Abstract
Plasma F2-isoprostanes (F2-isoPs) are reliable biomarkers of oxidative stress. Several possible F2-isoPs are generated by the oxidation of arachidonic acid esterified in phospholipids. The separation of these isomers represents a technical challenge for rapid and selective determination. We have developed a HPLC-MS/MS method for the simultaneous determination of seven plasma F2-isoPs, namely 8-iso-15(R)-prostaglandin F2α (PGF2α), 8-iso-PGF2α, 15(R)-PGF2α, iPF2α-IV, iPF2α-VI, 5-iPF2α-VI, and (±)5-8,12-iso-iPF2α-VI. We have validated this method in plasma of pregnant women, a mild physiological oxidative stress known to increase F2-isoPs. Thus, plasma samples of women collected at the third trimester of pregnancy (n = 20) were subjected to alkaline hydrolysis followed by liquid-liquid extraction in order to extract total F2-isoPs. The F2-isoPs were separated within 16.5 min using a column packed with core-shell particles. The class VI isomers were the most abundant, accounting for 65% of the total level of all quantified F2-isoPs in plasma of pregnant women (P < 0.05). The 15(R)-PGF2α was the most abundant of the class III isomers quantified. This method allowed fast and selective separation of seven isomers from three different classes of F2-isoP regioisomers.
Keywords: oxidative stress, pregnancy, mass spectrometry, 8-iso-PGF2α, F2-isoprostane, high-performance liquid chromatography
Oxidative stress is an independent risk factor for coronary heart disease (1, 2) and is a feature of many other pathologies (3) including hypercholesterolemia (4), inflammatory diseases (5), and endothelial dysfunctions (6, 7). F2-isoprostanes (F2-isoPs) found in tissues and biological fluids are reliable biomarkers of oxidative stress (8, 9). F2-isoPs result from the free radical-mediated peroxidation of arachidonic acid esterified in phospholipids. This reaction potentially generates 64 isomers of F2-isoPs divided into four classes of regioisomers, each composed of eight diastereoisomers (10). Once formed, these compounds can be released from phospholipids by phospholipase A2 (PLA2) or by the platelet activating factor acetylhydrolase (PAF-AH or lipoprotein-PLA2) (11). The F2-isoPs exert their biological activity, such as vasoconstriction, in their free form in plasma and are excreted in urine (12).
The 8-iso-prostaglandin F2α (PGF2α) has been the most studied isomer, and is known as an in vivo biomarker of oxidative stress (3). Urinary level of F2-isoPs is a good indicator of oxidative stress but relies on other factors such as the rate of hydrolysis of F2-isoPs from phospholipids, their metabolism as well as their excretion (13). However, valuable information could be obtained from the simultaneous analyses of the plasma concentrations of several isomers of F2-isoP, as they may be formed and eliminated differentially according to the physiological state or disorder (14–17).
Several methods have been developed to measure F2-isoPs including enzyme immunoassays and gas or liquid chromatography coupled to mass spectrometry. The methods using HPLC-MS/MS are usually the most selective for F2-isoP regioisomers and diastereoisomers (18). Generally, a C18 stationary phase composed of fully porous particles was used in these methods (18–22). However, new chromatographic columns packed with core-shell particles improve separation efficiency for several analytes in comparison to classical porous particles (23, 24). The benefits of these new columns for analysis of F2-isoPs were not demonstrated so far, to our knowledge.
Thus, the aim of this study was to develop a rapid and specific method to quantify F2-isoP isomers in plasma by HPLC-MS/MS using a column filled with core-shell particles. To test this new method, the plasma total levels (esterified + free) of several F2-isoP isomers were determined in women at the third trimester of pregnancy. In order to detect a wide range of physiologically produced F2-isoP isomers above the baseline, we chose to use plasma of pregnant women because pregnancy is a mild oxidative stress known to increase the levels of 8-iso-PGF2α(25–28).
MATERIAL AND METHODS
Materials
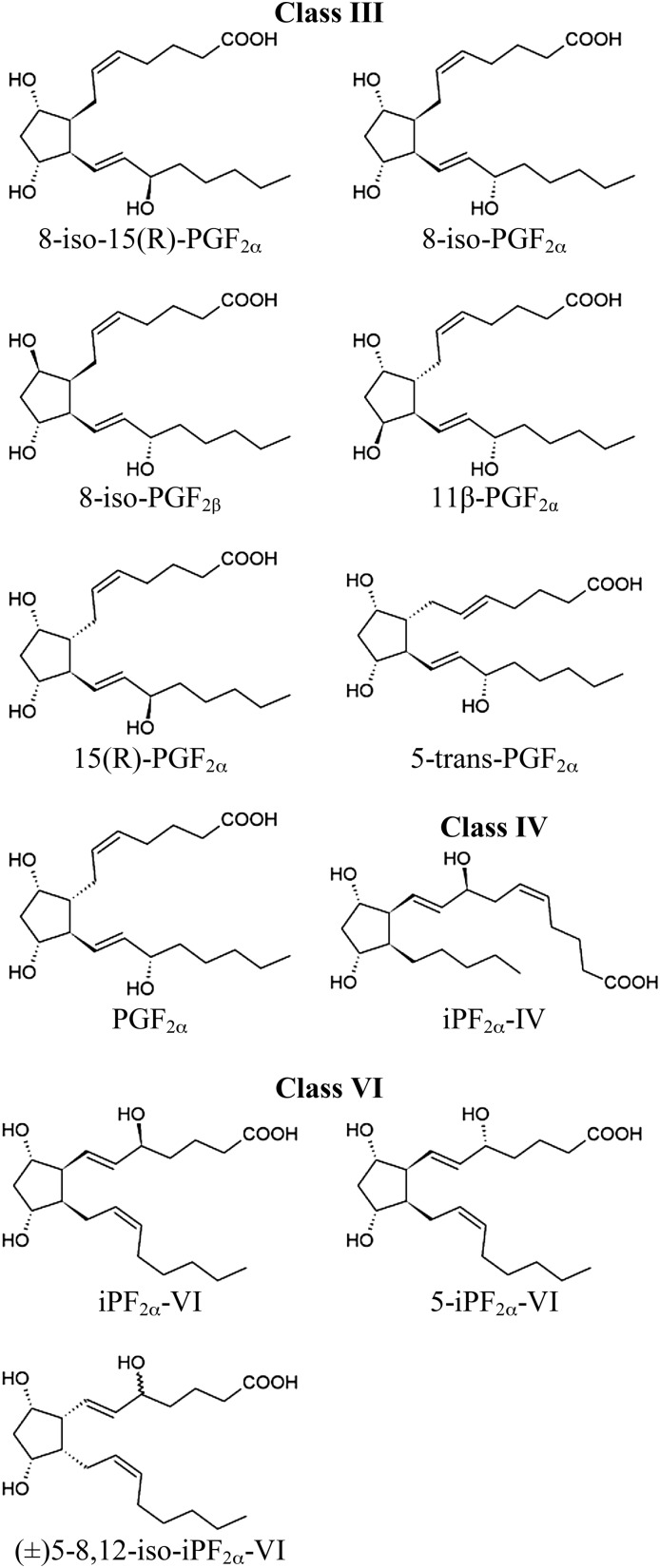
The 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 8-iso-PGF2β, 11β-PGF2α, 15(R)-PGF2α, 5-trans-PGF2α, PGF2α, iPF2α-IV, (±)5-iPF2α-VI, (±)5-8,12-iso-iPF2α-VI, and their deuterated counterparts 8-iso-PGF2α-d4, PGF2α-d4, iPF2α-IV-d4, iPF2α-VI-d4, (±)5-iPF2α-VI-d11, and (±)5-8,12-iso-iPF2α-VI-d11 were purchased from Cayman Chemical (Ann Arbor, MI). Butylated hydroxytoluene (BHT) was bought from Sigma-Aldrich (Oakville, ON, Canada) and sodium chloride was obtained from Laboratoire Mat (Québec, QC, Canada). All other reagents and solvents were HPLC grade and were purchased from VWR International (Ville Mont-Royal, QC, Canada).
Patient selection
We recruited 20 normotensive pregnant women at the Centre Mère-Enfant du Centre Hospitalier Universitaire de Québec. The institution approved the protocol and informed consent was obtained from all pregnant women participating in the study. Normotensive pregnancy was defined as a pregnancy in which the mother had normal blood pressure (≤140/90 mm Hg), absence of proteinuria, and absence of other medical complications such as thrombocytopenia (platelet count < 100,000 × 109/l), oliguria (<500 ml/day), pulmonary edema, elevated liver enzyme levels, severe nausea and vomiting, frontal headache, visual disturbances, persistent abdominal pain in right upper quadrant, chest pain or shortness of breath, suspected abruption placentae, hemolysis, elevated liver enzymes syndrome, intrauterine growth retardation, and oligohydramnios. Patients presenting any preexisting medical conditions such as chronic hypertension, diabetes mellitus, obesity (body mass index > 30 prior to pregnancy), kidney diseases, inflammatory intestinal diseases and blood clotting disorders, or any concurrent medical obstetrical complications, such as gestational diabetes, were excluded. Other exclusion factors were age (<18 years old or >40 years old) and the intake of anticoagulant drugs or drugs affecting lipid metabolism. No patient had symptoms associated with gestational hypertension with proteinuria (preeclampsia) as it is defined by the Canadian Hypertension Society Consensus (29). All women were nonsmokers.
Blood collection and processing
Twenty milliliters of blood were collected in heparinized tubes before the active phase of labor. The processing of blood samples was done within 2 h. Whole blood (500 μl) was immediately frozen on dry ice and kept at −80°C for further analysis. The remaining blood was centrifuged at 180 g for 10 min at 20°C. The supernatant (plasma) was recentrifuged at 1,300 g for 25 min to remove platelets from plasma, and was kept at −80°C for further analyses.
Preparation of standards for analysis of F2-isoPs
A solution of internal standards containing 50 ng/ml of each deuterated analyte (8-iso-PGF2α-d4, PGF2α-d4, iPF2α-IV-d4, iPF2α-VI-d4, (±)5-iPF2α-VI-d11, and (±)5-8,12-iso-iPF2α-VI-d11) in 0.01% acetic acid was prepared to be added to samples and standards. A stock solution containing 1 μg/ml of each compound (8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, 5-trans-PGF2α, PGF2α, iPF2α-IV, (±)5-iPF2α-VI, and (±)5-8,12-iso-iPF2α-VI) was prepared in 0.01% acetic acid. This last solution was used to prepare two sets of working solutions with concentrations ranging from 2 to 80 ng/ml in 0.01% acetic acid for the preparation of the standard curve, quality control, and method validation. The first set of working solutions was diluted to obtain the standard curves for each analyte (10 μl of working solution, 10 μl of internal standard, 80 μl of water containing 10% (v/v) acetonitrile, and 0.01% (v/v) acetic acid). The second set of working solutions was diluted the same way to obtain quality controls.
Extraction of F2-isoPs from plasma
F2-isoPs were extracted from plasma using a modified version of the method described by Taylor et al. (19). Ten microliters of a BHT solution (1% in ethanol) and 10 μl of the internal standard were added to 250 μl of freshly thawed plasma. Samples were completed to 500 μl with water. Then, 500 μl of hydrolysis solution (1 ml of 50% (w/w) KOH, 1 ml of water, and 10 ml methanol) were added. The resulting mixture was vortexed and incubated at 37°C for 60 min. The reaction was stopped with 100 μl of formic acid 0.05% (v/v) and acidified with 90 μl of hydrochloric acid 5 N. The tubes were then extracted twice with 1.5 ml of hexane. The organic phase was discarded. The aqueous phase was then extracted three times with 1.5 ml of 3:1 ethyl acetate:hexane. The resulting organic phase of the three extractions were combined and evaporated to dryness under nitrogen and reconstituted to 100 μl in 10% (v/v) acetonitrile and 0.01% (v/v) acetic acid in water.
Chromatography
The chromatography was carried out using a Shimadzu Prominence system (Columbia, MD). A Kinetex XB-C18 100 Å column (100 × 3.0 mm, 2.6 μm) was used preceded by a 4.0 × 2.0 mm C18 SecurityGuard cartridge. Both were from Phenomenex (Torrance, CA). The column oven temperature was controlled at 30°C. The injection volume was 40 μl. The separation was done using a gradient of three solvents at a flow rate of 0.45 ml/min. Solvent A was composed of 0.01% (v/v) acetic acid in water, solvent B consisted of 0.01% (v/v) acetic acid in acetonitrile, and solvent C was composed of 0.01% (v/v) acetic acid in methanol. First, solvent B was held at 17% for 1 min while solvent C was at 33%. The latter was followed by a linear gradient for over 8.9 min to 13.5% of B and 58.9% of C. The next step was a linear gradient over 0.5 min to 47.5% B and 47.5% C respectively. The previous conditions were maintained for 1.6 min and solvents B and C were decreased to 17% and 33% in 0.1 min respectively. This last condition was maintained for an additional 4.4 min to complete the 16.5 min run.
Mass spectrometry
The HPLC was coupled to a 3200 QTRAP® LC/MS/MS system from AB Sciex (Concord, ON, Canada) through a Turbo VTM ion source using the electrospray ionization probe. The mass spectrometer was operated in negative mode. Curtain gas, collision gas, ion source gas 1, and ion source gas 2 were set at 37, 7, 45 and 55 respectively. The ions spray voltage was set at −4,100 V and source temperature was set at 700°C. Class III F2-isoPs and their internal standard, 8-iso-PGF2α-d4 and PGF2α-d4 (class III-d4), were monitored in the multiple-reaction monitoring mode using the transitions 353.3 → 193.2 m/z and 357.3 → 197.2 m/z respectively. Class IV F2-isoPs and their internal standard, iPF2α-IV-d4 (class IV-d4), were monitored using the transitions 353.3 → 127.0 m/z and 357.0 → 127.0 m/z. Finally, class VI F2-isoPs and their internal standard, (±)5-iPF2α-VI-d11 and (±)5-8,12-iso-iPF2α-VI-d11 (class VI-d11), were analyzed using the transitions 353.0 → 115.0 m/z and 364.6 → 115.0 m/z respectively. Quantification was performed using Analyst® 1.4.2 software (AB Sciex).
Method validation
The lower limit of quantification (LLOQ) was defined as the concentration to which the signal-to-noise ratio was higher than five with a precision below 20% and an accuracy of ±20% of the nominal concentration (30). Determination of intra-day precision was done by analyzing a pool of plasma samples from three nonpregnant women (Innovative Research, Novi, MI) spiked with 10 μl of working solutions containing either 0 ng/ml, 7 ng/ml, or 20 ng/ml of each analyte (n = 4 per concentration). This validation procedure was carried out on three consecutive days in order to evaluate inter-day precision (n = 12 per concentration). Accuracy and recovery was determined using plasma samples spiked with the 7 and 20 ng/ml working solutions. The recovery was evaluated by comparing signal obtained for plasma spiked before extraction with signal obtained for plasma spiked after extraction with the corresponding working solutions. Matrix effects were evaluated by post-column infusion at 10 μl/min of a solution containing 100 ng/ml of each of the following molecules: 8-iso-PGF2α, 8-iso-PGF2α-d4, iPF2α-IV, iPF2α-IV-d4, (±)5-iPF2α-VI, and (±)5-iPF2α-VI-d11. During post-column infusion, an extract of plasma was injected concomitantly using the described HPLC-MS/MS method above.
Statistical analyses
Statistical analyses were performed with SigmaPlot 12.3 (Systat Software, Inc., San Jose, CA). The normality was tested using the Shapiro-Wilk test. The Kruskal-Wallis one-way ANOVA was used to compare levels of F2-isoPs. All pairwise multiple comparisons were done according to the Student-Newman-Keuls method. In all cases, a P-value lower than 0.05 was considered significant and a P-value between 0.05 and 0.1 was considered a tendency.
RESULTS
Analysis of F2-isoPs in plasma by selected reaction monitoring mass spectrometry
Compound-dependent mass spectrometric parameters were optimized for each class of regioisomers using split infusion at conditions close to the chromatographic separation. The 8-iso-PGF2α was used to optimize class III F2-isoP parameters (transitions 353.3 → 193.2 m/z and 357.3 → 197.2 m/z for deuterated standard respectively). The iPF2α-IV was used to define which parameters to employ for class IV F2-isoPs. The (±)5-iPF2α-VI was used to determine parameters for class VI regioisomers. It was not possible to measure class V F2-isoPs because no commercial standards were available. Declustering potential, entrance potential, collision energy, collision cell entrance potential, and collision cell exit potential for each transition are shown in Table 1.
TABLE 1.
Selected reaction monitoring parameters optimized for each class of F2-isoPs
| F2-isoPs | Transitions (m/z) | DP (V) | EP (V) | CEP (V) | CE (V) | CXP (V) |
| Class III | 353.3 → 193.2 | −50.0 | −7.0 | −20.0 | −34.0 | −4.0 |
| Class III-d4 | 357.3 → 197.2 | −50.0 | −7.0 | −20.0 | −34.0 | −4.0 |
| Class IV | 353.0 → 127.0 | −45.0 | −7.0 | −17.0 | −33.0 | −2.0 |
| Class IV-d4 | 357.0 → 127.0 | −45.0 | −7.0 | −17.0 | −33.0 | −2.0 |
| Class VI | 353.0 → 115.0 | −47.0 | −7.0 | −21.0 | −30.0 | −2.0 |
| Class VI-d11 | 364.6 → 115.0 | −47.0 | −7.0 | −21.0 | −30.0 | −2.0 |
DP, declustering potential; EP, entrance potential; CEP, collision cell entrance potential;
CE, collision energy; CXP, collision cell exit potential.
Chromatographic separation
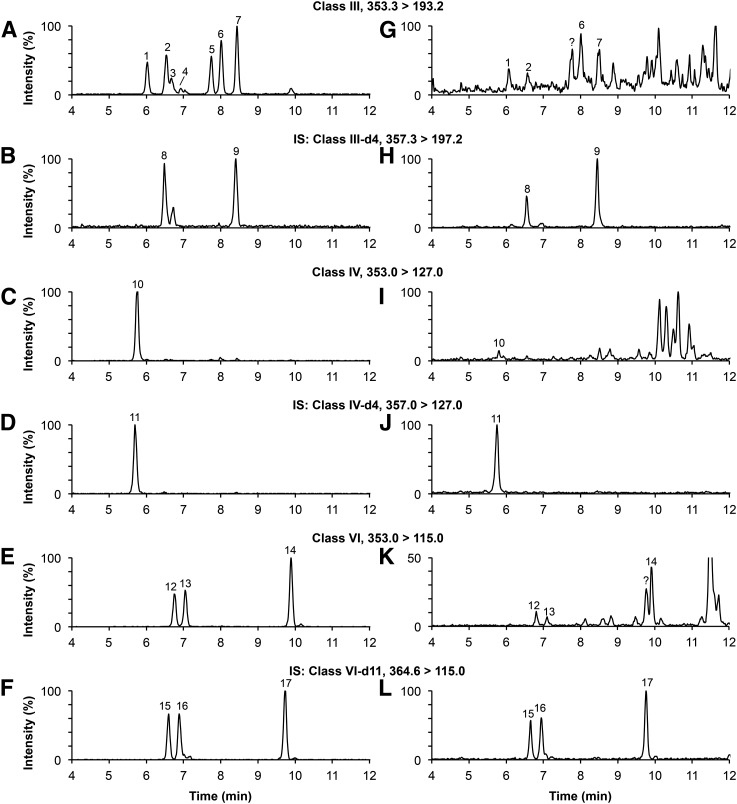
HPLC conditions were optimized to obtain a baseline separation of all F2-isoPs in plasma (Fig. 1). Mass chromatograms are shown for a pure standard solution (Fig. 2 A–F) and for a typical plasma sample (Fig. 2 G–L). All class III isomers were well resolved (Fig. 2A) except for the 8-iso-PGF2β. This last isomer, as well as the 11β-PGF2α isomer, were not detected in the plasma of third trimester pregnant women (Fig. 2G). The peak observed at 7.78 min (Fig. 2G) was considered an unknown plasma compound because it had the same retention time as the 5-trans-PGF2α, but eluted inconsistently under different chromatographic conditions (data not shown). The 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, and PGF2α were measurable in all plasma samples analyzed. The iPF2α-IV was well separated from impurities (Fig. 2I), but was not always detectable in our samples. Both iPF2α-VI and 5-iPF2α-VI were found in equal proportion in the (±)5-iPF2α-VI bought from the supplier. The iPF2α-VI-d4 (transition 357.0 → 115.0 m/z) was used to identify corresponding unlabeled isomers (data not shown). All class VI isomers were well resolved in pure standard solution and were all detected in the plasma samples analyzed (Fig. 2E, K). Both iPF2α-VI and 5-iPF2α-VI were well separated from plasma impurities (Fig. 2K). Only the (±)5-8,12-iso-iPF2α-VI barely coeluted with an unknown compound.
Fig. 1.
Chemical structures of analytes investigated by LC-MS/MS.
Fig. 2.
Mass chromatograms of a standard solution (A–F) and of a typical plasma sample spiked with 50 pg of each internal standard (G–L). Peak identification (see Fig. 1 for structure): 1, 8-iso-15(R)-PGF2α; 2, 8-iso-PGF2α; 3, 8-iso-PGF2β; 4, 11β-PGF2α; 5, 15(R)-PGF2α; 6, 5-trans-PGF2α; 7, PGF2α; 8, 8-iso-PGF2α-d4; 9, PGF2α-d4; 10, iPF2α-IV; 11, iPF2α-IV-d4; 12, iPF2α-VI; 13, 5-iPF2α-VI; 14, (±)5-8,12-iso-iPF2α-VI; 15, iPF2α-VI-d11; 16, 5-iPF2α-VI-d11; 17, (±)5-8,12-iso-iPF2α-VI-d11; ?, unknown compounds. IS, deuterated internal standards.
Method validation
Quantification of F2-isoPs was done using the ratio of peak area of each analyte to the peak area of corresponding internal standards. Class III isomers 8-iso-15(R)-PGF2α, 8-iso-PGF2α, and 15(R)-PGF2α were quantified using 8-iso-PGF2α-d4. The iPF2α-IV was calculated using iPF2α-IV-d4. The iPF2α-VI-d11, 5-iPF2α-VI-d11, and (±)5-8,12-iso-iPF2a-VI-d11 were used to measure respectively the iPF2α-VI, 5-iPF2α-VI, and (±)5-8,12-iso-iPF2a-VI. The calibration curves for each F2-isoP covered 0.2–8 ng/ml and were linear throughout this range (r2 > 0.99). LLOQ ranged between 11.3 and 21.0 pg injected on column (Table 2). At these concentrations, the precision was <20% [% coefficient of variation (CV)] and accuracy was below ±20% of the nominal concentration. Mean intra-day coefficients of variation (% CV) fluctuated from 2.6 to 5.2% and mean inter-day CVs varied between 3.4 and 8.2%. Mean intra-day and inter-day accuracy was below ±15% of the nominal concentration except for the 8-iso-PGF2α. The extraction recovery was also evaluated and ranged from 57.9 to 73.3%. No matrix effect was detected at the retention times of F2-isoPs except for 8-iso-PGF2α, which showed low ionic suppression (data not shown).
TABLE 2.
Results for the method validation of F2-isoPs
| LLOD (pg inj.) | LLOQ (pg inj.) | Recoverya (%) | Intradayd | Interdaye | |||
| Precisionb (% CV) | Accuracyc (%) | Precision (% CV) | Accuracy (%) | ||||
| 8-iso-15(R)-PGF2α | 9.1 | 13.8 | 62.6 | 3.1 | −3.9 | 4.7 | −5.8 |
| 8-iso-PGF2α | 10.6 | 16.1 | 71.1 | 4.6 | −21.6 | 5.3 | −22.6 |
| 15(R)-PGF2α | 11.0 | 16.6 | 73.3 | 2.9 | 1.6 | 4.1 | 0.3 |
| iPF2α-IV | 7.5 | 11.3 | 59.1 | 2.6 | −2.6 | 3.4 | −4.6 |
| iPF2α-VI | 9.2 | 13.9 | 58.4 | 5.2 | 1.5 | 8.2 | 2.3 |
| 5-iPF2α-VI | 8.2 | 12.4 | 57.9 | 3.4 | 1.8 | 6.5 | 0.1 |
| (±)5-8,12-iso-iPF2α-VI | 13.8 | 21.0 | 71.2 | 4.1 | 9.2 | 8.1 | 4.1 |
LLOD, lower limit of detection; injected (inj.), .
Recovery was expressed as the mean value of data obtained from plasma samples spiked with 10 μl of working solutions containing 7 ng/ml, or 20 ng/ml of each analyte.
Precision is expressed as the mean value of data obtained from plasma samples spiked with 10 μl of working solutions containing 0 ng/ml, 7 ng/ml, or 20 ng/ml of each analyte.
Accuracy is expressed as the mean value of data obtained from plasma samples spiked with the 7 or 20 ng/ml working solutions.
n = 4 per concentration.
n = 12 per concentration.
F2-isoPs in the plasma of pregnant women
The plasma from 20 nonsmoking women was retrieved at an average of 40.6 weeks of pregnancies before the active phase of labor. The total F2-isoPs were extracted from plasma by liquid-liquid extraction and measured by HPLC-MS/MS using the new validated method described above. The respective plasma median levels for 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, iPF2α-IV, iPF2α-VI, 5-iPF2α-VI, and 5-8,12-iso-iPF2α-VI are shown in Table 3. Statistical analyses indicated that class VI isomers were the most abundant, accounting for 65% of the total level of quantified F2-isoPs (P < 0.05, Table 3), and (±)5-8,12-iso-iPF2α-VI was the most abundant of all isomers (35% of the total level). The 15(R)-PGF2α was the most abundant of the class III isomers quantified. However, no significant differences were observed between 8-iso-15(R)-PGF2α, 8-iso-PGF2α, and iPF2α-IV. No significant differences were observed between iPF2α-VI and 5-iPF2α-VI as well.
TABLE 3.
F2-isoprostanes in the plasma of third trimester pregnant women
| Isoprostanes Levels (pg/ml plasma) | |
| Class III | |
| 8-iso-15(R)-PGF2α | 177 [150, 839]a |
| 8-iso-PGF2α | 195 [164, 551]a |
| 15(R)-PGF2α | 338 [285, 1758]b |
| Class IV | |
| iPF2α-IV | 137 [102, 984]a |
| Class VI | |
| iPF2α-VI | 416 [358, 4159]c |
| 5-iPF2α-VI | 393 [245, 5598]c |
| (±)5-8,12-iso-iPF2α-VI | 879 [487, 2643]d |
Values are medians and quartiles [Q1, Q3] (n = 20). Medians with different superscript letters (a–d) are statistically different (Kruskal-Wallis test followed by Student-Newman-Keuls, P < 0.05).
DISCUSSION
F2-isoPs found in tissues and biological fluids are reliable biomarkers of oxidative stress (8, 9). We have developed a new HPLC-MS/MS method for the simultaneous determination of seven F2-isoP isomers, namely the 8-iso-15(R)-PGF2α, 8-iso-PGF2α, 15(R)-PGF2α, iPF2α-IV, iPF2α-VI, 5-iPF2α-VI, and (±)5-8,12-iso-iPF2α-VI in plasma. The liquid chromatography coupled to mass spectrometry offers many advantages over immunoassays, allowing the analysis of many compounds in complex matrices with excellent selectivity, sensitivity, and dynamic range. The simultaneous analysis of several isomers of F2-isoPs can better characterize the oxidative stress of specific pathologies or physiological conditions. Indeed, in a comparative study, more than eight F2-isoP isomers were increased in urine of hypercholesterolemic patients, while only three isomers, including the 8-iso-PGF2α, were found to be increased in cases of congestive heart failure (17).
Several methods using HPLC-MS/MS have already been published for the determination of F2-isoPs (15, 18–22, 31, 32). Most chromatographic columns reported in these methods used a C18 stationary phase, although other types of stationary phases have also been used such as C8 or porous graphitic carbon. However, all columns used in previous methods were filled with fully porous particles. Our method is the first to use a chromatographic column filled with core-shell particles. This type of particle offers better efficiency than fully porous particles. In fact, as shown by the Van Deemter equation, many parameters contribute to peak broadening in HPLC, namely the longitudinal diffusion, the eddy dispersion, and the solid-liquid mass transfer resistance. Those three parameters are reduced when core-shell particles are used instead of the fully porous particles of about the same diameters (the eddy dispersion being the most affected) (33). This allowed us to achieve a better F2-isoP separation in only 16.5 min without loss of precision and accuracy. To our knowledge, the present chromatographic separation is the fastest among previously published methods.
The HPLC conditions were optimized in order to obtain a baseline separation of F2-isoPs for each class of regioisomers. The separation was performed using a gradient of three solvents, namely water, methanol, and acetonitrile, each containing 0.01% of acetic acid. The methanol allowed for a better selectivity of the F2-isoPs separation, but the use of acetonitrile was essential in order to avoid coelution between 8-iso-PGF2α and iPF2α-VI. Indeed, it is important to prevent coelution of isomers from class VI with isomers of class III because class VI isomers, when fragmented in the collision cells, produce both the main daughter ion 115 m/z, known to be specific to class VI, and the daughter ion 193 m/z that characterizes class III F2-isoPs. The signal for the 193 m/z ion represents about 10% of the signal of the 115 m/z ion produced from class VI fragmentation (22). That is why the proportion of acetonitrile in the eluent was higher at the beginning than at the end of the gradient.
In this study, we reported the total levels of the seven F2-isoP isomers in the plasma of 20 healthy women at the end of third trimester of pregnancy. The 8-iso-PGF2α is the most studied isomer used to characterize oxidative stress in vivo (3). The median level of 8-iso-PGF2α found in our study was 195 pg/ml of plasma. Literature values for the total level of 8-iso-PGF2α in plasma from healthy subjects ranged between 40 and 170 pg/ml (34, 35). Our measurement was slightly higher, but this was expected because pregnancy, per se, is known to be a mildly oxidative event (3, 25–28, 36). The class VI isomers were the most abundant, by 2- to 3-fold, when compared with the 8-iso-PGF2α. The class IV and VI isomers may be an underestimated marker of oxidative stress. This was the first study that quantified a wide array of F2-isoPs in the blood of pregnant women. This is why, except for 8-iso-PGF2α, it is not possible to compare our results with other studies.
In sum, we have developed a new HPLC-MS-MS method using a C18 core-shell column for the determination of seven F2-isoP isomers among classes III, IV, and VI in blood plasma. This new method has many advantages over the previously published method for the determination F2-isoPs such as speed and selectivity.
Footnotes
Abbreviations:
- BHT
- butylated hydroxytoluene
- CV
- coefficient of variation
- F2-isoP
- F2-isoprostane
- LLOQ
- lower limit of quantification
- PGF2α
- prostaglandin F2α
- PLA2
- phospholipase A2
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR, grant# MOP-84219 to J-F.B). J. Larose is a recipient of a Fonds de Recherche en Santé du Québec (FRSQ) award.
REFERENCES
- 1.Davies S. S., Roberts L. J., 2nd 2011. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 50: 559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwedhelm E., Bartling A., Lenzen H., Tsikas D., Maas R., Brummer J., Gutzki F. M., Berger J., Frolich J. C., Boger R. H. 2004. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 109: 843–848 [DOI] [PubMed] [Google Scholar]
- 3.Basu S. 2008. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid. Redox Signal. 10: 1405–1434 [DOI] [PubMed] [Google Scholar]
- 4.Reilly M. P., Pratico D., Delanty N., DiMinno G., Tremoli E., Rader D., Kapoor S., Rokach J., Lawson J., FitzGerald G. A. 1998. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 98: 2822–2828 [DOI] [PubMed] [Google Scholar]
- 5.Praticò D., Rokach J., Lawson J., FitzGerald G. A. 2004. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chem. Phys. Lipids. 128: 165–171 [DOI] [PubMed] [Google Scholar]
- 6.Arcaro G., Fava C., Dagradi R., Faccini G., Gaino S., Degan M., Lechi C., Lechi A., Minuz P. 2004. Acute hyperhomocysteinemia induces a reduction in arterial distensibility and compliance. J. Hypertens. 22: 775–781 [DOI] [PubMed] [Google Scholar]
- 7.Schulz E., Gori T., Munzel T. 2011. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 34: 665–673 [DOI] [PubMed] [Google Scholar]
- 8.Basu S. 2010. Bioactive eicosanoids: role of prostaglandin F(2alpha) and F-isoprostanes in inflammation and oxidative stress related pathology. Mol. Cells. 30: 383–391 [DOI] [PubMed] [Google Scholar]
- 9.Kadiiska M. B., Gladen B. C., Baird D. D., Germolec D., Graham L. B., Parker C. E., Nyska A., Wachsman J. T., Ames B. N., Basu S., et al. 2005. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 38: 698–710 [DOI] [PubMed] [Google Scholar]
- 10.Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., 2nd 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA. 87: 9383–9387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., McIntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J., 2nd 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281: 4616–4623 [DOI] [PubMed] [Google Scholar]
- 12.Milne G. L., Yin H., Hardy K. D., Davies S. S., Roberts L. J., 2nd 2011. Isoprostane generation and function. Chem. Rev. 111: 5973–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliwell B., Lee C. Y. 2010. Using isoprostanes as biomarkers of oxidative stress: some rarely considered issues. Antioxid. Redox Signal. 13: 145–156 [DOI] [PubMed] [Google Scholar]
- 14.Praticò D., Barry O. P., Lawson J. A., Adiyaman M., Hwang S-W., Khanapure S. P., Iuliano L., Rokach J., FitzGerald G. A. 1998. IPF2α-I: An index of lipid peroxidation in humans. Proc. Natl. Acad. Sci. USA. 95: 3449–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B., Saku K. 2007. Control of matrix effects in the analysis of urinary F2-isoprostanes using novel multidimensional solid-phase extraction and LC-MS/MS. J. Lipid Res. 48: 733–744 [DOI] [PubMed] [Google Scholar]
- 16.Basu S. 2004. Isoprostanes: novel bioactive products of lipid peroxidation. Free Radic. Res. 38: 105–122 [DOI] [PubMed] [Google Scholar]
- 17.Li H., Lawson J. A., Reilly M., Adiyaman M., Hwang S. W., Rokach J., FitzGerald G. A. 1999. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F(2)-isoprostanes in human urine. Proc. Natl. Acad. Sci. USA. 96: 13381–13386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin H., Porter N. A., Morrow J. D. 2005. Separation and identification of F2-isoprostane regioisomers and diastereomers by novel liquid chromatographic/mass spectrometric methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 827: 157–164 [DOI] [PubMed] [Google Scholar]
- 19.Taylor A. W., Bruno R. S., Frei B., Traber M. G. 2006. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-series F-isoprostanes. Anal. Biochem. 350: 41–51 [DOI] [PubMed] [Google Scholar]
- 20.Haschke M., Zhang Y. L., Kahle C., Klawitter J., Korecka M., Shaw L. M., Christians U. 2007. HPLC-atmospheric pressure chemical ionization MS/MS for quantification of 15-F2t-isoprostane in human urine and plasma. Clin. Chem. 53: 489–497 [DOI] [PubMed] [Google Scholar]
- 21.Korecka M., Clark C. M., Lee V. M., Trojanowski J. Q., Shaw L. M. 2010. Simultaneous HPLC-MS-MS quantification of 8-iso-PGF(2alpha) and 8,12-iso-iPF(2alpha) in CSF and brain tissue samples with on-line cleanup. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878: 2209–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan W., Byrd G. D., Ogden M. W. 2007. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J. Lipid Res. 48: 1607–1617 [DOI] [PubMed] [Google Scholar]
- 23.Gritti F., Leonardis I., Abia J., Guiochon G. 2010. Physical properties and structure of fine core-shell particles used as packing materials for chromatography. Relationships between particle characteristics and column performance. J. Chromatogr. A. 1217: 3819–3843 [DOI] [PubMed] [Google Scholar]
- 24.Gritti F., Leonardis I., Shock D., Stevenson P., Shalliker A., Guiochon G. 2010. Performance of columns packed with the new shell particles, Kinetex-C18. J. Chromatogr. A. 1217: 1589–1603 [DOI] [PubMed] [Google Scholar]
- 25.Myatt L., Cui X. 2004. Oxidative stress in the placenta. Histochem. Cell Biol. 122: 369–382 [DOI] [PubMed] [Google Scholar]
- 26.Agarwal A., Gupta S., Sharma R. K. 2005. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris J. M., Gopaul N. K., Endresen M. J., Knight M., Linton E. A., Dhir S., Anggard E. E., Redman C. W. 1998. Circulating markers of oxidative stress are raised in normal pregnancy and pre-eclampsia. Br. J. Obstet. Gynaecol. 105: 1195–1199 [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui I. A., Jaleel A., Tamimi W., Al Kadri H. M. 2010. Role of oxidative stress in the pathogenesis of preeclampsia. Arch. Gynecol. Obstet. 282: 469–474 [DOI] [PubMed] [Google Scholar]
- 29.Helewa M. E., Burrows R. F., Smith J., Williams K., Brain P., Rabkin S. W. 1997. Report of the Canadian Hypertension Society Consensus Conference: 1. Definitions, evaluation and classification of hypertensive disorders in pregnancy. CMAJ. 157: 715–725 [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration 2001. Guidance for Industry - Bioanalytical Method Validation. Accessed February 28, 2013 at http://www.fda.gov/downloads/Drugs/../Guidances/ucm070107.pdf [Google Scholar]
- 31.Bohnstedt K. C., Karlberg B., Basun H., Schmidt S. 2005. Porous graphitic carbon chromatography-tandem mass spectrometry for the study of isoprostanes in human cerebrospinal fluid. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 827: 39–43 [DOI] [PubMed] [Google Scholar]
- 32.Bohnstedt K. C., Karlberg B., Wahlund L. O., Jonhagen M. E., Basun H., Schmidt S. 2003. Determination of isoprostanes in urine samples from Alzheimer patients using porous graphitic carbon liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 796: 11–19 [DOI] [PubMed] [Google Scholar]
- 33.Gritti F., Guiochon G. 2012. Facts and legends on columns packed with sub-3-μm core-shell particles. LC GC N. Am. 30: 586–595 [Google Scholar]
- 34.Bastani N. E., Gundersen T. E., Blomhoff R. 2009. Determination of 8-epi PGF(2alpha) concentrations as a biomarker of oxidative stress using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 23: 2885–2890 [DOI] [PubMed] [Google Scholar]
- 35.Schwedhelm E., Boger R. H. 2003. Application of gas chromatography-mass spectrometry for analysis of isoprostanes: their role in cardiovascular disease. Clin. Chem. Lab. Med. 41: 1552–1561 [DOI] [PubMed] [Google Scholar]
- 36.Ishihara O., Hayashi M., Osawa H., Kobayashi K., Takeda S., Vessby B., Basu S. 2004. Isoprostanes, prostaglandins and tocopherols in pre-eclampsia, normal pregnancy and non-pregnancy. Free Radic. Res. 38: 913–918 [DOI] [PubMed] [Google Scholar]