performed, taking into account only factors known at the time of transplant and the immunosuppressive protocol (which changed over time—as defined in the Materials and Methods section) to better determine whether immunosuppression influenced donor age-related outcomes. In this analysis, the type of immunosuppression used was not found to be a significant risk factor for decreased graft or patient survival, whereas donor age more than 65 and more than five HLA mismatches remained significant risk factors for patient survival, graft survival, and death-censored graft survival.
Univariate Analyses
Donor Age
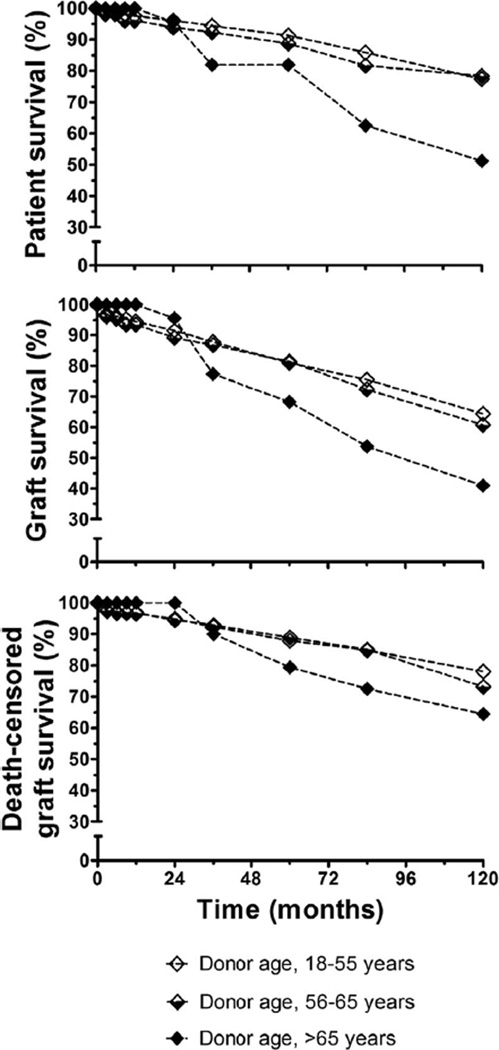
Donor age more than 65 years significantly affected patient survival (log-rank ≤0.0006) and graft survival (log-rank ≤0.0048), but not death-censored graft survival (Fig. 1). Because older donors may be more likely to donate to older recipients, we controlled for recipient age and studied survival. Although numbers are small, recipients aged 18 to 49 years receiving a kidney from a donor older than 65 years (n=13) had significantly reduced patient (P=0.013) and graft survival (P=0.032). In addition, recipients aged 18 to 49 years receiving a kidney from a donor aged 56 to 65 years (n=58) had significantly lower death-censored graft survival (P=0.033). For recipients 50 years or older, there were no significant differences in outcome relative to donor age.
FIGURE 1.
Kaplan-Meier actuarial survival analysis illustrating the effect of donor age on patient survival (top), graft survival (middle), and death-censored graft survival (bottom).
HLA Mismatch
To further quantify the effect of HLA mismatch, we analyzed outcomes for transplants with one or more HLA mismatches. Transplants with five to six mismatches had significantly worse graft survival than transplants with one to two mismatches (HR=1.5; P=0.007). However, there were no significant differences in patient (HR=1.38; P=0.092) or death-censored graft survival (HR=1.45; P=0.059). There were no significant differences in outcome between transplants with three to four mismatches versus one to two mismatches.
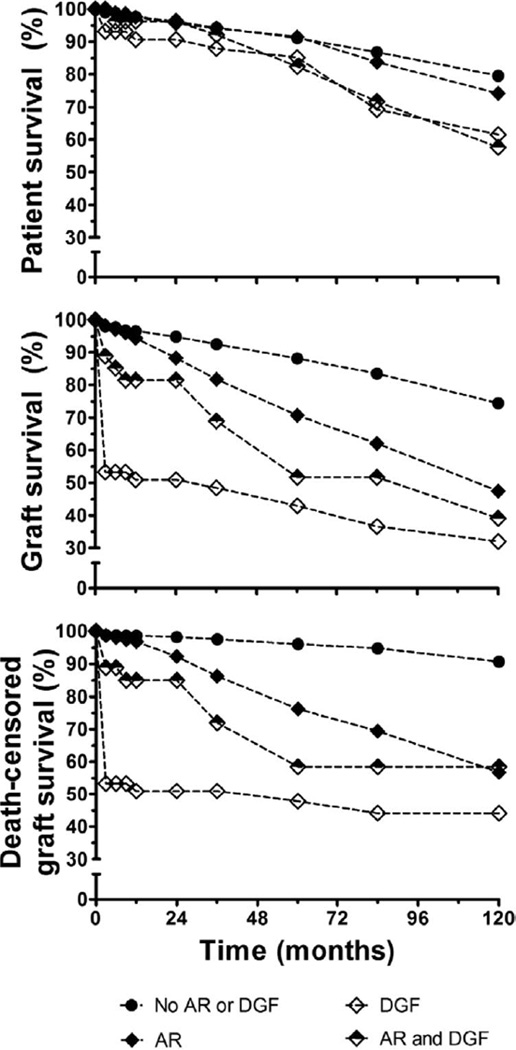
DGF and AR
Figure 2 shows the effect of DGF and AR on outcome. Kaplan-Meier actuarial survival analysis showed that DGF, AR, and the combination of DGF and AR significantly impacted recipient (log-rank ≤0.0009), graft (log-rank ≤0.0001), and death-censored graft survival (log-rank ≤0.0001).
FIGURE 2.
Kaplan-Meier actuarial survival analysis illustrating the effect of delayed graft function and acute rejection episodes on patient survival (top), graft survival (middle), and death-censored graft survival (bottom).
DISCUSSION
For a patient with end-stage renal disease, a LD transplant provides the best opportunity for a favorable outcome. LD transplants are associated with better patient and graft survival compared with deceased donor transplants (1–5). In addition, LD transplants can be performed preemptively and can minimize the time that a waitlisted patient undergoes dialysis (6–9).
When there is only one potential LD, the decision must be whether or not to proceed with LD transplantation, or to wait for a deceased donor kidney. However, when more than one potential LD exists, a decision must be made regarding which donor candidate should proceed with donation. The choice between donors is based on both medical and social factors. Our data provide guidance for the medical decision-making aspect—that is, if there are multiple potential donors, it may be ideal to select a donor who will offer the maximum possible medical benefit to the recipient.
We observed a significant effect of donor age on long-term outcome. Older donor age has been shown to result in worse outcomes in deceased donor kidney transplantation (10–14), and donor age is used in deceased donor allocation policy in the United States and Europe. For example, in the United States, an expanded criteria donor has been defined as a donor older than 60 years, or a donor between 50 and 60 years of age with two of the following: (1) hypertension; (2) a cerebrovascular cause of brain death; or (3) creatinine level more than 1.5 mg/dL (11, 15). Expanded criteria donor kidneys are allocated separately from standard criteria donor kidneys.
The effect of donor age on outcome after an LD transplant is less clear. An early study from our institution showed acceptable results with the use of donors older than 55 years (16), and this has been supported by several other studies (17–20). However, in a subsequent study with larger numbers, we suggested that advanced donor age was a risk factor for worse outcome (21).
Our current results suggest that donor age between 56 and 65 years is not a risk factor for recipient or graft survival. However, although our numbers were small, donor age more than 65 years was associated with worse outcomes. In an additional analysis of recipients 50 years or older, we found no differences in outcome related to donor age. However, this may be due to small numbers in the older donor age cohort (there were only 10 donors older than 65 years donating to recipients 50 years or older). For recipients 50 years or older, 10-year patient survival was lower for those receiving kidneys from donors older than 65 years (37.8%) compared with donor ages 18 to 44 (56.1%), 45 to 55 (69.1%), or 56 to 65 (71.4%) years. Ten-year graft survival was lower for recipients 50 years or older receiving kidneys from donors older than 65 years (38.5%) compared with donor ages 18 to 44 (50.6%), 45 to 55 (56.6%), or 56 to 65 (57.9%) years. Ten-year death-censored graft survival was also lower with donors older than 65 years (59.3%) compared with donor ages 18 to 44 (79.2%), 45 to 55 (78.3%), and 56 to 65 (77.2%) years.
Our results are consistent with other studies suggesting that receiving a LD kidney from an older donor is an independent predictor of graft loss or patient survival (22, 23). A meta-analysis by Iordanous et al. (24) suggested that recipients of kidneys from donors older than 60 years have worse outcomes, including instances when recipient age is taken into account. Interestingly, they found that the association of older donor age and worse outcome diminished in studies conducted after 1990; thus, it is important to restate that our data are from transplants performed from 1990 onward.
From the perspective of donor safety, we have been cautious in accepting donors older than 65 years. Thus, accepted donors were a selected subset of patients. Yet our data in combination with the data from the above studies suggest that when donors older than 65 years are used, long-term recipient outcome is worse. Clearly, this information should help choosing a donor when there are multiple willing donors. Additional studies need to be performed to determine whether recipients without an alternative donor would achieve better outcome with an older LD transplant versus remaining on the waiting list for a deceased donor organ.
In HLA-mismatched recipients, we found that transplants from five to six HLA-mismatched donors were associated with worse outcomes. These findings have been supported by others (25–29). Therefore, an organ with less than five HLA mismatches may be ideal for LD transplantation. Additional studies need to be performed to determine whether recipients without an alternative donor would achieve better outcome with a better-matched deceased donor organ.
Similar to previous studies (21, 30–36), we found that episodes of DGF, AR, or the combination of both were significant risk factors for worse outcomes. Our survival analysis shows that DGF has a major early effect on graft and death-censored graft survival. On the basis of HR analysis (Table 2), the combination of DGF and AR seem to negatively impact transplant outcome in a synergistic manner. This highlights the need to improve organ preservation techniques and to optimize immunosuppression as a means to minimize DGF and AR.
We identified a few other significant risk factors for worse LD transplant outcome, including living-related (vs. unrelated) donors, recipient age more than or equal to 50 years, male sex, pretransplant diabetes, and retransplant. Each of these variables has been shown to be a risk factor in other studies, and each is clearly established at the time of transplant. Interestingly, PRA level was not found to be a risk factor. This is consistent with other data from our center in which PRA in the absence of donor-specific antibody was not a risk factor for outcome (Dunn TB, Noreen H, Gillingham KJ, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant; submitted manuscript—Reviewed, awaiting author revisions). Finally, rapid discontinuation of prednisone did not have a negative effect on recipient outcome, which is consistent with our previous findings (37–39). This is important to note because long-term prednisone-free maintenance immunosuppression is associated with fewer steroid-related side effects (38, 40).
Our data come from an era in which the majority of donors had no history of hypertension. There was a predonation hypertension rate of 3.1% for all donors included in this study, defined as taking an antihypertensive at the time of donor evaluation. Textor et al. (41) recently reported no increased short-term morbidity after donor nephrectomy in patients with moderate, well-controlled hypertension. Criteria for acceptance in their study included white donors with well-defined hypertension treated using an angiotensin-receptor blocker with or without a diuretic, normal renal function and otherwise meeting the standard medical and social criteria defined by their institution. To date, there have been no long-term studies of recipient risks and outcome when receiving kidneys from such donors.
Our data suggest that in an instance when multiple potential LDs exist, it would be ideal to attempt to use a donor younger than 65 years and with less than five HLA mismatches. Paired exchange transplantation is an area where this information may be particularly useful. Paired exchanges and chains are being increasingly used by transplant centers and both have the potential to increase the number of LD transplants (42–44). Ideally, these exchanges should bring equivalent benefit to each recipient. Traditionally, paired exchanges and chains are used in instances of ABO incompatibility or the presence of donor-specific antibody (42–48). Our data suggest two points: first, donor age and degree of HLA mismatch should be considered in the paired exchange process; second, recipients with a compatible donor older than 65 years and an incompatible donor younger than 65 years may prefer the exchange process rather than proceeding with the older compatible donor. In addition, both DGF and AR were found to significantly impact patient and graft survival; therefore, newer protocols to minimize DGF and AR may prove exceptionally beneficial to LD kidney transplantation in the future.
A limitation of our study is that it is a single-center, nonrandomized retrospective cohort study and is subject to known drawbacks (49). Our patient population has a relatively low proportion of African Americans, living unrelated donors, retransplants, and patients with a high (>50%) PRA and a high proportion of recipients with body mass index >30 (Table 1). Additional studies will need to be performed to determine whether our observations can be applied to these subgroups.
In summary, identifying the best option when there are multiple potential LD requires knowledge of risk factors for outcome. Ideally, having this information available will help guide informed decision making. Two important risk factors in LD kidney transplantation are donor age and degree of HLA mismatch; these factors should be considered when selecting a LD if multiple willing potential donors exist.
MATERIALS AND METHODS
Between January 1, 1990, and October 1, 2009, 1768 recipients had a LD kidney transplant at the University of Minnesota. Recipient selection criteria and immunosuppressive protocols have been described in detail (50). In brief, until July 1996, all LD recipients were treated with triple therapy (a calcineurin inhibitor, an antimetabolite, and prednisone). After July 1996, LD transplant recipients were treated with antibody induction in a sequential therapy protocol (an antibody, an antimetabolite, and prednisone at transplant, with delayed introduction of a calcineurin inhibitor). Beginning in 1999, all primary transplants had rapid discontinuation of prednisone, tapered over 5 postoperative days; in 2000, this protocol was expanded to include second transplant recipients (51). Rejection episodes were confirmed by percutaneous allograft biopsy. Mild-to-moderate cellular rejection was treated with a steroid taper, whereas severe rejection was treated with antibody.
All donor and recipient data are maintained on an institutional review board approved database. Using Cox regression, we studied the effect of selected risk factors on patient, graft, and death-censored graft survival (α=0.05). We excluded from all analyses any recipient with a technical failure occurring within 7 days posttransplant and those that received an extrarenal transplant at any point during their lifetime (n=136).
Donor factors compared in the analyses were age (45–55, 56–65, and >65 years vs. 18–44 years), donor source (related vs. unrelated), and number of donor-recipient HLA mismatches (1–2, 3–4, 5–6 vs. 0). Recipient factors compared in the analyses were age (≥50 years vs. <50 years), sex, race (African American vs. other), pretransplant diabetes (yes vs. no), transplant number (primary vs. retransplant), and PRA level (≤50 vs. >50). Posttransplant clinical factors included in the analysis were rapid discontinuation of prednisone (yes vs. no) and the occurrence of DGF (yes vs. no), or an episode of AR (yes vs. no). We conducted a separate analysis including only factors known at the time of transplant (ie, including only donor-recipient demographics and immunosuppressive protocol) to control for differences in transplant era and immunosuppressive regimen.
We specifically studied the effect of donor age by controlling for recipient age. For those without a perfectly matched transplant, we studied the effect of number of donor-recipient HLA mismatches. We studied the effect of DGF, AR, and the combination of both DGF and AR in recipients. Ten-year actuarial survival was estimated using Kaplan-Meier methods, and groups were compared using the log-rank test.
ACKNOWLEDGMENT
The authors thank Ms. Stephanie Daily for her assistance with the manuscript.
Footnotes
M.D.R. participated in data analysis, research design, and writing of manuscript; T.M.S. participated in writing of manuscript and data analysis; K.J.G. participated in data analysis and writing of manuscript; and A.J.M. participated in research design, writing of manuscript, and mentorship.
REFERENCES
- 1.Boulware LE, Ratner LE, Sosa JA, et al. The general public’s concerns about clinical risk in live kidney donation. Am J Transplant. 2002;2:186. doi: 10.1034/j.1600-6143.2002.020211.x. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 3.Cecka JM. TheUNOSScientific Renal Transplant Registry—2000. Clin Transpl. 2000:1. [PubMed] [Google Scholar]
- 4.D’Alessandro AM, Sollinger HW, Knechtle SJ, et al. Living related and unrelated donors for kidney transplantation. A 28-year experience. Ann Surg. 1995;222:353. doi: 10.1097/00000658-199509000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terasaki PI, Cecka JM, Gjertson DW, et al. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Snyder JJ, Matas AJ, et al. Preemptive kidney transplantation: The advantage and the advantaged. J Am Soc Nephrol. 2002;13:1358. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 7.Segev DL, Gentry SE, Montgomery RA. Association between waiting times for kidney transplantation and rates of live donation. Am. J Transplant. 2007;7:2406. doi: 10.1111/j.1600-6143.2007.01922.x. [DOI] [PubMed] [Google Scholar]
- 8.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: A paired donor kidney analysis. Transplantation. 2002;74:1377. doi: 10.1097/00007890-200211270-00005. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa K, Terasaki PI. Outcome of preemptive renal transplantation versus waiting time on dialysis. Clin Transpl. 2002:367. [PubMed] [Google Scholar]
- 10.Chavalitdhamrong D, Gill J, Takemoto S, et al. Patient and graft outcomes from deceased kidney donors age 70 years and older: An analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing database. Transplantation. 2008;85:1573. doi: 10.1097/TP.0b013e31817059a1. [DOI] [PubMed] [Google Scholar]
- 11.Port FK, Bragg-Gresham JL, Metzger RA, et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 12.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589. doi: 10.1681/ASN.V123589. [DOI] [PubMed] [Google Scholar]
- 13.Cecka JM. The UNOS Scientific Renal Transplant Registry. Clin Transpl. 1996:1. [PubMed] [Google Scholar]
- 14.Knight RJ, Burrows L. The combined impact of donor age and acute rejection on long-term cadaver renal allograft survival. Surgery. 1999;125:318. [PubMed] [Google Scholar]
- 15.Metzger RA, Delmonico FL, Feng S, et al. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(suppl 4):114. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 16.Kerr SR, Gillingham KJ, Johnson EM, et al. Living donors >55 years: To use or not to use? Transplantation. 1999;67:999. doi: 10.1097/00007890-199904150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Sumrani N, Delaney V, Ding ZK, et al. Renal transplantation from elderly living donors. Transplantation. 1991;51:305. doi: 10.1097/00007890-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Morrissey PE, Gohh R, Yango A, et al. Renal transplant survival from older donors: A single center experience. Arch Surg. 2004;139:384. doi: 10.1001/archsurg.139.4.384. [DOI] [PubMed] [Google Scholar]
- 19.De La Vega LS, Torres A, Bohorquez HE, et al. Patient and graft outcomes from older living kidney donors are similar to those from younger donors despite lower GFR. Kidney Int. 2004;66:1654. doi: 10.1111/j.1523-1755.2004.00932.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Verma BS, Srivastava A, et al. Long-term followup of elderly donors in a live related renal transplant program. J Urol. 2000;163:1654. [PubMed] [Google Scholar]
- 21.Matas AJ, Payne WD, Sutherland DE, et al. 2,500 living donor kidney transplants: A single-center experience. Ann Surg. 2001;234:149. doi: 10.1097/00000658-200108000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuggle SV, Allen JE, Johnson RJ, et al. Factors affecting graft and patient survival after live donor kidney transplantation in the UK. Transplantation. 2010;89:694. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 23.Oien CM, Reisaeter AV, Leivestad T, et al. Living donor kidney transplantation: The effects of donor age and gender on short- and long-term outcomes. Transplantation. 2007;83:600. doi: 10.1097/01.tp.0000255583.34329.dd. [DOI] [PubMed] [Google Scholar]
- 24.Iordanous Y, Seymour N, Young A, et al. Recipient outcomes for expanded criteria living kidney donors: The disconnect between current evidence and practice. Am J Transplant. 2009;9:1558. doi: 10.1111/j.1600-6143.2009.02671.x. [DOI] [PubMed] [Google Scholar]
- 25.Held PJ, Kahan BD, Hunsicker LG, et al. The impact of HLA mismatches on the survival of first cadaveric kidney transplants. N Engl J Med. 1994;331:765. doi: 10.1056/NEJM199409223311203. [DOI] [PubMed] [Google Scholar]
- 26.Kaneku HK, Terasaki PI. Thirty year trend in kidney transplants: UCLA and UNOS Renal Transplant Registry. Clin Transpl. 2006:1. [PubMed] [Google Scholar]
- 27.Opelz G, Wujciak T, Dohler B. Is HLA matching worth the effort? Collaborative Transplant Study. Transplant Proc. 1999;31:717. doi: 10.1016/s0041-1345(98)01620-0. [DOI] [PubMed] [Google Scholar]
- 28.Pirsch JD, D’Alessandro AM, Sollinger HW, et al. The effect of donor age, recipient age, HLA match on immunologic graft survival in cadaver renal transplant recipients. Transplantation. 1992;53:55. doi: 10.1097/00007890-199201000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Seikaly M, Ho PL, Emmett L, et al. The 12th Annual Report of the North American Pediatric Renal Transplant Cooperative Study: Renal transplantation from 1987 through 1998. Pediatr. Transplant. 2001;5:215. [PubMed] [Google Scholar]
- 30.Matas AJ, Gillingham KJ, Humar A, et al. Immunologic and nonimmunologic factors: Different risks for cadaver and living donor transplantation. Transplantation. 2000;69:54. doi: 10.1097/00007890-200001150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Humar A, Kerr S, Gillingham KJ, et al. Features of acute rejection that increase risk for chronic rejection. Transplantation. 1999;68:1200. doi: 10.1097/00007890-199910270-00023. [DOI] [PubMed] [Google Scholar]
- 32.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation. 1997;63:968. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 33.Quiroga I, McShane P, Koo DD, et al. Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant. 2006;21:1689. doi: 10.1093/ndt/gfl042. [DOI] [PubMed] [Google Scholar]
- 34.Cacho DT, Pique AA, Cusi LI, et al. Living donor renal transplantation: Prognostic factors on graft survival. Transplant Proc. 2005;37:3679. doi: 10.1016/j.transproceed.2005.10.071. [DOI] [PubMed] [Google Scholar]
- 35.Lee SY, Chung BH, Piao SG, et al. Clinical significance of slow recovery of graft function in living donor kidney transplantation. Transplantation. 2010;90:38. doi: 10.1097/TP.0b013e3181e065a2. [DOI] [PubMed] [Google Scholar]
- 36.Suthanthiran M. Acute rejection of renal allografts: Mechanistic insights and therapeutic options. Kidney Int. 1997;51:1289. doi: 10.1038/ki.1997.176. [DOI] [PubMed] [Google Scholar]
- 37.Matas AJ, Gillingham K, Kandaswamy R, et al. Kidney transplant half-life (t½) after rapid discontinuation of prednisone. Transplantation. 2009;87:100. doi: 10.1097/TP.0b013e31818c25ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matas AJ, Kandaswamy R, Humar A, et al. Long-term immunosuppression, without maintenance prednisone, after kidney transplantation. Ann Surg. 2004;240:510. doi: 10.1097/01.sla.0000137140.79206.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humar A, Dunn T, Kandaswamy R, et al. Steroid-free immunosuppression in kidney transplant recipients: The University of Minnesota experience. Clin Transpl. 2007:43. [PubMed] [Google Scholar]
- 40.Matas AJ. Minimization of steroids in kidney transplantation. Transpl Int. 2009;22:38. doi: 10.1111/j.1432-2277.2008.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Textor SC, Taler SJ, Driscoll N, et al. Blood pressure and renal function after kidney donation from hypertensive living donors. Transplantation. 2004;78:276. doi: 10.1097/01.tp.0000128168.97735.b3. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari P, de Klerk M. Paired kidney donations to expand the living donor pool. J Nephrol. 2009;22:699. [PubMed] [Google Scholar]
- 43.Segev DL, Kucirka LM, Gentry SE, et al. Utilization and outcomes of kidney paired donation in the United States. Transplantation. 2008;86:502. doi: 10.1097/TP.0b013e3181812f85. [DOI] [PubMed] [Google Scholar]
- 44.Roodnat JI, Kal-van Gestel JA, Zuidema W, et al. Successful expansion of the living donor pool by alternative living donation programs. Am J Transplant. 2009;9:2150. doi: 10.1111/j.1600-6143.2009.02745.x. [DOI] [PubMed] [Google Scholar]
- 45.Delmonico FL, Morrissey PE, Lipkowitz GS, et al. Donor kidney exchanges. Am J Transplant. 2004;4:1628. doi: 10.1111/j.1600-6143.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 46.Waterman AD, Schenk EA, Barrett AC, et al. Incompatible kidney donor candidates’ willingness to participate in donor-exchange and non-directed donation. Am J Transplant. 2006;6:1631. doi: 10.1111/j.1600-6143.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 47.Woodle ES, Daller JA, Aeder M, et al. Ethical considerations for participation of nondirected living donors in kidney exchange programs. Am J Transplant. 2010;10:1460. doi: 10.1111/j.1600-6143.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 48.Gentry SE, Segev DL, Simmerling M, et al. Expanding kidney paired donation through participation by compatible pairs. Am J Transplant. 2007;7:2361. doi: 10.1111/j.1600-6143.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 49.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;37:3114. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 50.Matas AJ, Sutherland DE, Najarian JS. Evolution of immunosuppression at the University of Minnesota. Transplant Proc. 2004;36(2 suppl):64S. doi: 10.1016/j.transproceed.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 51.Matas AJ, Ramcharan T, Paraskevas S, et al. Rapid discontinuation of steroids in living donor kidney transplantation: A pilot study. Am J Transplant. 2001;1:278. doi: 10.1034/j.1600-6143.2001.001003278.x. [DOI] [PubMed] [Google Scholar]