Abstract
Schizophrenia is a severe mental illness with neurobiological bases that remain elusive. One hypothesis emphasizes disordered thalamic function. We previously used concurrent single pulse transcranial magnetic stimulation (spTMS) and functional magnetic resonance imaging (fMRI) to show that individuals with schizophrenia have a decreased spTMS-evoked response in the thalamus, and decreased effective connectivity between thalamus and insula and thalamus and superior frontal gyrus. To better understand the factors that may accompany or account for these findings, we investigated, in the same participants, resting state functional connectivity, white matter structural connectivity, and grey matter integrity. Patients with schizophrenia did not differ from healthy control subjects in resting state functional- or white matter structural connectivity, although they did show decreased measures of grey matter integrity in the insula. However, in this region, the spTMS-evoked response did not differ between groups. In a region of the thalamus that also had grey matter intensity abnormalities, although not at a level that survived correction for multiple comparisons, the spTMS-evoked response in patients was deficient. These results suggest that measures of structure and function are not necessarily complementary. Further, given its sensitivity for identifying deficits not evident with traditional imaging methods, these results highlight the utility of spTMS-fMRI, a method that directly and causally probes effective connectivity, as a tool for studying brain-based disorders.
Key words: connectivity, DTI, fMRI, thalamus, schizophrenia, VBM
Introduction
Schizophrenia is a severe mental illness that can have a devastating impact on the patient, caregivers, and society (Caqueo-Urizar et al., 2009; Corring, 2002; Nicholl et al., 2010). Although the etiology and pathology of the illness remain uncertain, considerable evidence implicates disordered thalamic function. The thalamus has been shown to be decreased in size in postmortem (Byne et al., 2002; Danos et al., 2005) and in vivo (Adriano et al., 2010; Brickman et al., 2004; Ellison-Wright and Bullmore, 2010; Glahn et al., 2008) human studies. Abnormal functional magnetic resonance imaging (fMRI) activation of the thalamus in patients with schizophrenia during sensory gating (Tregellas et al., 2007; Tregellas et al., 2009), working memory (Bor et al., 2011), and other executive function (Andrews et al., 2006; Barch, 2005; Minzenberg et al., 2009; Walter et al., 2007) tasks further highlights its role in the illness. Aberrant scalp-recorded electrophysiological indices of sensory gating in schizophrenia (Brockhaus-Dumke et al., 2008; Javitt, 2000; Javitt et al., 1995; Kim et al., 2009; Potter et al., 2006; Salisbury et al., 2002) have also been interpreted as evidence for thalamic dysfunction, given nonhuman research confirming the critical role of the thalamus in similar processes (Jones, 2007; Krause et al., 2003; McCormick and Bal, 1994; Sherman and Guillery, 2006; Wolf et al., 2010). Additionally, sleep studies have repeatedly identified a decrease in sleep spindle measures in patients with schizophrenia (Ferrarelli et al., 2007; Ferrarelli et al., 2010; Vukadinovic, 2011; Wamsley et al., 2012). Sleep spindles are waxing and waning 12 to 16 Hz oscillations that are initiated by the thalamic reticular nucleus (TRN) and regulated by reticulo-thalamo-cortical circuits (Fuentealba and Steriade, 2005; Steriade et al., 1985).
We recently provided further evidence for the role of the thalamus in schizophrenia by delivering single pulse transcranial magnetic stimulation (spTMS) to the precentral gyrus of patients with schizophrenia and of healthy control subjects (HCS) and simultaneously measuring the response to this perturbation with fMRI. We found that while a difference between groups was not present in the blood oxygen level-dependent (BOLD) response of the cortical tissue underlying the TMS coil, patients with schizophrenia had a severely decreased spTMS-evoked response in the thalamus, a site monosynaptically connected to the area directly stimulated by spTMS. Further, decreased spTMS-evoked responses were identified in the insula and the superior frontal gyrus (SFG).
In addition to the finding in the thalamus, we also observed that, compared to HCS, patients demonstrated decreased connectivity (correlation between spTSM-evoked time series) between the thalamus and insula and the thalamus and SFG, but not between the thalamus and precentral gyrus. Connectivity between the precentral gyrus and insula or the precentral gyrus and SFG also did not differ between groups (Guller et al., 2012). This method of assessing coupling between regions is known as effective connectivity. In neurophysiology, a distinction can be made between measures of functional connectivity versus effective connectivity. The former refers to a spontaneous, unmanipulated correlation between time series and provides no information regarding the cause or directionality of the connectivity. The latter refers to activity in one region causing activity in another region due to a direct manipulation (Friston, 1994), although it can also refer to an analytic approach such as dynamic causal modeling that infers causal relationships based on models (Friston, 2009). The spTMS-fMRI perturb-and-measure procedure can yield estimates of effective connectivity because it measures responses that are caused by stimulation of a region of the cortex.
Our findings suggested that the decreased spTMS-evoked response in the thalamus was not due to decreased connectivity with the site of stimulation. Rather, the findings suggested that the decreased thalamic response may have driven the decreased connectivity between the thalamus and insula and SFG and the decreased response in these cortical regions. Therefore, the results corroborated the hypothesis of a thalamic dysfunction in schizophrenia, using a novel method of direct cortical perturbation.
Some studies have suggested that schizophrenia may be characterized by abnormal resting state connectivity (Welsh et al., 2010), structural white matter connectivity (Oh et al., 2009; Rose et al., 2006), and grey matter morphometry (Corradi-Dell'acqua et al., 2011; Ellison-Wright and Bullmore, 2010; Saze et al., 2007; Takahashi et al., 2004) measures. To better understand whether the decreased spTMS-evoked thalamic response in patients with schizophrenia may be related to such abnormalities, here we examine resting state fMRI and structural (white and grey matter) data from the same subjects who participated in the spTMS-fMRI study described above (Guller et al., 2012). Based on the previous literature and the suggestion that the thalamus is defective in patients with schizophrenia, we developed and tested 4 hypotheses: (1) resting state connectivity data analysis will show group differences similar to those revealed by the effective connectivity analysis described above, (2) white matter integrity will not differ by group in tracts connecting the thalamus and cortex (because we believe the deficit to be localized to and driven by the thalamus and not its gross structural connectivity with cortex), (3) patients with schizophrenia will have decreased measures of grey matter in the thalamus compared with HCS, and (4) the region(s) that show decreases in grey matter measures in patients with schizophrenia will also show a decreased spTMS-evoked response.
Materials and Methods
Subjects
Fourteen healthy subjects and 14 subjects with schizophrenia, recruited from local mental health providers, through newspaper and Internet advertisements and by word of mouth, participated in the study (Table 1). A psychiatrist interviewed all subjects to obtain psychiatric and medical histories and to exclude (healthy controls) or confirm (patients with schizophrenia) diagnosis using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Revised (DSM-IV-TR) (2000) criteria. Exclusion criteria included substance abuse/dependence within the prior 6 months, diagnosed neurological disorders, insulin-dependent diabetes, seizure history, recent heart attack or cancer, diagnosed sleep disorders, night-shift work, and standard MRI contraindications (e.g., ferrous implants). Healthy subjects were additionally excluded if they were taking psychotropic medications or had first-degree relatives with a psychiatric diagnosis. The Structured Clinical Interview for DSM-IV Disorders (First et al., 2002) was also administered. Symptom severity was evaluated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987).
Table 1.
Demographics and Clinical Characteristics of Healthy Control Subjects and Patients with Schizophrenia
| Healthy Control Group n=14 | Schizophrenia Group n=14 | Analysisa | |
|---|---|---|---|
| Age (years)-Range | 20–45 | 25–48 | |
| Age (years)-Mean±SD | 34+8.04 | 32.93+7.53 | n.s. |
| Male/Female | 10/4 | 10/4 | |
| Education (years, starting with high school±SD) | 6.0+2.51 | 5.21+2.12 | n.s. |
| Positive and Negative Syndrome Scale (PANSS) score Mean±SD | 70.1+17.7 | ||
| Positive Mean±SD | 15.6+6.0 | ||
| Negative Mean±SD | 20.7+6.0 | ||
| General Mean±SD | 33.8+10.5 |
Student's t-test.
SD, standard deviation; n.s., not significant.
Patients were diagnosed as paranoid (n=11), residual (n=1), catatonic (n=1), or undifferentiated (n=1) subtype. They were receiving second-generation (n=10), first- and second-generation (n=2), or first-generation (n=2) antipsychotic medications. All were outpatients with a stable chronic illness (M=11 years, SD=7).
Procedures were approved by the University of Wisconsin—Madison Health Sciences Institutional Review Board. Procedures were in accord with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from all subjects.
Image acquisition
All scans were acquired with a 3 tesla General Electric 750 Discovery scanner (Waukesha, WI) and an 8-channel head coil. The following scans were obtained: axial, T1-weighted high resolution structural scans (echo time=3.2 ms; repetition time=8.2 ms; field of view=25.6 cm; matrix=256×256; 156×1.0 mm slices, no inversion recovery); axial diffusion tensor imaging (DTI) scans using echo-planar imaging acquisition (repetition time=8000 ms, echo time=83.9 ms; field of view=25.6 cm; matrix=128×128; 54×2.0 mm slices; flip angle=90o; number of gradient directions=54, b-value=1000 sec/mm2); and sagittal resting state echo-planar functional scans (echo time=25 ms; repetition time=2250 ms; field of view=22.4 cm; matrix=64×64; 40×3.5 mm slices, flip angle=60o). spTMS-fMRI data were acquired either in the same session as the resting state data (n=9: 6 patients; 3 HSC) or on a separate day but during approximately the same time of day (early afternoon; mean duration between scans=27 days, SD=18 days). spTMS-fMRI data collection methods are described in Guller and colleagues, 2012. Two schizophrenia patients' DTI scans had slightly different parameters: 61×2.2 mm slices, number of gradient directions=55.
Resting state connectivity: single-subject level
We used the same method for analyzing resting state connectivity data as was used to analyze the effective connectivity data described in spTMS-fMRI study (Guller et al., 2012). Briefly, after removing the first 4 volumes, resting state data were slice-time corrected, motion corrected, and demeaned. A corrected time series was extracted from voxels (in the precentral gyrus, thalamus, insula, and SFG) that showed the greatest response to spTMS during the spTMS-fMRI runs. The time series across the voxels in each region was averaged. For each subject, the averaged time series in each region was correlated with the time series in each of the other areas. Pearson's r-values were transformed to Z-scores using the Fisher technique. Z-scores were compared between groups using two-tailed, two-sample t tests. Two subjects, one schizophrenia patient and one HCS, did not participate in the resting state session, thus the data from 13 subjects in each group were analyzed. Analysis was conducted using the software package Analysis of Functional NeuroImaging (AFNI) (Cox, 1996) (afni.nimh.nih.gov/afni). Statistical testing for this and subsequent analyses was performed with Microsoft Excel (2007).
Resting state connectivity: group-level
With the exception of steps related to registration, which were conducted using the tool Functional MRI of the Brain Software Library Linear Image Registration Tool (FLIRT) (Jenkinson and Smith, 2001) in the software package Analysis Group at the Oxford Centre for Functional MRI of the Brain Software Library (FSL) (Smith et al., 2004; Woolrich et al., 2009) (www.fmrib.ox.ac.uk/fsl/), processing steps were completed using AFNI (Cox, 1996). Each subject's high resolution T1 image was skull-stripped, segmented into cerebrospinal fluid, white matter, and grey matter, and linearly transformed to an MNI152 (Montreal Neurological Institute average of 152 normal structural MRI scans) template image. Resting state scans were directly obtained before T1 images, and thus they were already in alignment. To confirm proper functional and structural image alignment we visually inspected each set of images for each subject. Then, four volumes of resting state data were removed. Resting state scans were time shifted and motion corrected. The transformation matrix resulting from the T1 to MNI152 registration was applied to the corrected functional data to align them with the standard MNI152 template. Time series from the cerebrospinal fluid and white matter were extracted. These time series, and the motion parameters obtained from motion correction, were regressed out of the resting state data. The data were also detrended with a second order polynomial. Resting state data were spatially smoothed with a 6 mm Gaussian kernel and bandpass filtered from 0.01–0.1 Hz. An averaged time series from a thalamic mask (based on the Harvard–Oxford subcortical structural atlas provided in FSL (Smith et al., 2004)) was extracted for use as the seed in the connectivity analysis. For each subject the time series in every voxel was correlated with the average thalamic time series. A group-level statistical map was obtained by conducting a two-sample, two-tailed t test contrasting HCS with schizophrenia patients. We corrected for multiple comparisons using false-discovery rate (FDR) correction, the correction approach typically implemented with AFNI.
DTI: group-level, voxel-wise whole brain analysis
Group-level DTI analysis was performed using FSL (Smith et al., 2004). Eddy-current correction was applied to reduce stretches and shears that result from the gradient coils and to correct for simple head motion. A brain mask was created using Brain Extraction Tool (BET). A diffusion tensor model was fit to the data with DTIFIT. Voxel-wise statistical analysis of the resulting FA images was carried out using Tract-Based Spatial Statistics. All subjects' FA data were aligned into a common space using the nonlinear registration tool FNIRT (FMRIB's nonlinear image registration tool), which uses a b-spline representation of the registration warp field. Next, the mean FA image was created and thinned to create a mean FA skeleton that represented the centers of all tracts common to the group. Each subject's aligned FA data were then projected onto this skeleton and the resulting data analyzed using voxelwise between-subject statistics. Multiple comparisons were controlled by correcting for family-wise error (FWE), as is standard with these FSL routines.
DTI: single-subject, probabilistic tractography
Single-subject DTI analysis was performed using FSL (Smith et al., 2004). Using Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques (BEDPOST) (Behrens et al., 2003), a probabilistic diffusion model was fit to the corrected diffusion data. Probabalistic tractography (using probtrackx) was run on the output of BEDPOST. Tracts were constructed in one of two ways. First, we constructed tracts using a hand-drawn left precentral gyrus and bilateral thalamus region of interest (ROI) as the initiation and waypoint/termination, respectively. In other words, tracts were drawn from all of the voxels in the left precentral gyrus to all of the voxels of the thalamus. Tracts extending past the thalamus were excluded. The precentral gyrus ROI was limited to the left-hemisphere and began dorsally with the first occurrence of grey matter in the MRI image and continued ventrally until the hand knob (Yousry et al., 1997) was no longer identifiable. The ROI extended from the edge of the brain to the interhemispheric fissure. Rostrally, the ROI extended to the edge of the gyrus and caudally it included the central sulcus. The thalamic ROI extended bilaterally in the ventral–dorsal direction from the slice in which the vermis of the cerebellum was no longer visible to the slice in which the frontal horn and atrium of the lateral ventricles were no longer distinct. Laterally, the ROI followed the grey-and white matter intersection of thalamus and the internal capsule. Medially, the ROI followed the contour of the lateral ventricles. The thalamic ROI included all nuclei within these boundaries. ROIs were drawn by the first author and ROI sizes did not significantly differ between groups.
We also constructed tracts between the same precentral gyrus, thalamus, SFG, and insula voxels as those analyzed in the previously cited spTMS-fMRI study (Guller et al., 2012) (precentral gyrus initiation point to thalamus waypoint/termination point; precentral gyrus initiation point to SFG waypoint/termination point; precentral gyrus initiation point to insula waypoint/termination point; thalamus initiation point to SFG waypoint/termination point, and thalamus initiation point to insula waypoint/termination point). In all cases (those involving the entire left precentral gyrus and thalamus as the ROIs and those involving select voxels as the ROIs,) the constructed tracts were binarized into white matter ROIs and used to assess FA (using the output of DTIFIT) in each subject. A two-tailed, two-sample t test was used to assess statistical significance between groups.
Voxel-based morphometry
Structural grey matter data were analyzed with FSL-voxel-based morphometry (VBM) (Ashburner and Friston, 2000), a VBM style analysis. First, structural images were brain-extracted using BET. Next, tissue-type segmentation was carried out using FAST4 (Functional MRI of the Brain Software Library Automated Segmentation Tool) (Zhany et al., 2001). The resulting grey matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT. The resulting images were averaged to create a study-specific template, to which the native grey matter images were then nonlinearly re-registered. The registered partial volume images were then modulated (to correct for local expansion or contraction) by dividing by the Jacobian of the warp field. The modulated segmented images were smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. Finally, a voxel-wise general linear model was applied using permutation-based nonparametric testing, correcting for multiple comparisons across space using threshold-free cluster enhancement. A similar analysis was carried out using SPM8 (statistical parametric mapping, www.fil.ion.ucl.ac.uk/spm/) and produced nearly identical results. Only the results of analysis using FSL are presented here.
The spTMS-evoked response in voxels identified by VBM as differing between patients and HCS was examined by transforming this region back into single-subject space (using the reverse of the transform between single-subject and standard space) and following the same procedure described in the spTMS-fMRI study (Guller et al., 2012). Briefly, a hemodynamic response from these ROIs was parameterized using the sum of 2 gamma functions (in units of percent signal change). The gamma functions were upsampled from 2000 ms to 10 ms resolution. The amplitude of the peak of the hemodynamic response curve was then extracted and compared across groups. Hemodynamic response curves and their associated 95% confidence intervals (computed using group mean and standard errors at each time-point) were plotted using in-house code written in R (http://r-project.org).
Results
Resting state connectivity
Previous spTMS-evoked effective connectivity analyses revealed that connectivity between thalamus and SFG and between thalamus and insula were significantly reduced in patients with schizophrenia compared with healthy subjects, whereas connectivity between the precentral gyrus and thalamus, precentral gyrus and SFG, and precentral gyrus and insula were preserved. An identical analysis conducted in the same voxels but during a resting state scan revealed no significant group differences in the connectivity between any of these areas. We did not find group differences in the resting state functional connectivity between the thalamus and precentral gyrus (p>0.94), thalamus and mSFG (p>0.23), thalamus and insula (p>0.49), SFG and precentral gyrus (p>0.72), or insula and precentral gyrus (p>0.68, Table 2).
Table 2.
Resting State Functional Connectivity Group Mean Correlation Coefficients Between Region of Interest Time Series
| ROI | Precentral Gyrus | mSFG | Thalamus | Insula |
|---|---|---|---|---|
| Precentral Gyrus | ||||
| mSFG | HCS: 0.54 | |||
| SCZ: 0.51 | ||||
| Thalamus | HCS: 0.36 | HCS: 0.49 | ||
| SCZ: 0.33 | SCZ: 0.33 | |||
| Insula | HCS: 0.37 | HCS: 0.37 | ||
| SCZ: 0.38 | SCZ: 0.27 | |||
None of the comparisons were significant in evaluations of resting state functional connectivity.
In the same group of patients, effective connectivity between thalamus and SFG and thalamus and insula was impaired. These results are reported in the spTMS-fMRI study (Guller et al. 2012).
SCZ, patients with schizophrenia; HCS, healthy control subjects; ROI, region of interest; SFG, superior frontal gyrus.
We further conducted a whole-brain, group-level analysis of the resting state, using an averaged thalamic time series as the ROI. We did not find group differences in the functional connectivity between the thalamus and any other brain area (p>0.05, FDR corrected).
Diffusion tensor imaging
To determine whether white matter structural connectivity differences may relate to previous findings of thalamic dysfunction in schizophrenia, we performed three DTI analyses. First, we conducted a whole-brain, voxel-wise, group-level analysis of white matter structural connectivity using FA. We did not find group differences with this metric (p>0.05, FWE corrected). Next, we used probabilistic tractography to identify the tracts connecting the entire left precentral gyrus and bilateral thalami. We compared the FA in these tracts between groups and found no difference (p>0.10). Finally, we used probabilistic tractography to identify tracts connecting the voxels most responsive to spTMS, as reported in the spTMS-fMRI study (Guller et al., 2012). Using the tracts as ROI's, we compared the FA between groups. No significant group differences were found in the FA of the tracts connecting the spTMS-responsive voxels of the thalamus and precentral gyrus (p>0.15), thalamus and SFG (p>0.59), thalamus and insula (p>0.32), precentral gyrus and SFG (p>0.34), or precentral gyrus and insula (p>0.49).
Voxel-based morphometry
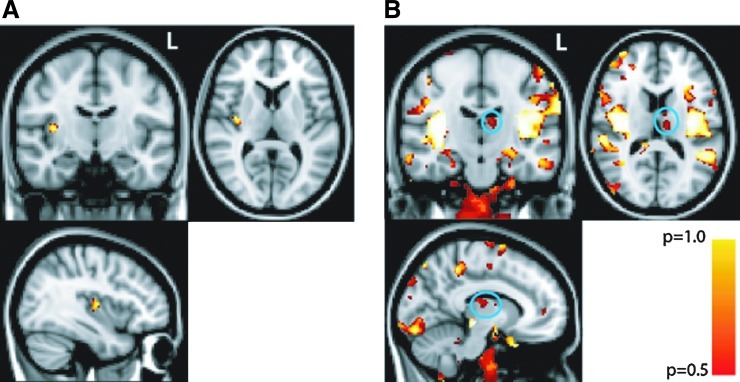
Finally, to address whether there were grey matter structural differences between our samples of HCS and patients with schizophrenia, we conducted a VBM study. This analysis, when corrected for multiple comparisons (FWE), showed our patients with schizophrenia to have reduced VBM measures in the right posterior insula (voxel with maximum difference is at x y z coordinate 26 57 40) compared with healthy subjects (p<0.05, corrected) (Fig. 1A). Because our hypothesis specifically addressed the role of the thalamus in schizophrenia, we investigated whether VBM differences were identifiable in the thalamus at a relaxed threshold that was not corrected for multiple comparisons. Indeed, at the uncorrected threshold, we found a region in the left medial dorsal thalamus (voxel with maximum difference is at x y z coordinate 49 61 42) with reduced VBM measures in patients with schizophrenia compared with healthy subjects (p<0.05, uncorrected, [Fig. 1B]). Note that there was no overlap between these voxels and the voxels most responsive to spTMS assessed in the spTMS-fMRI study (Guller et al., 2012).
FIG. 1.
Insula and thalamus show decreased voxel-based morphometry (VBM) measures (A) area in the right posterior insula where measures of VBM are decreased in patients with schizophrenia compared with healthy control subjects (HCS), p<0.05, corrected. (B) regions that show decreased VBM measures, including the left medial dorsal thalamus (inscribed in teal circle), at p<0.05, uncorrected.
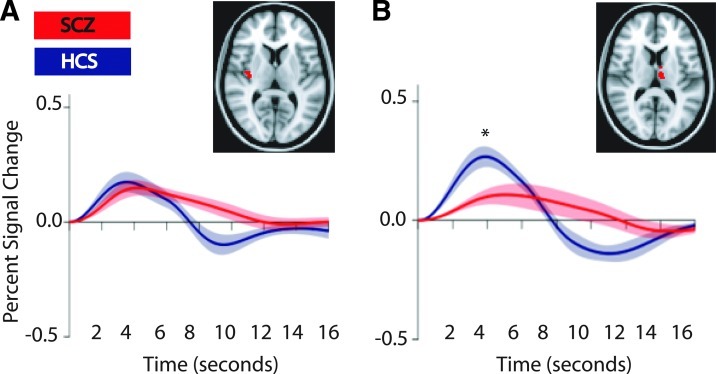
We interrogated the spTMS-evoked response in voxels (in the thalamus and insula) identified by VBM as differing between patients and HCS in grey matter integrity. We found that, as with the voxels interrogated in the spTMS-fMRI study (Guller et al., 2012), the amplitude at the peak of the spTMS-evoked response in voxels in medial dorsal thalamus was significantly smaller for patients with schizophrenia than for HCS (p<0.02). A similar result was not obtained in insula voxels identified by VBM as differing between patients and HCS (p>0.98). [Fig. 2]. The spTMS-evoked response in the thalamic VBM ROI correlated with the spTMS-evoked response in the previously assessed thalamic ROI (based on voxels most responsive to TMS, r=0.41, p<0.03). The spTMS-evoked response in the insula VBM ROI did not correlate with the spTMS-evoked response in the previously assessed insular ROI (based on voxels most responsive to TMS, r=0.39, p<0.17). PANSS scores did not significantly correlate with any VBM or spTMS-fMRI measures presented here.
FIG. 2.
Single pulse transcranial magnetic stimulation (spTMS)-evoked response in insula and thalamus (A) spTMS-evoked response in the insula region that shows decreased VBM measures does not differ between groups (B) spTMS-evoked response in the thalamic region that shows decreased VBM measures is decreased in patients with schizophrenia (SCZ) compared with HCS; *p<0.02. Shaded areas represent the 95% confidence interval. Inset: red voxels are the regions in insula (A) and thalamus (B) that show decreased VBM measures.
Discussion
Understanding the neurobiological underpinnings of schizophrenia is critical for the development of better tools for disease diagnosis and treatment. One prominent hypothesis of schizophrenia pathology is that of disordered thalamic function. Few tools exist for directly probing thalamic function in human subjects. The present study provides more details about the nature of this deficit.
Effective, but not functional, connectivity is impaired in schizophrenia
When connectivity was probed with a concurrent spTMS-fMRI method (effective connectivity) we found impairment in patients with schizophrenia in connectivity between thalamus and insula and thalamus and SFG (Guller et al., 2012). However, when connectivity was assessed during the resting state (functional connectivity), we did not find evidence for impairment at the single-subject level or at the group-averaged level. Although we anticipated a group difference in the resting state connectivity analysis, several factors may contribute to our finding of no group differences. First, a decrease in connectivity may be more evident when connectivity is directly assayed with spTMS. It may be that perturbing the system with spTMS increases the sensitivity of connectivity analyses by increasing the dynamic range of fluctuations in the BOLD signal. In addition, the pulses of TMS may force the slow oscillations of the BOLD signal in different brain areas to reset in phase, allowing for easier detection of a difference if one group is not reset as much or deviates more quickly from the reset phase angle. During rest, time courses of different regions may be randomly distributed in phase and without an external reset the difference between groups may be too small to detect. A second possibility is that we did not detect a difference in resting state connectivity because in our sample such a difference does not exist. A difference in connectivity may exist only when the neural circuits are actively engaged (with TMS or with a task), and not when they are at rest. A third possibility is that, with our relatively small n, we simply lacked sufficient power to detect differences at the group level.
In addition to the factors considered above, the possibility of a confound exists in our rsfMRI results, in that, for a subset of the subjects, rsfMRI data were collected during the same session but after the spTMS-fMRI scans had been run. This would be a concern if aftereffects of spTMS interacted with rsfMRI measures. We do not, however, believe that this complicates the interpretation of our results, for several reasons. First, a subset of subjects from both groups experienced same-session collection of these two types of data. Second, the rsfMRI analyses failed to find group-level differences in resting state connectivity between regions that both had and had not shown weaker effective connectivity in patients. If, however, a nonspecific residual effect of spTMS was responsible for erasing the differences in effective connectivity observed between thalamus and insula and between thalamus and SFG, might one not expect that this same effect might produce differences between regions that had not shown weaker effective connectivity in patients, such as between precentral gyrus and thalamus or precentral gyrus and SFG? Finally, and perhaps most convincingly, our spTMS protocol featured many fewer pulses, and much longer interpulse intervals, than have any TMS protocols that report residual physiological or behavioral effects that last longer than the stimulation itself (Thut and Pascual-Leone, 2010).
No evidence for white matter abnormality in patients with schizophrenia
Although some studies have found differences in measures of white matter integrity in schizophrenia (Oh et al., 2009; Rose et al., 2006), others have not (Foong et al., 2002; Steel et al., 2001). Our null finding may be a result of several factors. First, DTI analysis at the group level depends on registration of diffusion images to a standard template. This step may “smooth away” any small differences that exist between groups, especially in a small-n study such as this one. Second, we tested the FA, at the single-subject level, in white matter tracts connecting the entire left precentral gyrus and bilateral thalamus and in the voxels that we analyzed in the spTMS-fMRI study (Guller et al., 2012). It may be that in these specific tracts a difference in white matter integrity does not exist. This would suggest that thalamic dysfunction is not explainable by abnormalities in its structural connectivity with other brain regions. Rather, it may be that the pathophysiology is localized to a substructure of the thalamus itself, such as the TRN (Ferrarelli and Tononi, 2011; Pinault, 2011; Zhang et al., 2009).
Grey matter integrity is impaired in the insula in patients with schizophrenia but a functional deficit is evident only in thalamic voxels identified by VBM as differing between patients and HCS
We identified an area of the insula that has decreased VBM measures in schizophrenia subjects compared with HCS. This finding is supported by several other studies (Corradi-Dell'acqua et al., 2011; Ellison-Wright and Bullmore, 2010; Saze et al., 2007; Takahashi et al., 2004). However, this region of structural deficit did not evince a commensurate functional deficit as assessed with spTMS-fMRI.
Because our hypothesis is specifically focused on the thalamus, we interrogated a region in the left medial dorsal thalamus that demonstrated a VBM deficit, but only when the data were not corrected for multiple comparisons. In this region, as in the thalamic region assessed in the spTMS-fMRI study (Guller et al., 2012), we identified a functional deficit. Thus, the more strongly structurally aberrant voxels of the insula did not evince a functional abnormality when the precentral gyrus (an area with which the insula is not heavily connected) was stimulated, whereas the less strongly structurally aberrant voxels of the thalamus (an area heavily connected with the stimulation site) did. These results provide further support for the idea that a direct “perturb-and-measure” approach, such as spTMS-fMRI, may be more sensitive than traditional imaging methods for identifying deficits in function and effective connectivity in patient groups. Indeed, it is only when we consider the spTMS-fMRI response in the regions identified with VBM that the hypothesis for a thalamic dysfunction in schizophrenia is supported.
The TRN—a hypothesis for the thalamic abnormality in schizophrenia
A few studies have implicated the TRN in schizophrenia. Sleep studies that have identified sleep spindle abnormalities in patients provide evidence for physiological dysfunction in the TRN (Ferrarelli et al., 2010) because the TRN is known to be the pacemaker of spindles and is necessary for their production (Steriade et al., 1985). Animal studies support the idea that the TRN plays a central role in, sensory gating and the control of attention (McAlonan et al., 2006; Zikopoulos and Barbas, 2007), behaviors impaired in schizophrenia (Freedman et al., 2003; Luck and Gold, 2008; Salisbury et al., 2002; Zhang et al., 2009).
The TRN is a thin (∼1 mm in cross-section (Morel et al., 1997)), shell-like structure surrounding the dorsal and lateral portions of the thalamus proper. All thalamic projections to the cortex pass through the TRN. Likewise, the vast majority of cortical projections to the thalamus pass through the TRN. Excitatory glutamatergic cortico-reticular synapses outnumber cortico-thalamic synapses 3.7 times and excitatory postsynaptic currents are 2.5 times larger in the TRN than in thalamo-cortical neurons (Golshani et al., 2001). The TRN itself is a GABA-ergic nucleus. All of its outputs synapse on the thalamus proper, providing strong inhibitory control over all thalamic primary nuclei.
If the TRN is indeed impaired in schizophrenia, its decreased input onto the thalamus could lead to a decrease in the hemodynamic response in this region, and to a decrease in the strength of thalamic connectivity with cortical structures. Perhaps this impairment is only evident when the TRN is stimulated, as happens when spTMS is applied to the cortex. Conventional structural MRI and fMRI techniques cannot resolve the TRN, thus studies will need to investigate this hypothesis more fully in animal models, or when higher resolution human imaging becomes available.
Future directions
Future studies should examine connectivity measures and grey matter integrity measures in larger sample sizes. Although our sample size was sufficient for identifying spTMS-evoked and effective connectivity deficits in patients with schizophrenia, perhaps an increased sample size would uncover further deficits in functional and structural connectivity and VBM measures. In addition, future studies should explore the effect of medication on connectivity by recruiting patients with similar medication regimes or by recruiting first-episode or high-risk subjects.
Conclusions
Our data support two conclusions: (1) effective, but not functional, connectivity is impaired in patients with schizophrenia and (2) a functional thalamic deficit in schizophrenia cannot be attributed to gross white matter structural connectivity abnormalities between the thalamus and cortex or to grey matter structural abnormalities in the thalamus. Thus, the overall hypothesis of impaired thalamic function in schizophrenia is supported. Further, this study underscores the advantage of a direct perturb-and-measure approach such as spTMS-fMRI for understanding brain function. If solely traditional brain imaging methods were used, we would have concluded that connectivity is not impaired in our group of schizophrenia patients but that the insula has abnormal structural integrity. With the additional information provided by spTMS-fMRI we can conclude that, when the brain is probed in a controlled manner, connectivity is indeed impaired in patients with schizophrenia. Additionally, we failed to identify a functional deficit in the insula that corresponded with the structural deficit. On the other hand, we identified a functional deficit in the thalamus in voxels that may have deceased grey matter integrity. These findings would have remained undetected if solely standard imaging methods were applied.
Acknowledgments
This work was funded by the National Institutes of Health grants R01-MH064498 (Bradley R. Postle), 20MH-077967-01A (Giulio Tononi), and T31-GM007507 (Neuroscience Training Program). We thank Kristina Bolduc, Jenelle Fuller, Marti Garcia, and DJ Nephew for technical assistance. We extend further gratitude to Alexander J. Shackman and Fabio Ferrarelli for assistance with conducting experiments and Rasmus M. Birn, Andeas Buchmann, and Gregory R. Kirk for consultation on data analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 2000. [Google Scholar]
- Adriano F. Spoletini I. Caltagirone C. Spalletta G. Updated meta-analyses reveal thalamus volume reduction in patients with first-episode and chronic schizophrenia. Schizophr Res. 2010;123:1–14. doi: 10.1016/j.schres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Andrews J. Wang L. Csernansky JG. Gado MH. Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. Am J Psychiatry. 2006;163:463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Ashburner J. Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Behrens TE. Woolrich MW. Jenkinson M. Johansen-Berg H. Nunes RG. Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bor J. Brunelin J. Sappey-Marinier D. Ibarrola D. d'Amato T. Suaud-Chagny MF, et al. Thalamus abnormalities during working memory in schizophrenia. An fMRI study. Schizophr Res. 2011;125:49–53. doi: 10.1016/j.schres.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Brickman AM. Buchsbaum MS. Shihabuddin L. Byne W. Newmark RE. Brand J, et al. Thalamus size and outcome in schizophrenia. Schizophr Res. 2004;71:473–484. doi: 10.1016/j.schres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A. Schultze-Lutter F. Mueller R. Tendolkar I. Bechdolf A. Pukrop R, et al. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008;64:376–384. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Byne W. Buchsbaum MS. Mattiace LA. Hazlett EA. Kemether E. Elhakem SL, et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Caqueo-Urizar A. Gutierrez-Maldonado J. Miranda-Castillo C. Quality of life in caregivers of patients with schizophrenia: a literature review. Health Qual Life Outcomes. 2009;7:84. doi: 10.1186/1477-7525-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi-Dell'acqua C. Tomelleri L. Bellani M. Rambaldelli G. Cerini R. Pozzi-Mucelli R, et al. Thalamic-insular dysconnectivity in schizophrenia: Evidence from structural equation modeling. Hum Brain Mapp. 2012;33:740–752. doi: 10.1002/hbm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corring DJ. Quality of life: perspectives of people with mental illnesses and family members. Psychiatr Rehabil J. 2002;25:350–358. doi: 10.1037/h0095002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Danos P. Schmidt A. Baumann B. Bernstein HG. Northoff G. Stauch R, et al. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res. 2005;140:281–289. doi: 10.1016/j.pscychresns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I. Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F. Huber R. Peterson MJ. Massimini M. Murphy M. Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F. Peterson MJ. Sarasso S. Riedner BA. Murphy MJ. Benca RM, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F. Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37:306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. Spitzer R. Gibbon M. Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- Foong J. Symms MR. Barker GJ. Maier M. Miller DH. Ron MA. Investigating regional white matter in schizophrenia using diffusion tensor imaging. Neuroreport. 2002;13:333–336. doi: 10.1097/00001756-200203040-00017. [DOI] [PubMed] [Google Scholar]
- Freedman R. Olincy A. Ross RG. Waldo MC. Stevens KE. Adler LE, et al. The genetics of sensory gating deficits in schizophrenia. Curr Psychiatry Rep. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 2009;7:e33. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Fuentealba P. Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Glahn DC. Laird AR. Ellison-Wright I. Thelen SM. Robinson JL. Lancaster JL, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshani P. Liu XB. Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci U S A. 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guller Y. Ferrarelli F. Shackman AJ. Sarasso S. Peterson MJ. Langheim FJ, et al. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry. 2012;69:662–671. doi: 10.1001/archgenpsychiatry.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Doneshka P. Grochowski S. Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995;52:550–558. doi: 10.1001/archpsyc.1995.03950190032005. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. 2nd. Cambridge; New York: Cambridge University Press; 2007. [Google Scholar]
- Kay SR. Fiszbein A. Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim DI. Mathalon DH. Ford JM. Mannell M. Turner JA. Brown GG, et al. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M. Hoffmann WE. Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry. 2003;53:244–253. doi: 10.1016/s0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K. Cavanaugh J. Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Bal T. Sensory gating mechanisms of the thalamus. Curr Opin Neurobiol. 1994;4:550–556. doi: 10.1016/0959-4388(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ. Laird AR. Thelen S. Carter CS. Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A. Magnin M. Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Nicholl D. Akhras KS. Diels J. Schadrack J. Burden of schizophrenia in recently diagnosed patients: healthcare utilisation and cost perspective. Curr Med Res Opin. 2010;26:943–955. doi: 10.1185/03007991003658956. [DOI] [PubMed] [Google Scholar]
- Oh JS. Kubicki M. Rosenberger G. Bouix S. Levitt JJ. McCarley RW, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull. 2011;37:238–243. doi: 10.1093/schbul/sbq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter D. Summerfelt A. Gold J. Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE. Chalk JB. Janke AL. Strudwick MW. Windus LC. Hannah DE, et al. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006;32:16–22. doi: 10.1016/j.neuroimage.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Salisbury DF. Shenton ME. Griggs CB. Bonner-Jackson A. McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Saze T. Hirao K. Namiki C. Fukuyama H. Hayashi T. Murai T. Insular volume reduction in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:473–479. doi: 10.1007/s00406-007-0750-2. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Guillery RW. Exploring the Thalamus and Its Role in Cortical Function. 2nd. Cambridge, Mass: MIT Press; 2006. [Google Scholar]
- Smith SM. Jenkinson M. Woolrich MW. Beckmann CF. Behrens TE. Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steel RM. Bastin ME. McConnell S. Marshall I. Cunningham-Owens DG. Lawrie SM, et al. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Res. 2001;106:161–170. doi: 10.1016/s0925-4927(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Steriade M. Deschenes M. Domich L. Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Suzuki M. Hagino H. Zhou SY. Kawasaki Y. Nohara S, et al. Bilateral volume reduction of the insular cortex in patients with schizophrenia: a volumetric MRI Study. Psychiatry Res. 2004;132:187–196. doi: 10.1016/j.pscychresns.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Thut G. Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR. Davalos DB. Rojas DC. Waldo MC. Gibson L. Wylie K, et al. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92:262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR. Ellis J. Shatti S. Du YP. Rojas DC. Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry. 2009;166:354–360. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukadinovic Z. Sleep abnormalities in schizophrenia may suggest impaired trans-thalamic cortico-cortical communication: towards a dynamic model of the illness. Eur J Neurosci. 2011;34:1031–1039. doi: 10.1111/j.1460-9568.2011.07822.x. [DOI] [PubMed] [Google Scholar]
- Walter H. Vasic N. Hose A. Spitzer M. Wolf RC. Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: evidence from event-related fMRI. Neuroimage. 2007;35:1551–1561. doi: 10.1016/j.neuroimage.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Wamsley EJ. Tucker MA. Shinn AK. Ono KE. McKinley SK. Ely AV, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RC. Chen AC. Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. Matzke K. Paelchen K. Dobrowolny H. Bogerts B. Schwegler H. Reduction of Prepulse Inhibition (PPI) after neonatal excitotoxic lesion of the ventral thalamus in pubertal and adult rats. Pharmacopsychiatry. 2010;43:99–109. doi: 10.1055/s-0029-1242823. [DOI] [PubMed] [Google Scholar]
- Woolrich MW. Jbabdi S. Patenaude B. Chappell M. Makni S. Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yousry TA. Schmid UD. Alkadhi H. Schmidt D. Peraud A. Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Llinas RR. Lisman JE. Inhibition of NMDARs in the Nucleus Reticularis of the Thalamus Produces Delta Frequency Bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhany Y. Brady M. Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans on Medcal Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B. Barbas H. Circuits formultisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci. 2007;18:417–438. doi: 10.1515/revneuro.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]