Abstract
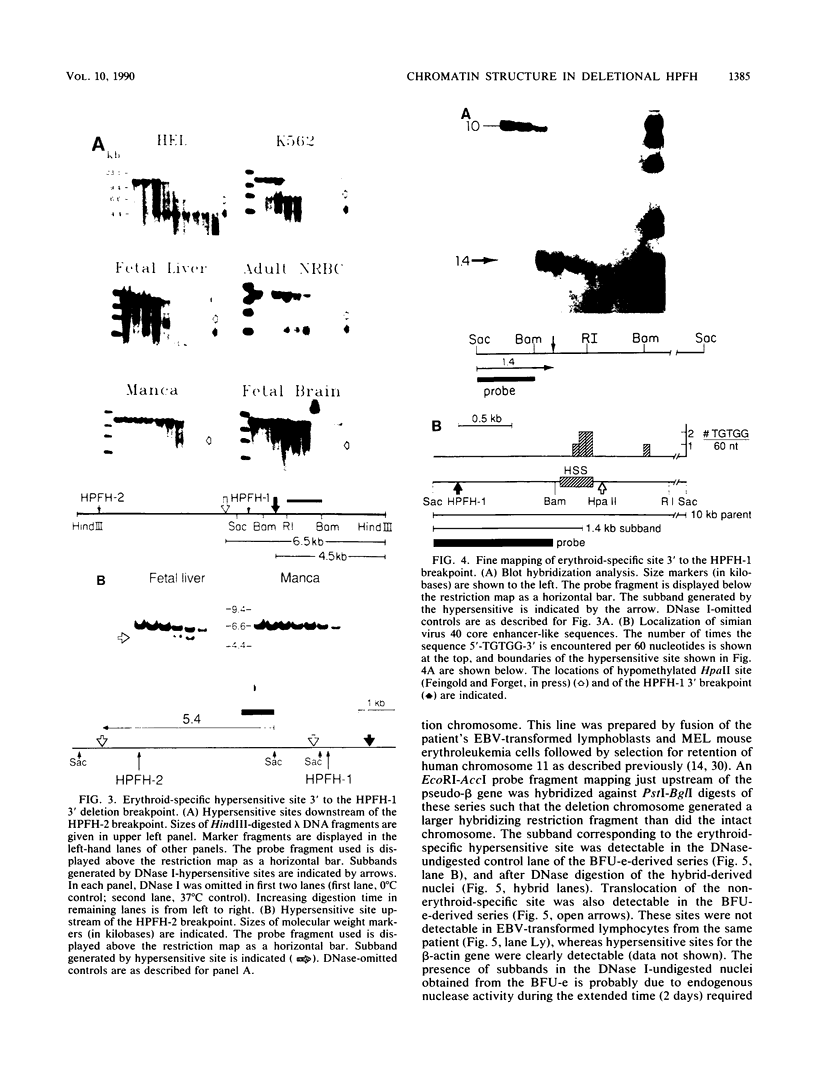
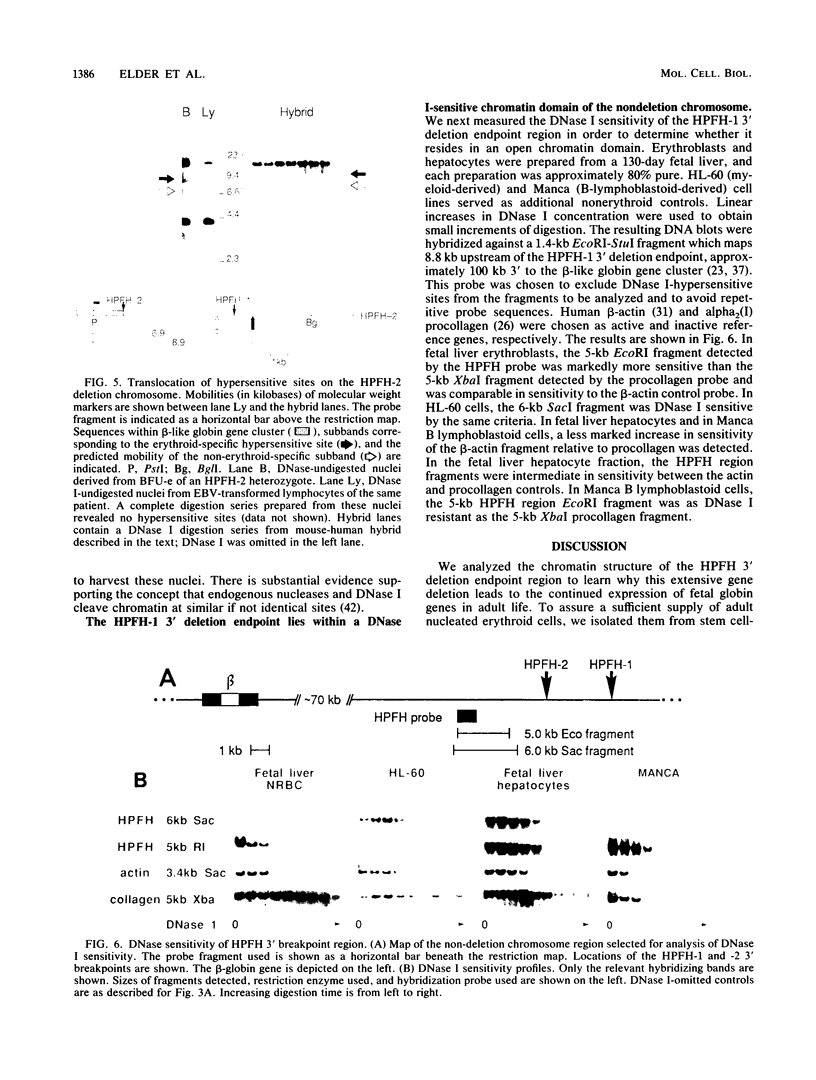
Hereditary persistence of fetal hemoglobin (HPFH) can involve large deletions which eliminate the 3' end of the beta-like globin gene cluster and more than 70 kilobases (kb) of flanking DNA. Blot hybridization revealed a DNase I-hypersensitive site extending from 1.1 to 1.4 kb downstream of the HPFH-1 3' deletion endpoint. The site was found in normal fetal and adult nucleated erythroid cells and in two erythroleukemia cell lines but not in nonerythroid cells and tissues. Simian virus 40 core enhancer-like sequences were found nonrandomly distributed within the boundaries of the site, which is contained in a fragment of known enhancer activity (E. A. Feingold and B. G. Forget, Blood, in press). A second hypersensitive site was found 0.5 kb upstream of the HPFH-1 3' deletion endpoint but was not erythroid specific. A third site, most prominent in fetal liver-derived erythroid cells, was found 1 kb upstream of the HPFH-2 deletion endpoint. As predicted by the locations of the deletion endpoints, the first two sites were translocated to within 12 kb of the A gamma gene in erythroid colonies derived from an HPFH-2 heterozygote and in hybrid mouse-human erythroid cells carrying the HPFH-2 deletion chromosome. Further analysis of this region showed that it was DNase I sensitive in erythroid and myeloid cells, indicating that it resides in an open chromatin domain. These observations suggest that alterations of chromatin structure flanking the fetal globin genes may contribute to abnormal gene regulation in deletion-type HPFH.
Full text
PDF

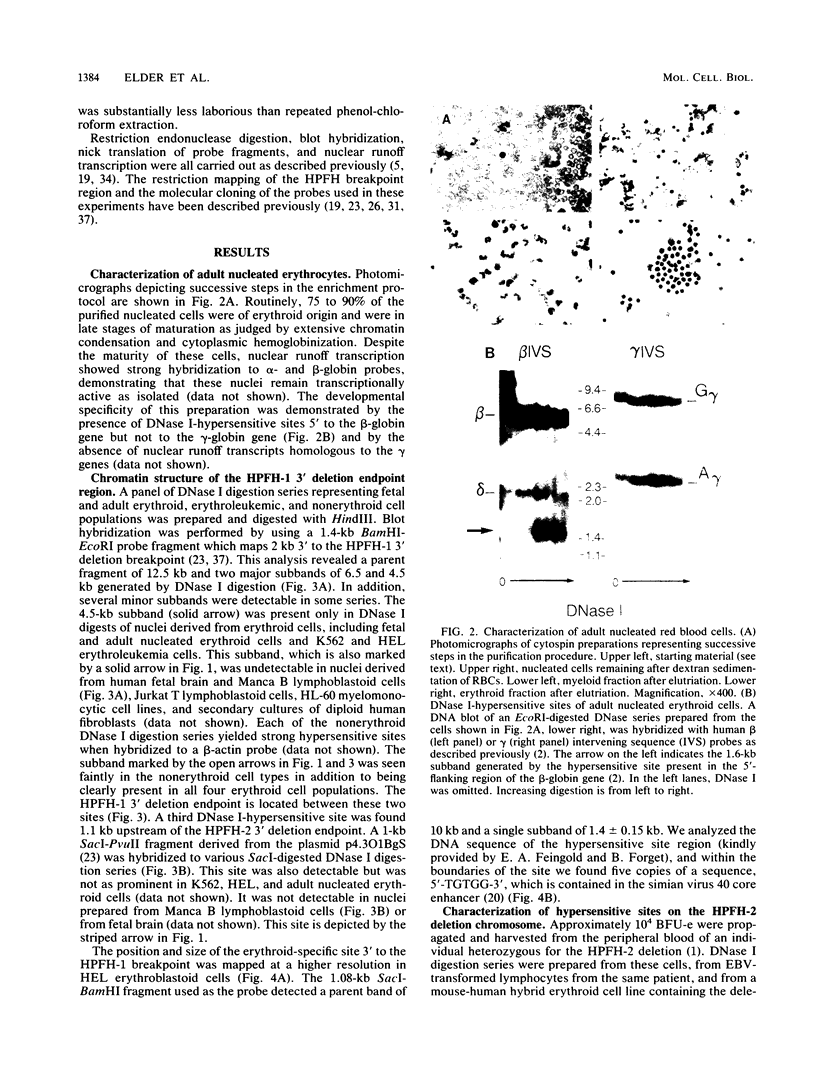



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnou N. P., Papayannopoulou T., Stamatoyannopoulos G., Nienhuis A. W. Structurally diverse molecular deletions in the beta-globin gene cluster exhibit an identical phenotype on interaction with the beta S-gene. Blood. 1985 May;65(5):1245–1251. [PubMed] [Google Scholar]
- Arapinis C., Elion J., Labie D., Krishnamoorthy R. Differences in DNase I sensitivity and methylation within the human beta-globin gene domain and correlation with expression. Eur J Biochem. 1986 Apr 1;156(1):123–129. doi: 10.1111/j.1432-1033.1986.tb09556.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Brunvand M. W., Schmidt A., Siebenlist U. Nuclear factors interacting with the mitogen-responsive regulatory region of the interleukin-2 gene. J Biol Chem. 1988 Dec 15;263(35):18904–18910. [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Camaschella C., Serra A., Saglio G., Baiget M., Malgaretti N., Mantovani R., Ottolenghi S. The 3' ends of the deletions of Spanish delta beta zero-thalassemia and black HPFH 1 and 2 lie within 17 kilobases. Blood. 1987 Aug;70(2):593–596. [PubMed] [Google Scholar]
- Collins F. S., Cole J. L., Lockwood W. K., Iannuzzi M. C. The deletion in both common types of hereditary persistence of fetal hemoglobin is approximately 105 kilobases. Blood. 1987 Dec;70(6):1797–1803. [PubMed] [Google Scholar]
- Collins F. S., Stoeckert C. J., Jr, Serjeant G. R., Forget B. G., Weissman S. M. G gamma beta+ hereditary persistence of fetal hemoglobin: cosmid cloning and identification of a specific mutation 5' to the G gamma gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4894–4898. doi: 10.1073/pnas.81.15.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Curtin P., Pirastu M., Kan Y. W., Gobert-Jones J. A., Stephens A. D., Lehmann H. A distant gene deletion affects beta-globin gene function in an atypical gamma delta beta-thalassemia. J Clin Invest. 1985 Oct;76(4):1554–1558. doi: 10.1172/JCI112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Felsenfeld G. Specific factor conferring nuclease hypersensitivity at the 5' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):95–99. doi: 10.1073/pnas.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas R., Endlich B., Pfeiffer C., Yagi M., Stamatoyannopoulos G. G to A substitution in the distal CCAAT box of the A gamma-globin gene in Greek hereditary persistence of fetal haemoglobin. Nature. 1985 Jan 24;313(6000):323–325. doi: 10.1038/313323a0. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Groudine M., Kohwi-Shigematsu T., Gelinas R., Stamatoyannopoulos G., Papayannopoulou T. Human fetal to adult hemoglobin switching: changes in chromatin structure of the beta-globin gene locus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7551–7555. doi: 10.1073/pnas.80.24.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Vanin E., deLange T., Flavell R. A., Grosveld F. G. Beta-globin gene inactivation by DNA translocation in gamma beta-thalassaemia. Nature. 1983 Dec 15;306(5944):662–666. doi: 10.1038/306662a0. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S., Smithies O. A Chinese G gamma + (A gamma delta beta)zero thalassemia deletion: comparison to other deletions in the human beta-globin gene cluster and sequence analysis of the breakpoints. Nucleic Acids Res. 1985 Sep 25;13(18):6559–6575. doi: 10.1093/nar/13.18.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood. 1983 Jul;62(1):1–13. [PubMed] [Google Scholar]
- Myers J. C., Chu M. L., Faro S. H., Clark W. J., Prockop D. J., Ramirez F. Cloning a cDNA for the pro-alpha 2 chain of human type I collagen. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3516–3520. doi: 10.1073/pnas.78.6.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikori M., Hansen H., Jhanwar S., Fried J., Sordillo P., Koziner B., Lloyd K., Clarkson B. Establishment of a near-tetraploid B-cell lymphoma line with duplication of the 8;14 translocation. Cancer Genet Cytogenet. 1984 May;12(1):39–50. doi: 10.1016/0165-4608(84)90006-2. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Taramelli R., Comi P., Mazza U., Saglio G., Camaschella C., Izzo P., Cao A., Galanello R. Molecular comparison of delta beta-thalassemia and hereditary persistence of fetal hemoglobin DNAs: evidence of a regulatory area? Proc Natl Acad Sci U S A. 1982 Apr;79(7):2347–2351. doi: 10.1073/pnas.79.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Enver T., Takegawa S., Anagnou N. P., Stamatoyannopoulos G. Activation of developmentally mutated human globin genes by cell fusion. Science. 1988 Nov 18;242(4881):1056–1058. doi: 10.1126/science.2461587. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Geha R., Rappeport J. M., Wilson M., Penta A. C., Hussey R. E., Fitzgerald K. A., Daley J. F., Levine H., Rosen F. S. Reconstitution after transplantation with T-lymphocyte-depleted HLA haplotype-mismatched bone marrow for severe combined immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6047–6051. doi: 10.1073/pnas.79.19.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Sukumaran P. K. A second type of hereditary persistence of foetal haemoglobin in India. Br J Haematol. 1973 Jul;25(1):131–135. doi: 10.1111/j.1365-2141.1973.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Taramelli R., Kioussis D., Vanin E., Bartram K., Groffen J., Hurst J., Grosveld F. G. Gamma delta beta-thalassaemias 1 and 2 are the result of a 100 kbp deletion in the human beta-globin cluster. Nucleic Acids Res. 1986 Sep 11;14(17):7017–7029. doi: 10.1093/nar/14.17.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Murnane M. J., deRiel J. L., Forget B. G. Heterogeneity in the molecular basis of hereditary persistence of fetal haemoglobin. Nature. 1980 May 29;285(5763):335–337. doi: 10.1038/285335a0. [DOI] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]
- Wigmore D. J., Eaton R. W., Scott W. A. Endonuclease-sensitive regions in SV40 chromatin from cells infected with duplicated mutants. Virology. 1980 Jul 30;104(2):462–473. doi: 10.1016/0042-6822(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Clegg J. B., Weatherall D. J. Hereditary persistence of fetal haemoglobin (HPFH) and delta beta thalassaemia. Br J Haematol. 1979 Dec;43(4):509–520. doi: 10.1111/j.1365-2141.1979.tb03784.x. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Cereghini S. Structure of transcriptionally active chromatin. CRC Crit Rev Biochem. 1986;21(1):1–26. doi: 10.3109/10409238609113607. [DOI] [PubMed] [Google Scholar]