Abstract
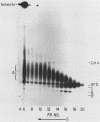
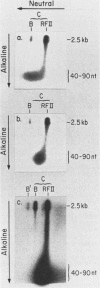
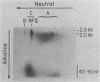
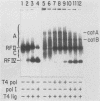
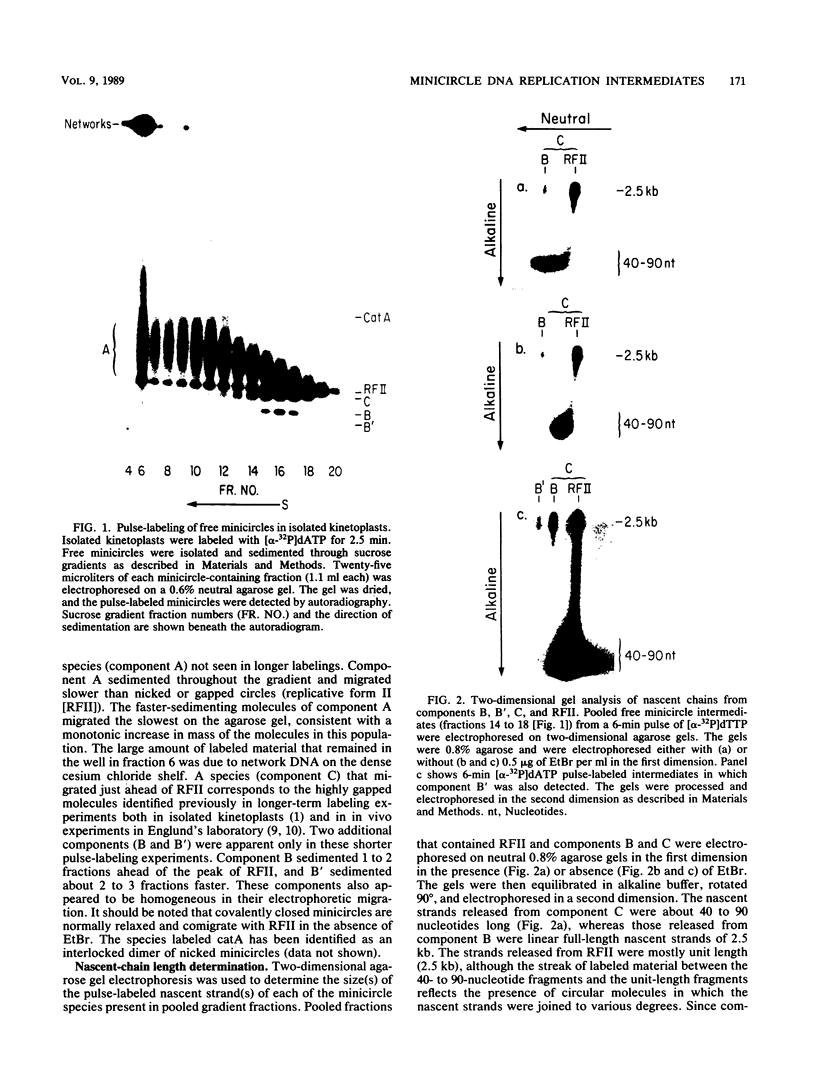
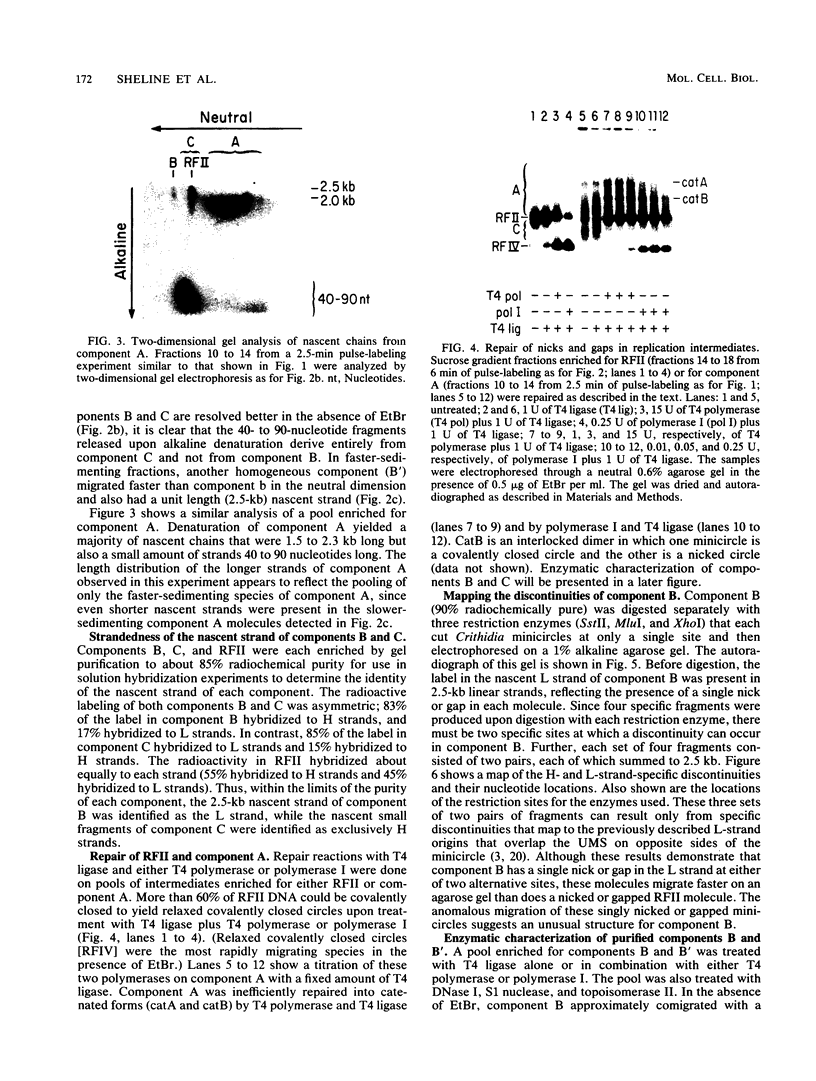
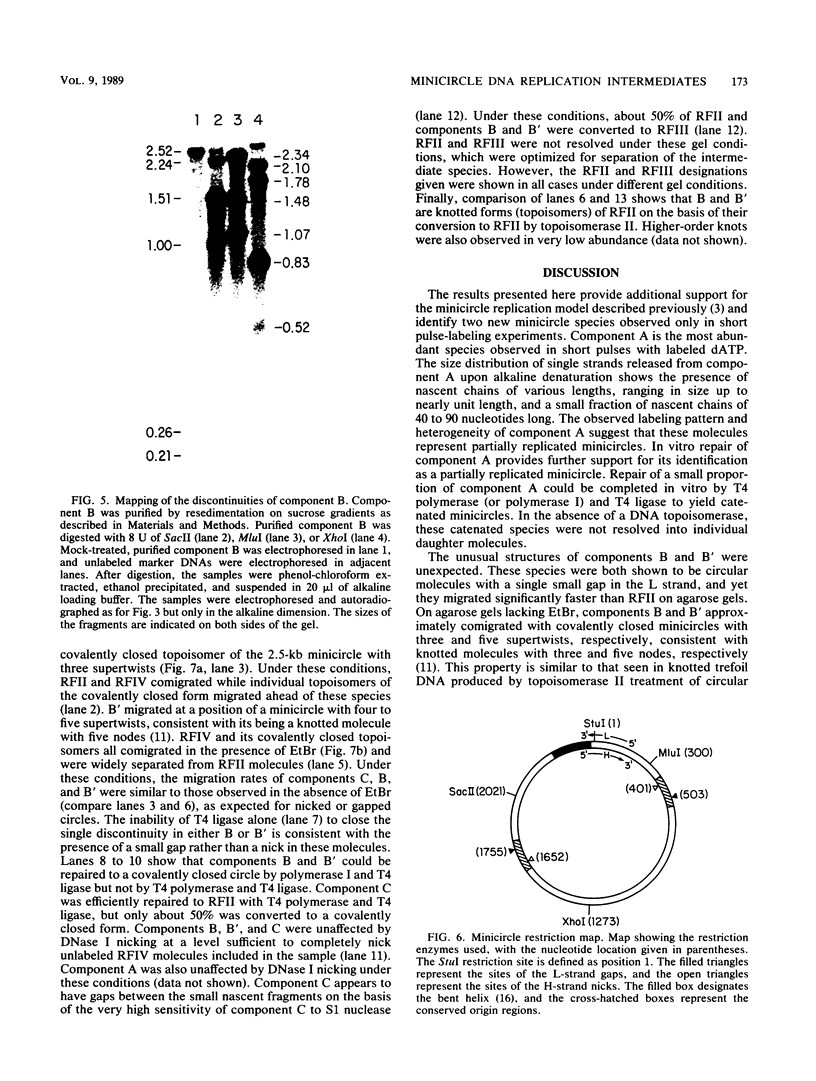
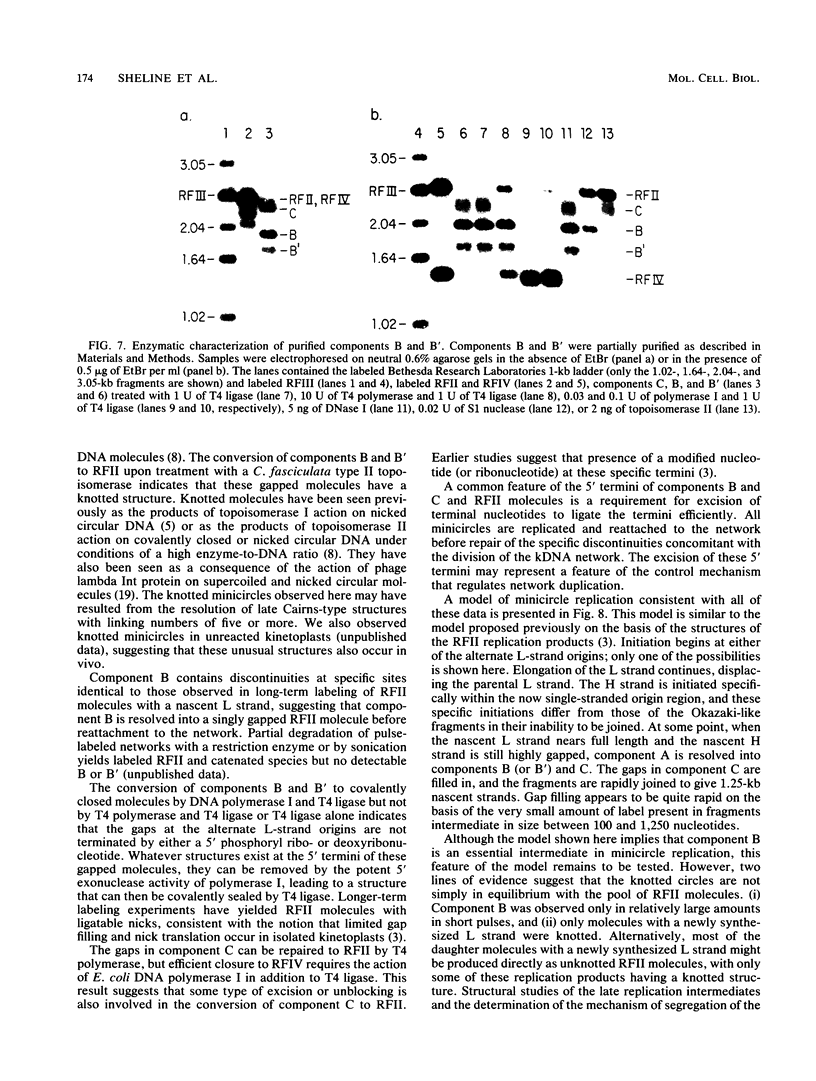
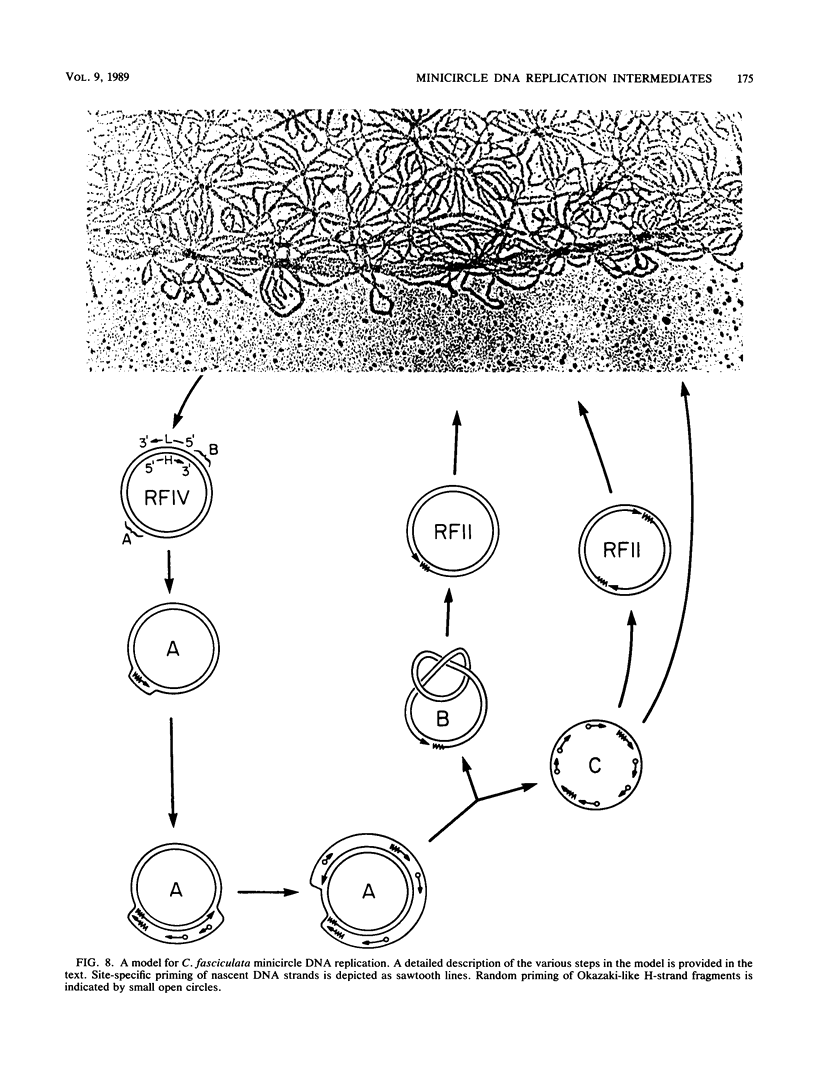
We have previously described an isolated kinetoplast system from Crithidia fasciculata capable of ATP-dependent replication of kinetoplast DNA minicircles (L. Birkenmeyer and D.S. Ray, J. Biol. Chem. 261: 2362-2368, 1986). We present here the identification of two new minicircle species observed in short pulse-labeling experiments in this system. The earliest labeled minicircle species (component A) contains both nascent H and L strands and is heterogeneous in sedimentation and electrophoretic migration. Component A has characteristics consistent with a Cairns-type structure in which the L strand is the leading strand and the H strand is the lagging strand. The other new species (component B) has a nascent 2.5-kilobase linear L strand with a single discontinuity that mapped to either of two alternative origins located 180 degrees apart on the minicircle map. Component B could be repaired to a covalently closed form by Escherichia coli polymerase I and T4 ligase but not by T4 polymerase and T4 ligase. Even though component B has a single gap in one strand, it had an electrophoretic mobility on an agarose gel (minus ethidium bromide) similar to that of a supercoiled circle with three supertwists. Treatment of component B with topoisomerase II converted it to a form that comigrated with a nicked open circular form (replicative form II). These results indicate that component B is a knotted topoisomer of a kinetoplast DNA minicircle with a single gap in the L strand.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeyer L., Ray D. S. Replication of kinetoplast DNA in isolated kinetoplasts from Crithidia fasciculata. Identification of minicircle DNA replication intermediates. J Biol Chem. 1986 Feb 15;261(5):2362–2368. [PubMed] [Google Scholar]
- Birkenmeyer L., Sugisaki H., Ray D. S. Structural characterization of site-specific discontinuities associated with replication origins of minicircle DNA from Crithidia fasciculata. J Biol Chem. 1987 Feb 15;262(5):2384–2392. [PubMed] [Google Scholar]
- Birkenmeyer L., Sugisaki H., Ray D. S. The majority of minicircle DNA in Crithidia fasciculata strain CF-C1 is of a single class with nearly homogeneous DNA sequence. Nucleic Acids Res. 1985 Oct 11;13(19):7107–7118. doi: 10.1093/nar/13.19.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Dean F. B., Cozzarelli N. R. Mechanism of strand passage by Escherichia coli topoisomerase I. The role of the required nick in catenation and knotting of duplex DNA. J Biol Chem. 1985 Apr 25;260(8):4984–4994. [PubMed] [Google Scholar]
- Englund P. T. Free minicircles of kinetoplast DNA in Crithidia fasciculata. J Biol Chem. 1979 Jun 10;254(11):4895–4900. [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Hsieh T. Knotting of the circular duplex DNA by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8413–8420. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Fein B. I., Englund P. T. Gapped Minicircles. A novel replication intermediate of kinetoplast DNA. J Biol Chem. 1984 Dec 25;259(24):15532–15539. [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Marini J. C., Bangs J. D., Hajduk S. L., Jimenez H. E., Kitchin P. A., Klein V. A., Ryan K. A., Englund P. T. Presence of a bent helix in fragments of kinetoplast DNA minicircles from several trypanosomatid species. Mol Biochem Parasitol. 1984 Jul;12(3):273–286. doi: 10.1016/0166-6851(84)90084-7. [DOI] [PubMed] [Google Scholar]
- Ntambi J. M., Shapiro T. A., Ryan K. A., Englund P. T. Ribonucleotides associated with a gap in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1986 Sep 5;261(25):11890–11895. [PubMed] [Google Scholar]
- Ray D. S., Hines J. C., Sugisaki H., Sheline C. kDNA minicircles of the major sequence class of C. fasciculata contain a single region of bent helix widely separated from the two origins of replication. Nucleic Acids Res. 1986 Oct 24;14(20):7953–7965. doi: 10.1093/nar/14.20.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Griffith J. D., Englund P. T. A knotted free minicircle in kinetoplast DNA. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5844–5848. doi: 10.1073/pnas.85.16.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. Kinetoplast DNA in trypanosomid flagellates. Int Rev Cytol. 1986;99:119–179. doi: 10.1016/s0074-7696(08)61426-6. [DOI] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Spengler S. J., Stasiak A., Cozzarelli N. R. The stereostructure of knots and catenanes produced by phage lambda integrative recombination: implications for mechanism and DNA structure. Cell. 1985 Aug;42(1):325–334. doi: 10.1016/s0092-8674(85)80128-8. [DOI] [PubMed] [Google Scholar]
- Sugisaki H., Ray D. S. DNA sequence of Crithidia fasciculata kinetoplast minicircles. Mol Biochem Parasitol. 1987 Apr;23(3):253–263. doi: 10.1016/0166-6851(87)90032-6. [DOI] [PubMed] [Google Scholar]