Abstract
Background
It has previously been shown that during gestation, the mother's brain has an increase in glial fibrillary acidic protein (GFAP)-immunoreactivity (-ir) and a decrease in the mRNA level of A1 adenosine receptor. Little is known about the A2A adenosine receptor in the maternal brain, and whether caffeine consumption throughout gestational period modifies GFAP and adenosine receptor density in specific brain areas. This study was undertaken to investigate the protein density of GFAP and adenosine receptors (A1 and A2A subtypes) in different regions of pregnant rat brain and the possible effect of caffeine on these proteins.
Methods
For this purpose, we examined the GFAP-, A1- and A2A-ir in the cingulate cortex (Cg2), dentate gyrus (DG), medial preoptic area (mPOA), secondary somatosensory cortex (S2), and striatum (Str) of pregnant Wistar rats (drug-free tap water or water with 1g/L diluted caffeine).
Results
We show a consistent and highly significant reduction of GFAP-ir in caffeine-treated pregnant rats in most of the areas analyzed. Our data demonstrate that caffeine consumption induces a significant increase of A2A-ir in Str. Concerning A1 receptor, the observed changes are dependent on the region analyzed; this receptor density is increased in Cg2, DG, and mPOA and decreased in the somatosensory cortex and Str. The results were confirmed by Western blotting.
Conclusions
Our results suggest that chronic caffeine exposure could modify the physiolological situation of gestation by a reorganization of the neural circuits and the adenosine neuromodulator system.
Introduction
Caffeine is the most widely consumed psychoactive substance in the world, with nearly 70% of pregnant women consuming products containing caffeine (including beverages such as coffee, tea and soft drinks, chocolate, and some medications; the average intake is 125 mg/day).1,2 The multiple effects of caffeine are mainly due to antagonism of endogenous adenosine that activates four specific receptors (A1, A2A, A2B, and A3). A1 and A2A adenosine receptors are the most predominantly expressed in the brain, and they are the preferential targets for caffeine. A1 receptors are widely expressed in the brain, whereas A2A receptors are mainly concentrated in the striatum (Str).3–5 Although several clinical investigations have shown that high caffeine intake during pregnancy represents a danger to offspring and may be associated with a higher risk of growth retardation and sudden death,6,7 little evidence of effects of this substance on the adenosine neuromodulator system of the maternal brain has been produced to date.
Pregnancy is a physiological state characterized by behavioral changes, hormonal level variations, and modifications in the number and sensitivity of receptors.8–10 One of the most notable changes in the maternal brain, responsible for behavioral and physiological alterations during pregnancy, is the modification of neurotransmitter and receptor expression and the increase in astrocyte numbers.11 It has been shown that adrenergic receptors are increased in the rat hippocampus,12 and benzodiazepine and progesterone receptors are increased in the hippocampus and the medial preoptic nucleus, respectively, during pregnancy.13,14 Changes in monoamine levels in the prefrontal area, hippocampus, and medial preoptic area (mPOA) have been reported in pregnant rats.15 We previously described how metabotropic glutamate receptors (mGluR) are increased and adenosine A1 receptors decreased in the whole rat brain at the end of pregnancy, associated with increased endogenous adenosine levels.16,17 Nevertheless, there is no detailed study of protein immunoreactivity differentiating anatomical regions.
Astrocytes play a critical role in neuromodulation and neuroprotection in the central nervous system,18 and quantitative changes in the expression of glial fibrillary acidic protein (GFAP), an exclusive protein of astrocytes,19 have been observed in late pregnancy.20 Furthermore, the passage from quiescent to reactive astrocytes, producing upregulation of GFAP, has been associated with neurodegenerative conditions and is used as a marker of neuronal damage.21,22 However, the astrocytic response to caffeine during pregnancy remains unknown.
Therefore, the aim of the present study was to analyze the effect of caffeine during pregnancy on the distribution and density of A1 and A2A receptors and GFAP protein in various brain areas. For this purpose, a previously established animal model of caffeine consumption in rat was used, and the protein expression in five regions was examined: cingulate cortex (Cg2), dentate gyrus (DG), mPOA, secondary somatosensory cortex (S2), and Str.
Materials and Methods
Animals
Eighteen adult Wistar rats (3-months old) were used (12 for immunohistochemistry [IHC] assay and 6 for Western blotting assay). All animals were housed individually in a temperature-controlled room (23°C). The animals were subjected to a 12-hour light/dark cycle (08:00–20:00 lights on) and given free access to food and tap water. Following a previous protocol of caffeine administration by our group, nine pregnant rats were treated with caffeine (1 g/L) in the drinking water from gestational day 2 and during the entire gestational period. Nine control pregnant rats received drug-free tap water. As we previously reported,23 daily water intake and rat weight during the gestational period were of the same order in the two animal groups (pregnant rats not treated and treated with caffeine).
To perform the IHC assay, the last day of gestation, embryonic day 21, six animals per experimental group were intracardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; 0.08 M K2HPO4, 0.02 M NaH2PO4; pH 7.4), and the brains were removed. All the brains were immersed in a graded sucrose solution after fixation and stored in a cryprotectant solution at −20°C. Serial sections (50-μm thick) of the cortical tissue were obtained using a vibratome, and the sections were processed for immunohistochemical staining. Sections (at least six per animal and region) were selected and matched for anatomical location. Alphabetically, the areas chosen for study were the cingulate cortex, the polymorph layer of the dentate gyrus, medial preoptic area, secondary somatosensory cortex, and striatum (Cg2, DG, mPOA, S2, and Str according to Paxinos and Watson24).
For Western blot assay, six pregnant rats (three of them receiving drug-free tap water and the other three with caffeine) were sacrificed, and the brains were removed, frozen in N2 liquid, and stored at −80°C.
All experiments were performed in accordance with the guidelines established by the European Union regarding the use and care of laboratory animals, the Spanish law on animal experiments, and the Animal Research Ethics Committee of the Castilla-La Mancha University.
Immunohistochemistry
Free-floating selected sections were pretreated for 30 minutes with a solution of 0.5% hydrogen peroxide and 50% ethanol in PB to remove the possible endogenous peroxidase activity. Subsequently, sections were rinsed in PB and preincubated for 1 hour at room temperature in a stock solution containing 3% normal serum (normal horse serum for slices used with those primary antibodies made in mouse and normal goat serum for rabbit anti-A1; Vector Laboratories) in PB with 0.25% Triton X-100. Thereafter, the sections were incubated for 48 hours at 4°C in the same stock solution containing mouse antineuron-specific nuclear protein (neuronal nuclei [NeuN]), mouse anti-GFAP, rabbit anti-A1, and mouse anti-A2A (1:2000; Chemicon, 1:400; Sigma-Aldrich, 1:250; Calbiochem, and 1:1000; Upstate, respectively; dilutions used and immunostaining procedure were the same as described in detail elsewhere25,26 or recommended for IHC application included on the datasheet of the company). The sections were then washed in PB, incubated in secondary biotinylated antibodies (1:200; Vector), and processed using the horse anti-mouse or goat anti-rabbit avidin-biotin-peroxidase method of the Vectastain ABC immunoperoxidase kit (Vector). Staining was visualized histochemically with 0.05% 3,3′-diaminobenzidine tetrahydrochloride (Sigma) and 0.01% hydrogen peroxide, and the sections were rinsed in PB, mounted onto glass slides, dehydrated, cleared with xylene, and cover-slipped. Adjacent sections stained with thionine (staining of Nissl bodies) or immunostained with the neuronal marker NeuN were used to reveal the borders between different areas and quantified Nissl- or NeuN-stained cells. To demonstrate specificity, separate sections were treated following the IHC protocol, but omitting primary antibody incubation (see Supplementary Fig. S1 for further details; Supplementary Data are available online at www.liebertpub.com/caf).
Microscopy and quantitative analysis
For quantitative analysis of IHC, sections from each animal were analyzed in two parameters: the intensity of staining and the area occupied by the staining. The experimenter was unaware of the experimental group. Photomicrographs (6–10 fields per animal and region of analysis) for figures and quantification were captured using a digital camera (Olympus DP70) set up on an Olympus light microscope (Olympus BX51). First, the light and contrast settings were adjusted to normalize the distribution of gray levels in the digitized image using Adobe Photoshop software (version 11.0; Adobe Systems). Brightness levels of each image histogram were adjusted pushing the black-and-white-point sliders of the level tool to the edges of the histogram. Once these settings were adjusted, they remained constant for all measurements of a given section. Intensity measurements were taken using National Institutes of Health (NIH) ImageJ software version 1.44p.27 As previously described by other groups,28,29 we also analyzed the surface area occupied by the staining in the same regions and fields used in the analysis of intensity. Briefly, we estimated the surface area occupied by stained microstructures per μm2 of the tissue. For this purpose, images were analyzed using same software as in the intensity parameter. The surface area occupied by staining was expressed as a percentage of the reference area (image area).
The total number of cells (including pyramidal neurons, nonpyramidal neurons, and glia) was averaged in Nissl-stained sections, and the total number of neurons (including pyramidal and nonpyramidal neurons) was estimated by positive NeuN immunostaining. Concisely, adjacent sections were used for these two stainings. Photomicrographs (six fields per animal and region of analysis) were captured using a digital camera (Olympus DP70) set up on an Olympus light microscope (Olympus BX51). An unbiased counting frame with an area of 2044.7 μm2 was used. The counting frame was systematically and randomly moved through the region of interest until a total of six fields were acquired. Cells were manually counted using NIH ImageJ software version 1.44p (Plugin: cell counter).
Plasma membrane preparation
The frontal cortex, Str, and hippocampus were dissected according to Chiu's procedure.30 Plasma membranes from these regions were purified through sucrose gradients as described previously.31 Briefly, previously dissected tissue was homogenized in 20 volumes of isolation buffer (10 mM Tris–HCl, pH 7.4, 0.1 g/L bacitracin, and 100 μM phenyl methyl sulfonyl fluoride) containing 8.5% sucrose using a Potter-Elvehjem homogenizer. The crude homogenate was centrifuged for 2 minutes at 1500 g in a Beckman J2-21 centrifuge. The supernatant was spun for 10 minutes at 20,000 g, and the pellet (P2 fraction) was resuspended in an isolation buffer. The suspension obtained was gently homogenized in a Dounce homogenizer (10× A, 10× B) and stored at 0°C for 2 hours to permit lyzing. The homogenate was layered over a discontinuous sucrose gradient (40%/28%) in the isolation buffer and spun for 45 minutes at 65,000 g in a Beckman L7-55 centrifuge. The interface was collected as the plasma membrane fraction and spun for 60 minutes at 100,000 g. The pellet was finally resuspended in the isolation buffer.
Western blotting assays
For Western blot assays, 5 μg of purified plasma membranes was mixed with the Laemmli buffer containing 0.125 M Tris (pH 6.8), 20% glycerol, 10% β-mercaptoethanol, 4% sodium dodecyl sulfate (SDS), and 0.002% bromophenol blue; the mixture was then heated at 95°C for 5 minutes (each gel always included samples from different groups for direct comparison). The protein was electrophoresed on a 10% SDS-PAGE gel using a miniprotean system (Bio-Rad) with prestained molecular weight markers (Bio-Rad). The protein was transferred to nitrocellulose membranes in the iBlot™ Dry Blotting System (Invitrogen). Membranes were blocked with phosphate-buffered saline (PBS; 14 mM NaCl, 2 mM KCl, 12 mM Na2HPO4, 1.7 mM KH2PO4; pH 7.4) containing 0.1% Tween 20 (PSB-T) and 5% skimmed milk for 1 hour at room temperature and then incubated with the same primary antibodies as for IHC at 4°C overnight (1:1000 dilution for anti-A1, anti-A2A, and GFAP; 1:5000 dilution for anti-β-actin; Abcam). After three washing periods for 10 minutes with PBS-T, the membranes were incubated with the horseradish peroxidase-conjugated anti-rabbit or anti-mouse (Bio-Rad) at a dilution of 1:5000 in PBS-T containing 5% skimmed milk for 30 minutes. The antigen was visualized using the Lumi-Light Western Blotting Substrate (Roche). Luminescent signal was recorded and quantified with the Syngene G:Box (Syngene) monitored with Gene Snap (Syngene) Software (version 7.08). Specific bands were quantified with densitometry using Gene Tools Software (version 4.01; Syngene). Membranes were stripped for a second probe by incubating them three times with a medium stripping buffer (0.15% glycine, 0.1% SDS, 1% Tween 20, pH 2.2) for 5 minutes at room temperature. After removing the stripping buffer and washing them twice with PBS, membranes were ready for the blocking stage.
Protein determination
Protein concentration was measured with Lowry's method,32 using bovine serum albumin as standard.
Statistical analysis
Data are mean±standard error of the mean. SPSS version 15.0 for Windows (SPSS, Inc.) was used for two-way analysis of variance with Tukey's post hoc test. Differences between mean values were considered statistically significant at p<0.05.
Results
IHC assay
Adenosine A1 and A2A receptors
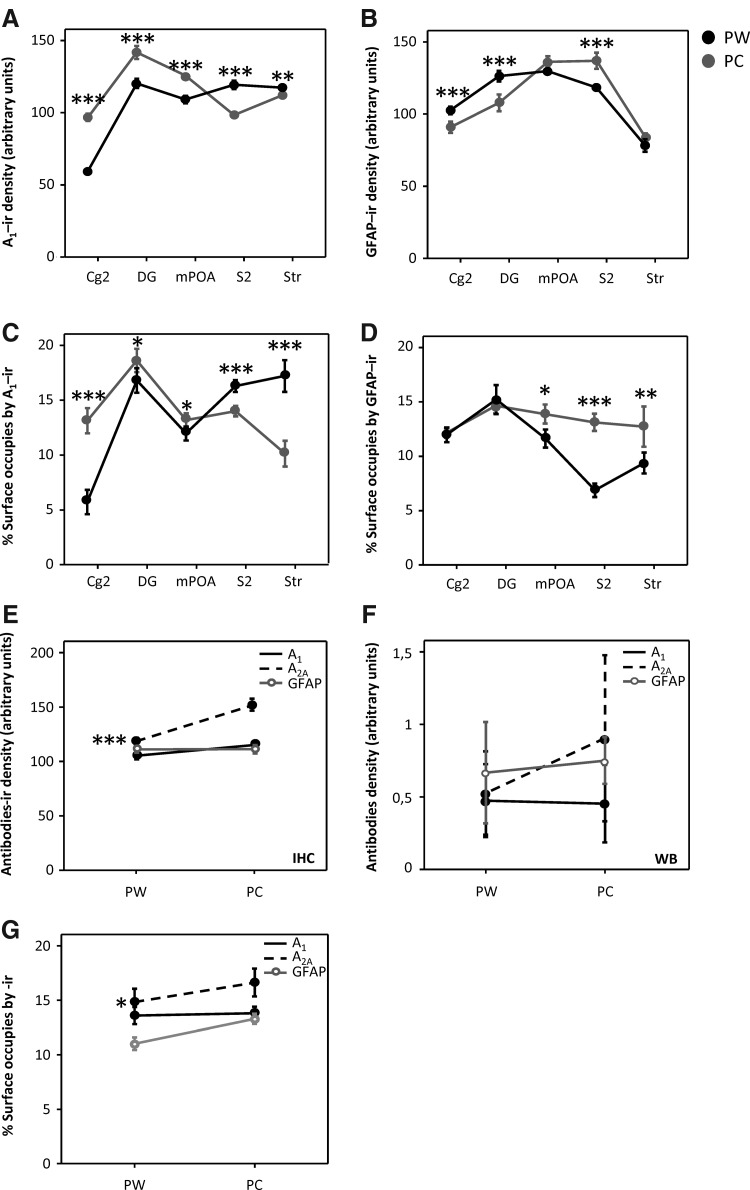
When we analyzed A1 receptor density, the caffeine-treated experimental group (pregnant caffeine [PC]) exhibited a significant increase in optical density for Cg2, DG, and mPOA regions (61%, 16%, and 15%, respectively), whereas for Str and S2 areas, there was a significant decrease in the labeling intensity (5% and 18%, respectively) when compared with control rats taking drug-free tap water (pregnant water [PW]). We tried to elucidate whether these observed changes in the density of labeling were parallel to the variation in the amount of stained microstructures. In this case, every comparison between different areas of the PW and PC groups showed a similar profile (Fig. 1A, C; see Supplementary Table S1 and Supplementary Fig. S2 for representative photomicrographs).
FIG. 1.
Graphs representing mean±SEM of A1-(A, C), A2A-(E–G), and GFAP-staining (B, D) by IHC. Optical density in arbitrary measurement (A, B, E) and percentage of the occupied surface area by staining (C, D, G) in every analyzed region and the combined totals (E–G). (A) An increase in the A1 receptor density in Cg2, DG, and mPOA and a decrease in S2 and Str, as effects of caffeine treatment during gestation. These changes are parallel to changes in the surface occupied by immunoreactivity (C). In Str, the A2A receptor density (E) and its percentage of occupied surface area (G) are increased with caffeine treatment. GFAP-ir is decreased in Cg2 and DG and increased in S2 (B), in PC animals, with an increase in the percentage of the occupied surface area by immunoreactivity in mPOA, S2, and Str (D). Combining the results in totals by technical approaches, IHC and Western blot (WB), we observed the same total effect of caffeine; *p<0.05; **p<0.01; ***p<0.001 significantly different from PW rats. GFAP, glial fibrillary acidic protein; Cg2, cingulate cortex; S2, secondary somatosensory cortex; DG, dentate gyrus; mPOA, medial preoptic area; PC, pregnant caffeine; PW, pregnant water; ir, immunoreactivity; SEM, standard error of the mean; WB, Western blot; IHC, immunohistochemistry.
A detailed examination of the A2AR-immunoreactivity (A2AR-ir) in the Str revealed that there was a notable increase in the immunostaining density as an effect of caffeine treatment in pregnant rats (Fig. 1E). With respect to the surface area occupied by this staining, there was a significant increase in the size of labeled regions modified by the caffeine treatment (see Fig. 1G and Supplementary Fig. S2 for representative photomicrographs). We were unable to detect A2A-ir signal in the other regions analyzed in this study.
GFAP immunostaining
When we analyzed the effect of caffeine during gestation in the labeling intensity of GFAP protein, there was observed an increase in the S2 cortical area (16%), and a significant decrease in the Cg2 and DG regions (11% and 15%, respectively). However, no significant changes were detected in the Str and mPOA regions between treated and untreated pregnant rats (Fig. 1B).
When we analyzed the surface area occupied by GFAP-ir, we observed a significant increase in the occupied surface of GFAP-ir in mPOA, S2, and Str. Cg2 and DG did not present this parameter modified in PC animals (see Fig. 1D and Supplementary Fig. S3 for representative photomicrographs).
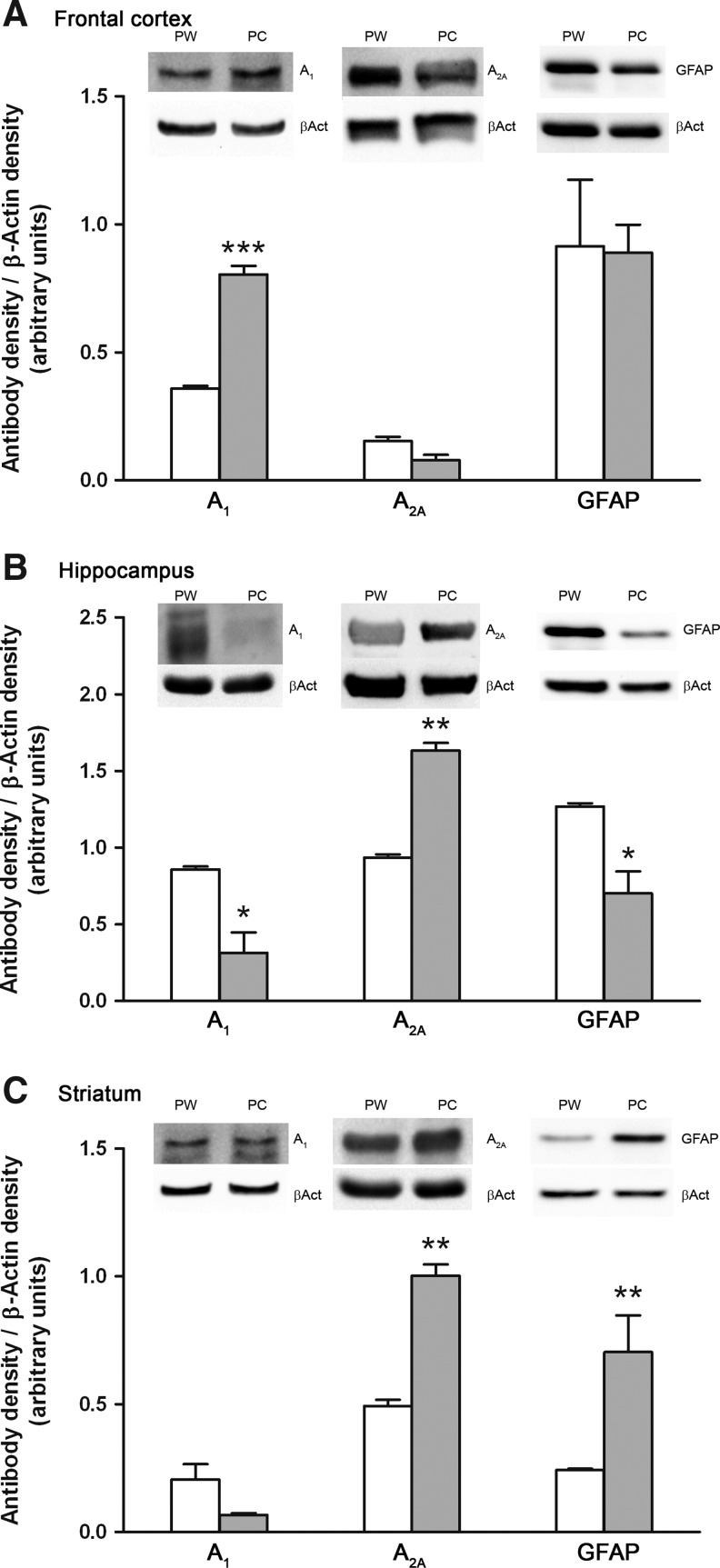
Western blotting assay
We wanted to confirm the IHC results with Western blotting assays of the plasma membrane. We found that the density of the three analyzed proteins was different in the analyzed groups depending on the area studied (Fig. 2). The linearity of the Western blotting method was checked using Na+/K+-ATPase as a specific plasma membrane marker (see Supplementary Fig. S4 for further details).
FIG. 2.
WB of A1, A2A, and GFAP performed in plasma membranes isolated from the frontal cortex (A), hippocampus (B) and Str (C) of nontreated pregnant rats, PW, or treated with caffeine, PC, Upper panel shows a representative WB assay, showing the bands corresponding to A1 37 kDa, A2A 45 kDa, and GFAP 50 kDa. β-actin 42 kDa, which was used as loading control, is shown below. Lower panel shows the histogram obtained from densitometric analysis of bands. In summary, caffeine treatment during gestation produces a significant increase in the A1 receptor density in the frontal cortex and the A2A receptor density in the hippocampus and Str, and a significant decrease in the A1 receptor and GFAP densities in hippocampus. The results are mean±SEM using arbitrary units, of three animals in each group. *p<0.05, **p<0.01, and ***p<0.001 significantly different from PW rats. Str, striatum.
When we measured adenosine A1 receptors in the plasma membranes obtained from the frontal cortex, a significant increase (124%) in the A1 receptor density was observed in rats that received caffeine during gestation as compared to control rats. On the other hand, caffeine consumption induced a significant decrease (63%) of these receptors in the hippocampus. Nevertheless, a nonsignificant decrease of these receptors was reported when the A1 receptor density was measured in Str.
Changes opposite to those reported in the density of A1 receptors were found in the density of the adenosine A2A receptors. In frontal cortex plasma membranes, a decreasing, but not significant, tendency (49%) was observed when PC membranes were compared to PW membranes. On the other hand, when A2A receptor densities were studied in the hippocampus and Str, caffeine consumption during pregnancy induced an increase in the receptor density as compared to PW rats (74% for hippocampus and 103% for Str), which was statistically significant in both cases (Fig. 2).
Finally, when GFAP was studied in these brain areas, we observed that caffeine consumption during pregnancy induced different changes in this protein depending on the area analyzed. In the frontal cortex, caffeine consumption did not induce any quantitative changes in the density of GFAP. However, in rats treated with caffeine during pregnancy, the density of GFAP was altered in an opposite way in the hippocampus and Str: increased (190%) in Str and decreased (45%) in hippocampus.
When the optical density data obtained by IHC and Western blot were put together in an overall quantity per experimental group, the comparison between methods demonstrated the same profile (Fig. 1E, F).
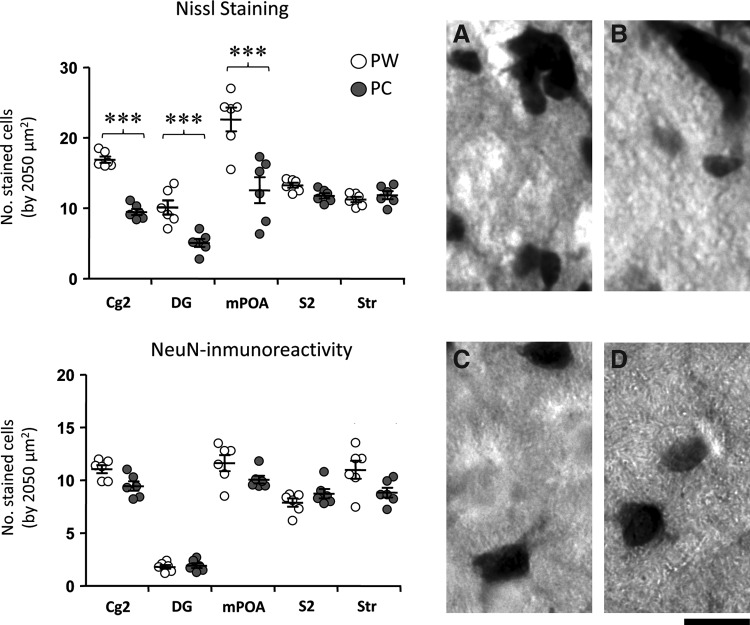
Number of cells/neurons
Counting of Nissl-stained cells demonstrated a significant loss of cellular bodies in Cg2, DG, and mPOA (44%, 50%, and 45%, respectively) in animals that consumed caffeine during gestation as compared to those that drank caffeine-free water. In brain sections stained with an anti-NeuN (neuronal marker) with IHC assay, we observed a slight, nonsignificant loss of neuronal bodies in Cg2 and Str (Fig. 3 and Supplementary Table S1).
FIG. 3.
Graphs representing mean±SEM of the number of Nissl-stained or NeuN-immunopositive cells in the five analyzed regions. Representative photomicrographs of Nissl-stained (A, B) or NeuN-immunopositive (C, D) cells of the polymorph layer of the DG in control situation (A, C) and caffeine-treated (B, D) rats. In summary, there is a significant loss of cellular bodies in Cg2, DG, and mPOA. ***p<0.001 significantly different from PW rats. NeuN, neuronal nuclei.
Discussion
Treatment with caffeine during pregnancy produces dissimilar changes in adenosine receptor and GFAP levels depending on the brain region analyzed. Antagonism of adenosine receptors induces loss of cells in Cg2, DG, and mPOA, associated with a decrease in glial cells and astroglial immunoreactivity in Cg2 and DG. Together, these data provide evidence that chronic caffeine exposure produces an area-specific reorganization of the adenosine neuromodulator system in the maternal brain, which could be involved in the restructuring of cellular circuits.
Caffeine during pregnancy produces dissimilar changes in the adenosynergic system depending on the brain region analyzed
Concerning adenosine receptors during gestation, previous work in the whole brain by our group established a downregulation of A1 receptors in the same animal model of caffeine treatment.16 In addition, the present study reveals that this downregulation is area specific. A decrease in A1 receptor is observed in the somatosensory cortex and Str, while in Cg2, DG, and mPOA, there is an increase in these receptors. Additionally, the protein density of A2A receptor, mainly concentrated in the Str, is increased by caffeine treatment at the end of pregnancy in this region.
Results of IHC are also consistent with Western blotting analysis, in which the same profile of changes is confirmed, except for the optical density of A1 receptor in the hippocampus. With respect to this apparent discrepancy in the results, several studies have demonstrated opposing changes in hippocampal Ammon's horn subfields versus DG in diverse situations; i.e., estrogen receptor expression is significantly increased in the CA1 and CA3 regions in lactating rats after treatment with kainic acid, but not in the DG.33 Similarly, there is an increase in the number of granular cells in DG, but not in other hippocampal subfields after maternal separation.34 Consequently, the significant increase observed in A1-ir of DG after caffeine treatment would be masked in the overall decrease when it is analyzed as a part of the whole hippocampal formation using Western blot.
A number of behavioral, physiological, and neural changes occur in mammal females during pregnancy.35 Maternal behavior, such as nest building, and the accompanying hormonal alterations affect various measures of plasticity in brain areas that are necessary for the full expression of maternal behaviors. The maternal experience is associated with, among other signs of neural plasticity, suppression of cell proliferation in DG,36 plasticity in the spine density, and cell architecture in the hippocampus37,38 and medial prefrontal cortex, or glial plasticity in Cg2 and mPOA.20,39 For example, there is evidence from early studies that an intact Cg2 is critical for the rapid onset of maternal behavior in rats,40,41 and this region is involved in recognition of the offspring and the onset of maternal behavior.42 The increase demonstrated here in A1-ir receptor in DG, Cg2, and mPOA reveals a new putative indicator of plasticity in regions directly involved in the maternal behavior.
To sample another brain area clearly involved in gestation, we analyzed S2. In this neocortical region, changes in the A1 adenosine receptor density, as a result of caffeine treatment, are in contrast to those observed in the other cortical region analyzed, Cg2. This might explain a specific effect of caffeine in an adult brain and a dependence on regional function.
Changes in the optical density of immunostaining and the absence of modifications in the surface area occupied may imply that the same microstructures are expressing different quantities of protein, which suggests that the distribution of protein expression in brain areas remains constant, unlike the amount. Modification of the stained surface in the same way as the intensity parameter denotes a change in the optical density of staining that could be due to an increase/decrease in the amount of labeled microstructures. Further studies using electron microscopy are necessary to verify this hypothesis.
Changes in glial processes are opposite to those in A1 receptor protein expression
It is well known that changes in ovarian steroids (estrogen and progesterone) and lactogenic (prolactin) hormone levels occur during gestation.43,44 These sex hormone modifications have been related to quantitative changes in the expression of GFAP in three of the five analyzed regions (Cg2, DG, and mPOA42,45). For example, Salmaso et al. linked the fluctuations in GFAP protein in Cg2 of pregnant rats with recognition of the offspring and the onset of maternal behavior.39
The change of the GFAP protein pattern is linked to modifications in the number of synaptic inputs to neurons. This growth of astrocytic processes, identified by a GFAP-ir increase, produces a covering of neuronal membranes,46,47 regulating the functional activity of neighboring neurons and affecting synaptic transmission.48–53 Therefore, the decrease observed here in GFAP-ir in two regions from the maternal brain could be related to a decrease in the astrocytic processes that might affect the formation and maintenance of synaptic contacts.
Curiously, regions where a decrease in GFAP-ir are demonstrated in this article presents a parallel increase of A1 receptor-ir. These data suggest a kind of inverse relationship between the two proteins. The polymerization and depolymerization of GFAP are modulated by cytosolic Ca2+, and the agonist stimulation of group II metabotropic glutamate receptors (mGluR II) produces a phosphorylation of GFAP that is inhibited by caffeine (nonspecific agonist of ryanodine receptors and nonselective antagonist of adenosine receptors).54 Previous work by our group demonstrated that chronic caffeine exposure during the gestational period can affect transduction pathways other than adenosine receptors, such as the transduction pathway of mGluR/PLC.55 Thus, the changes in GFAP-ir after caffeine treatment during gestation might be explained as a direct effect on RyRs, for high caffeine doses, and/or a relationship with mGluR II via crosstalk with the adenosynergic system.
Moreover, various studies have demonstrated a relationship between drug exposure and cytogenesis in several regions of adult rat brain.56,57 In particular, exposure to moderate-to-high doses of caffeine has been shown to alter adult neurogenesis in DG, by depressing proliferation and reducing the number of activated microglia.58,59 Here, we have demonstrated, with the comparison of Nissl and NeuN quantification, a decrease in the number of glial cells in animals with caffeine consumption, in just three areas, in two of which, Cg2 and DG, we observed an increase in A1 receptor-ir and a decrease in GFAP-ir. These data suggest that results in GFAP-ir are due to a decrease in the number of cells.
Caffeine effect during gestational period: neurotoxicity or neuroprotection? That is the question
It has been suggested that chronic (but not acute) caffeine treatment attenuates brain injury, by adenosine receptor-mediated suppression of glutamate release, mediated by the A2A receptor in Parkinson's and Alzheimer's diseases,60–63 by A1 receptors in ischemic and immunological brain injury models,64,65 and through a downregulation of glial activation.66 Antagonists of adenosine receptors, particularly A2A receptor antagonists, have the ability to restore memory dysfunction associated with aging and neurodegenerative diseases.67–69 However, caffeine, when given acutely, potentiates the increase of GFAP in the Str and enhances the acute toxicity and lethalness of 3,4-methylenedioxymethamphetamine (MDMA).70,71 Moreover, it has been demonstrated that other psychostimulant drugs affect the expression of GFAP and induce strong changes in the astrocyte proliferation rate (cocaine and MDMA exposure),71–73 generating many reactive species enabling neurodegeneration.73,74
Besides the double effect of caffeine on glial cells, up- or downregulation, it is postulated that an upregulation of adenosine receptors takes place after chronic caffeine exposure.64,75–78 Nevertheless, the effect of caffeine on these receptors depends on the dose, administration method, and frequency of treatment. With our results of chronic caffeine treatment during gestation, we add a new role for this psychoactive substance in the adenosine receptors in a nonpathological situation. We may conclude that the regular microanatomical changes produced by pregnancy in the maternal brain (which partially explain maternal behavior and the neuroprotection of the mother's brain in a stressful situation) are modified by caffeine consumption. Taken together, our findings provide data reflecting the vulnerability of an adult brain. There are studies describing effects on fetuses' or neonates' brain whose mothers have consumed caffeine during gestation or lactation. However, the effects on mothers' brain seem to be forgotten. Metabolism of caffeine is slow during pregnancy, mainly during the third trimester of gestation, increasing caffeine concentration in mother's blood. This maintained increase can affect some transduction pathways and areas in the brain that are necessary to sustain the status of gestation, and in turn can affect not only fetuses and neonates but also mother`s health. Perhaps, the same caution that applies to cigarette smoking during pregnancy could be taken in consideration to caffeine. Nevertheless, epidemiological studies are needed to evaluate the effects of caffeine consumption on the brain of pregnant women.
Supplementary Material
Acknowledgments
We thank Dr. J. DeFelipe for providing some technical tools. IB-Y thanks the Juan de la Cierva program of the Ministerio de Ciencia e Innovación (JCI-2007-41-276) and the Vice-Chancellorship of Research of the Castilla-La Mancha University (PL20112089). This study was supported by the grant BFU2011-23034 from the Ministerio de Economía y Competitividad.
Author Disclosure Statement
No competing financial interest exists for any of the authors.
References
- 1.Olsen J. Cigarette smoking, tea and coffee drinking, and subfecundity. Am J Epidemiol. 1991;133:734–739. doi: 10.1093/oxfordjournals.aje.a115948. [DOI] [PubMed] [Google Scholar]
- 2.Frary CD. Johnson RK. Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105:110–113. doi: 10.1016/j.jada.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Fredholm BB. Irenius E. Kull B. Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 4.Ferre S. Ciruela F. Borycz J. Solinas M. Quarta D. Antoniou K. Quiroz C. Justinova Z. Lluis C. Franco R. Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- 5.Schiffmann SN. Fisone G. Moresco R. Cunha RA. Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford RP. Schluter PJ. Mitchell EA. Taylor BJ. Scragg R. Stewart AW. Heavy caffeine intake in pregnancy and sudden infant death syndrome. New Zealand Cot Death Study Group. Arch Dis Child. 1998;78:9–13. doi: 10.1136/adc.78.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klebanoff MA. Levine RJ. Clemens JD. Wilkins DG. Maternal serum caffeine metabolites and small-for-gestational age birth. Am J Epidemiol. 2002;155:32–37. doi: 10.1093/aje/155.1.32. [DOI] [PubMed] [Google Scholar]
- 8.Cathey TM. Chung KW. The relationship between ovarian steroids and uterine estrogen receptors during late pregnancy. Life Sci. 1991;49:293–298. doi: 10.1016/0024-3205(91)90016-5. [DOI] [PubMed] [Google Scholar]
- 9.Fang X. Wong S. Mitchell BF. Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during late gestation and at parturition. Endocrinology. 1996;137:3213–3219. doi: 10.1210/endo.137.8.8754742. [DOI] [PubMed] [Google Scholar]
- 10.Smiley RM. Finster M. Do receptors get pregnant too? Adrenergic receptor alterations in human pregnancy. J Matern Fetal Med. 1996;5:106–114. doi: 10.1002/(SICI)1520-6661(199605/06)5:3<106::AID-MFM2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Kinsley CH. Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- 12.Glaser J. Russell VA. Taljaard JJ. Rat brain hypothalamic and hippocampal monoamine and hippocampal beta-adrenergic receptor changes during pregnancy. Brain Res. 1992;577:293–299. doi: 10.1016/0006-8993(92)90286-i. [DOI] [PubMed] [Google Scholar]
- 13.Weizman R. Dagan E. Snyder SH. Gavish M. Impact of pregnancy and lactation on GABAA receptor and central-type and peripheral-type benzodiazepine receptors. Brain Res. 1997;752:307–314. doi: 10.1016/s0006-8993(96)01489-8. [DOI] [PubMed] [Google Scholar]
- 14.Francis K. Meddle SL. Bishop VR. Russell JA. Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res. 2002;927:18–26. doi: 10.1016/s0006-8993(01)03318-2. [DOI] [PubMed] [Google Scholar]
- 15.Macbeth AH. Gautreaux C. Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. 2008;1241:136–147. doi: 10.1016/j.brainres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leon D. Albasanz JL. Fernandez M. Ruiz MA. Martin M. Down-regulation of rat brain adenosine A1 receptors at the end of pregnancy. J Neurochem. 2004;88:993–1002. doi: 10.1046/j.1471-4159.2003.02220.x. [DOI] [PubMed] [Google Scholar]
- 17.Leon D. Castillo CA. Ruiz MA. Albasanz JL. Martin M. Metabotropic glutamate receptor/phospholipase C pathway is increased in rat brain at the end of pregnancy. Neurochem Int. 2007;50:681–688. doi: 10.1016/j.neuint.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Kimelberg HK. Norenberg MD. Astrocytes Sci Am. 1989;260:66–72. doi: 10.1038/scientificamerican0489-66. 74, 76. [DOI] [PubMed] [Google Scholar]
- 19.Bignami A. Eng LF. Dahl D. Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 20.Salmaso N. Nadeau J. Woodside B. Steroid hormones and maternal experience interact to induce glial plasticity in the cingulate cortex. Eur J Neurosci. 2009;29:786–794. doi: 10.1111/j.1460-9568.2009.06627.x. [DOI] [PubMed] [Google Scholar]
- 21.Cordeau P., Jr. Lalancette-Hebert M. Weng YC. Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke. 2008;39:935–942. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- 22.Nagele RG. Wegiel J. Venkataraman V. Imaki H. Wang KC. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Leon D. Albasanz JL. Ruiz MA. Fernandez M. Martin M. Adenosine A1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem. 2002;82:625–634. doi: 10.1046/j.1471-4159.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G. Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 2007. [Google Scholar]
- 25.Benavides-Piccione R. DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Canals M. Marcellino D. Fanelli F. Ciruela F. de Benedetti P. Goldberg SR. Neve K. Fuxe K. Agnati LF. Woods AS. Ferré S. Lluis C. Bouvier M. Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 27.Del Bigio MR. Enno TL. Effect of hydrocephalus on rat brain extracellular compartment. Cerebrospinal Fluid Res. 2008;5:12. doi: 10.1186/1743-8454-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonta C. Imbert M. Vascularization in the primate visual cortex during development. Cereb Cortex. 2002;12:199–211. doi: 10.1093/cercor/12.2.199. [DOI] [PubMed] [Google Scholar]
- 29.Kastanauskaite A. Alonso-Nanclares L. Blazquez-Llorca L. Pastor J. Sola RG. DeFelipe J. Alterations of the microvascular network in sclerotic hippocampi from patients with epilepsy. J Neuropathol Exp Neurol. 2009;68:939–950. doi: 10.1097/NEN.0b013e3181b08622. [DOI] [PubMed] [Google Scholar]
- 30.Chiu K. Lau WM. Lau HT. So KF. Chang RC. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J Vis Exp. 2007;7:269. doi: 10.3791/269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan KS. Balaram P. Mammalian brain plasma membranes. Isolation, enzymatic, and chemical characterization. Exp Cell Res. 1976;101:299–306. doi: 10.1016/0014-4827(76)90381-5. [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH. Rosebrough NJ. Farr AL. Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Vanoye-Carlo A. Mendoza-Rodriguez CA. Morales T. Langley E. Cerbon M. Estrogen receptors increased expression during hippocampal neuroprotection in lactating rats. J Steroid Biochem Mol Biol. 2009;116:1–7. doi: 10.1016/j.jsbmb.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Oreland S. Nylander I. Pickering C. Prolonged maternal separation decreases granule cell number in the dentate gyrus of 3-week-old male rats. Int J Dev Neurosci. 2010;28:139–144. doi: 10.1016/j.ijdevneu.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Leuner B. Glasper ER. Gould E. Parenting and plasticity. Trends Neurosci. 2010;33:465–473. doi: 10.1016/j.tins.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawluski JL. Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 37.Furuta M. Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pawluski JL. Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 39.Salmaso N. Cossette M-P. Woodside B. Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS One. 2011;6:e23529. doi: 10.1371/journal.pone.0023529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slotnick BM. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29:204–236. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- 41.Slotnick BM. Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- 42.Salmaso N. Woodside B. Fluctuations in astrocytic basic fibroblast growth factor in the cingulate cortex of cycling, ovariectomized and postpartum animals. Neuroscience. 2008;154:932–939. doi: 10.1016/j.neuroscience.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 43.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 44.Grattan DR. Steyn FJ. Kokay IC. Anderson GM. Bunn SJ. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol. 2008;20:497–507. doi: 10.1111/j.1365-2826.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 45.Salmaso N. Woodside B. Upregulation of astrocytic basic fibroblast growth factor in the cingulate cortex of lactating rats: time course and role of suckling stimulation. Horm Behav. 2006;50:448–453. doi: 10.1016/j.yhbeh.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Segura LM. Chowen JA. Parducz A. Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994;44:279–307. doi: 10.1016/0301-0082(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 47.Theodosis DT. Trailin A. Poulain DA. Remodeling of astrocytes, a prerequisite for synapse turnover in the adult brain? Insights from the oxytocin system of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1175–R1182. doi: 10.1152/ajpregu.00755.2005. [DOI] [PubMed] [Google Scholar]
- 48.Araque A. Carmignoto G. Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 49.Volterra A. Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 50.Jourdain P. Bergersen LH. Bhaukaurally K. Bezzi P. Santello M. Domercq M. Matute C. Tonello F. Gundersen V. Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 51.Ni Y. Malarkey EB. Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- 52.Perea G. Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 53.Wigley R. Hamilton N. Nishiyama A. Kirchhoff F. Butt AM. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210:661–670. doi: 10.1111/j.1469-7580.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oppelt D. Rodnight R. Horn J. Fitarelli D. Kommers T. Oliveira D. Wofchuk S. Role of intracellular calcium stores on the effect of metabotropic glutamate receptors on phosphorylation of glial fibrillary acidic protein in hippocampal slices from immature rats. Neurochem Res. 2004;29:1541–1545. doi: 10.1023/b:nere.0000029567.68068.ab. [DOI] [PubMed] [Google Scholar]
- 55.Leon D. Albasanz JL. Ruíz MA. Iglesias I. Martín M. Effect of chronic gestational treatment with caffeine or theophylline on Group I metabotropic glutamate receptors in maternal and fetal brain. J Neurochem. 2005;94:440–451. doi: 10.1111/j.1471-4159.2005.03211.x. [DOI] [PubMed] [Google Scholar]
- 56.Mao L. Wang JQ. Gliogenesis in the striatum of the adult rat: alteration in neural progenitor population after psychostimulant exposure. Brain Res Dev Brain Res. 2001;130:41–51. doi: 10.1016/s0165-3806(01)00195-x. [DOI] [PubMed] [Google Scholar]
- 57.Kachroo A. Irizarry MC. Schwarzschild MA. Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp Neurol. 2010;223:657–661. doi: 10.1016/j.expneurol.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wentz CT. Magavi SS. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology. 2009;56:994–1000. doi: 10.1016/j.neuropharm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brothers HM. Marchalant Y. Wenk GL. Caffeine attenuates lipopolysaccharide-induced neuroinflammation. Neurosci Lett. 2010;480:97–100. doi: 10.1016/j.neulet.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guerreiro S. Toulorge D. Hirsch E. Marien M. Sokoloff P. Michel PP. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol Pharmacol. 2008;74:980–989. doi: 10.1124/mol.108.048207. [DOI] [PubMed] [Google Scholar]
- 61.Popoli P. Pintor A. Domenici MR. Frank C. Tebano MT. Pezzola A. Scarchilli L. Quarta D. Reggio R. Malchiodi-Albedi F. Falchi M. Massotti M. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22:1967–1975. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dall'Igna OP. Porciuncula LO. Souza DO. Cunha RA. Lara DR. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol. 2003;138:1207–1209. doi: 10.1038/sj.bjp.0705185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nobre HV., Jr. Cunha GM. de Vasconcelos LM. Magalhaes HI. Oliveira Neto RN. Maia FD. de Moraes MO. Leal LK. Viana GS. Caffeine and CSC, adenosine A2A antagonists, offer neuroprotection against 6-OHDA-induced neurotoxicity in rat mesencephalic cells. Neurochem Int. 2010;56:51–58. doi: 10.1016/j.neuint.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Bona E. Aden U. Fredholm BB. Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38:312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Xu H. Aibiki M. Nagoya J. Neuroprotective effects of hyperthermic preconditioning on infarcted volume after middle cerebral artery occlusion in rats: role of adenosine receptors. Crit Care Med. 2002;30:1126–1130. doi: 10.1097/00003246-200205000-00028. [DOI] [PubMed] [Google Scholar]
- 66.McCarty MF. Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med Hypotheses. 2006;67:251–269. doi: 10.1016/j.mehy.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Prediger RD. Batista LC. Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 68.Dall'Igna OP. Fett P. Gomes MW. Souza DO. Cunha RA. Lara DR. Caffeine and adenosine A2a receptor antagonists prevent beta-amyloid 25–35-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Canas PM. Porciuncula LO. Cunha GM. Silva CG. Machado NJ. Oliveira JM. Oliveira CR. Cunha RA. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McNamara R. Kerans A. O'Neill B. Harkin A. Caffeine promotes hyperthermia and serotonergic loss following co-administration of the substituted amphetamines, MDMA ‘Ecstasy’ and MDA ‘Love’. Neuropharmacology. 2006;50:69–80. doi: 10.1016/j.neuropharm.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Khairnar A. Plumitallo A. Frau L. Schintu N. Morelli M. Caffeine enhances astroglia and microglia reactivity induced by 3,4-methylenedioxymethamphetamine ‘ecstasy’ in mouse brain. Neurotox Res. 2010;17:435–439. doi: 10.1007/s12640-009-9125-y. [DOI] [PubMed] [Google Scholar]
- 72.Fattore L. Puddu MC. Picciau S. Cappai A. Fratta W. Serra GP. Spiga S. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience. 2002;110:1–6. doi: 10.1016/s0306-4522(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 73.Thomas DM. Walker PD. Benjamins JA. Geddes TJ. Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- 74.Granado N. O'Shea E. Bove J. Vila M. Colado MI. Moratalla R. Persistent MDMA-induced dopaminergic neurotoxicity in the striatum and substantia nigra of mice. J Neurochem. 2008;107:1102–1112. doi: 10.1111/j.1471-4159.2008.05705.x. [DOI] [PubMed] [Google Scholar]
- 75.Fredholm BB. Adenosine actions and adenosine receptors after 1 week treatment with caffeine. Acta Physiol Scand. 1982;115:283–286. doi: 10.1111/j.1748-1716.1982.tb07078.x. [DOI] [PubMed] [Google Scholar]
- 76.Guillet R. Kellogg C. Neonatal exposure to therapeutic caffeine alters the ontogeny of adenosine A1 receptors in brain of rats. Neuropharmacology. 1991;30:489–496. doi: 10.1016/0028-3908(91)90011-y. [DOI] [PubMed] [Google Scholar]
- 77.Hettinger-Smith BD. Leid M. Murray TF. Chronic exposure to adenosine receptor agonists and antagonists reciprocally regulates the A1 adenosine receptor-adenylyl cyclase system in cerebellar granule cells. J Neurochem. 1996;67:1921–1930. doi: 10.1046/j.1471-4159.1996.67051921.x. [DOI] [PubMed] [Google Scholar]
- 78.Johansson B. Georgiev V. Lindstrom K. Fredholm BB. A1 and A2A adenosine receptors and A1 mRNA in mouse brain: effect of long-term caffeine treatment. Brain Res. 1997;762:153–164. doi: 10.1016/s0006-8993(97)00378-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.