Abstract
Background
The parasite Toxoplasma gondii influences the behaviour of infected animals and probably also personality of infected humans. Subjects with a Rhesus-positive blood group are protected against certain behavioural effects associated with Toxoplasma infection, including the deterioration of reaction times and personality factor shift.
Methodology/Principal Findings
Here, we searched for differences in the toxoplasmosis-associated effects between RhD-positive and RhD-negative subjects by testing 502 soldiers with two personality tests and two intelligence tests. The infected subjects expressed lower levels of all potentially pathognomic factors measured with the N-70 questionnaire and in neurasthenia measured with NEO-PI-R. The RhD-positive, Toxoplasma-infected subjects expressed lower while RhD-negative, Toxoplasma-infected subjects expressed higher intelligence than their Toxoplasma-free peers. The observed Toxoplasma-associated differences were always larger in RhD-negative than in RhD-positive subjects.
Conclusions
RhD phenotype plays an important role in the strength and direction of association between latent toxoplasmosis and not only psychomotor performance, but also personality and intelligence.
Introduction
The trophically transmitted parasites often modify the behavior of their intermediate host to increase its susceptibility to predation [1], [2]. By this they increase the probability of their transmission from intermediate to definitive host. A popular model for studying such manipulation activity of parasites in a mammal host is Toxoplasma gondii, for review see [3], [4]. In its life cycle, Toxoplasma needs to be transmitted from the intermediate host, e.g. an infected rodent, to the definitive host, i.e. any representative of the Felinidae family, including the domestic cat. It is known that infected rodents are hyperactive in the open field [5], [6], exhibit increased voluntary wheel running [7], [8] and longer exploration times in the hole board test [9], are deficient in motor performance and coordination [8], [10], and have longer reaction times [11], impaired working memory [12], and impaired ability to recognise novel stimuli [8], [13]. The most specific and also the most spectacular toxoplasmosis-associated change reported in rodents is the so-called Fatal attraction phenomenon, i.e. the conversion of the rats' and mice's innate fear of cat odour into attraction to cat odour (but not to the odour of other predators). This phenomenon was observed in several laboratories [12], [14]–[16] and was dependent on activation of the brain regions that respond to sexual stimuli in normal mice by the odour of a particular predator, the cat in infected rodents [16]. Current results suggest that changed concentrations of testosterone [17] and dopamine probably play an important role in the differences in the personality and behavior between Toxoplasma-infected and Toxoplasma-free subjects. It was found that the Toxoplasma gondii genome contains two genes for enzymes (tyrosine hydroxylases) implicated in the synthesis of dopamine [18] and increased concentration of this neurotransmitter was observed in the infected rodent brain areas [19].
Any warm-blooded animal, including humans, can be infected with Toxoplasma and the prevalence of this infection in different countries varies between 5 and 80% depending on climate, hygienic standards and kitchen habits [20]. After a short phase of acute toxoplasmosis, the infection proceeds to its latent stage when tissue cysts with bradyzoites are formed and these survive for the rest of the host's life mainly in neural and muscular tissues. In immunocompetent subjects, the latent phase of infection was considered asymptomatic and harmless from the clinical point of view, however, results of many recent studies suggested that this form of the infection could have many serious clinical implications [21]–[24]. However, practically all studies performed in the past 20 years have demonstrated behavioural changes including the Fatal attraction phenomenon [25], observed earlier in laboratory animals, also in humans, for recent reviews, see [3], [26].
It is well known that the gene pool of the local human population is strongly influenced by the selection pressure of parasites. Recent studies have shown that the association between latent toxoplasmosis and human reaction times, personality and physiology depend on RhD phenotype of the infected subject [27]–[31]. It has even been suggested that the spreading of the deletion responsible for RhD negativity in the Caucasian population can be caused by increased psychomotor performance of RhD-negative, Toxoplasma-free subjects in Europe where the cats and therefore also toxoplasmosis were rare before the advent of the domestic cat [27]. The association between toxoplasmosis and the personality of RhD-negative and RhD-positive subjects was studied using Cattell's 16PF and Cloninger's TCI questionnaires [29]. In the present study, we searched for the difference between RhD-positive and RhD-negative subjects using the NEO-PI-R questionnaire that is based on the modern Big Five model of personality. Moreover, we searched for similar RhD phenotype- and toxoplasmosis-associated differences in verbal and nonverbal intelligence and also in pathognomic traits measured with the N-70 questionnaire.
Materials and Methods
Ethics Statement
All participants provided their written informed consent. The recruitment of study subjects and data handling were performed in compliance with the Czech legislation in force and were approved by the Institutional Review Board of the Faculty of Science, Charles University.
Sample and Participant Selection
All psychological testing was performed at the Military University Hospital Prague. The study population consisted of 502 male soldiers of Czech nationality (age: 18–52, mean 27.25, S.D. 6.71, median 25.94) who attended the Military University Hospital Prague to take entrance psychological examinations for military missions in 2005 and consented to participate in the research project. The subjects were examined with standardized panel of psychological and performance tests, essayed for RhD and ABO phenotype during the health examination and also provided 5 ml of blood for a serology test. In the informed consent form, the general aim of the project (a study of the influence of environmental factors on human psychology and performance) and the need for obtaining their consent to using the results of their psychological and clinical examinations were explained. The consent rate was about 65%.
N-70 questionnaire
The N-70 is a questionnaire constructed for the assessment of seven areas of clusters - anxiety, depression, phobia, hysteria, hypochondria, psychosomatic symptoms and psychastenia [32]. The purpose of this method is to detect individuals who may be too sensitive for military operations [33]. Subjects are asked to answer 70 questions using a 3-point agreement scale. Scores in each cluster range from 0–30. The total N-70 score is the number of non-negative answers for all 70 questions.
NEO-PI-R questionnaire
The electronic version of NEO-PI-R (Costa & Mccrae, 1992) translated to Czech and validated by Hřebíčková (2001) [34] was used.
Wiener Matrizen-Test of intelligence
The Wiener Matrizen-Test (WMT) [35], a nonverbal intelligence test, is an adapted version of the Raven progressive matrices which conforms to the Rasch model [36]. The WMT assesses general intelligence by measuring reasoning ability. The test requires the completion of 24 matrices with increasing task difficulty and was administered without an explicit time limit. The intention and conceptualization of the WMT are largely based on Raven's Matrices [37]–[39]. The correlation between the WMT and Standard Progressive Matrices is about r = 0.92 [35]. Construction and item selection, however, follow the standards of Rasch scaling. For these reasons, and due to the fact that the WMT showed comparable validity characteristics but had a considerably higher administration economy, we prefer the WMT to the Raven matrices in clinical practice. The split-half reliability of the WMT is 0.83 [35]. The 1993 Czech adopted version [40], distributed by Psychodiagnostika (Brno), was used in the present study. Both the raw score and the IQ adjusted for age of the participant were compared in statistical tests.
OTIS test of intelligence
The OTIS test is a test of verbal intelligence which was derived from the original test [41]. Seven types of items were taken from the original test:
term or object definition by choosing the most suitable characteristics
term or object definition by choosing the most suitable description
the choice of an object based on common attributes
the choice of the opposite
the identifying of “foreign” (unrelated) terms
logical or ethical solution of the situations
the interpretation of the adage
The test contains 32 items (0–32). The maximum score is therefore 32 points. Both the raw score and the IQ (adjusted for the educational level, see [32]) were compared in statistical tests.
Immunological tests for toxoplasmosis
All serological tests were carried out in the National Reference Diagnostic Laboratory for Toxoplasmosis, National Institute of Public Health, Prague. Specific IgG and IgM antibody titres were determined by ELISA (IgG: SEVAC, Prague, IgM: TestLine, Brno), optimized for early detection of acute toxoplasmosis (Pokorný et al., 1989) and by complement fixation tests (CFT) (SEVAC, Prague) which are more sensitive and therefore more suitable for the detection of old T. gondii infection (Warren & Sabin, 1942). The titre of anti-Toxoplasma antibodies in sera was measured in dilutions between 1∶8 and 1∶1024. The subjects with negative results of IgM ELISA (positivity index<0.9) and both CFT titres higher than 1∶8 and IgG ELISA >250 optical units, i.e. approximately 10 IU/ml, were considered latent toxoplasmosis positive. The individuals with ambiguous diagnosis, e.g. different result of CFT and ELISA, were excluded from the study.
Statistical analysis
The Statistica 8.0 was used for descriptive statistics, General Linear Model tests and computing t aus by standard Kendall correlation tests. Partial Kendall correlation test suggested by Siegel and Castellan [42] based on taus computed with standard Kendall correlations was used for nonparametric analyses [17]; the Excel sheet for this analysis is available at http://web.natur.cuni.cz/flegr/programy.php.
Results
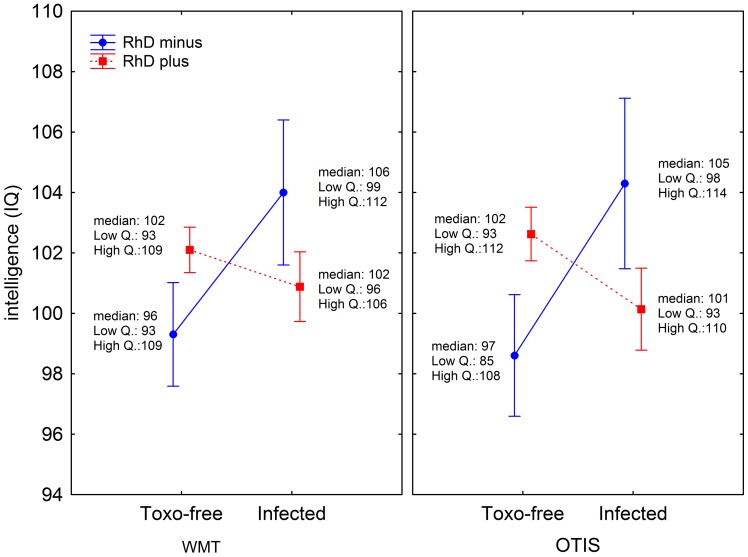
We obtained scores for the N-70, NEO-PI-R, WMT and Otis tests from 502 subjects tested for RhD and latent toxoplasmosis. One hundred and fifty-four (154, i.e. 31.4%) of 491 subjects with unambiguous results of the test for toxoplasmosis were Toxoplasma infected and 87 (17.3%) of 502 subjects were RhD negative. No association between toxoplasmosis and RhD phenotype was observed (Chi2 = 0.14, p = 0.707). Descriptive statistics for the population under study are shown in Tables 1 and 2. For the analysis of correlation of toxoplasmosis and RhD phenotype with the personality profile of soldiers (ordinal variables), we used a robust nonparametric test. To control for the effect of age, partial Kendal correlation tests were performed with age as a covariate and to control for the effect of RhD phenotype, RhD-positive and RhD-negative subjects were tested separately. Table 1 shows that Toxoplasma-infected subjects scored lower in the total N-70 score and also in anxiety, depression, phobia, hysteria, and vegetative lability and in the BigFive trait neuroticism. The differences were much stronger in RhD-negative than RhD-positive subjects. No relation between latent toxoplasmosis and nonverbal (WMT) or verbal (Otis) intelligence was observed in RhD nonsorted population. However, separate analyses performed for RhD-positive and RhD-negative populations showed negative association between intelligence and toxoplasmosis in RhD-positive subjects and positive association between intelligence and toxoplasmosis in RhD-negative subjects, see Fig. 1. Again, the correlation of intelligence with toxoplasmosis (estimated with partial tau) was much stronger for RhD-negative subjects.
Table 1. Descriptive statistics and results of testing differences in personality traits and intelligence between Toxoplasma-infected and Toxoplasma-free RhD-negative and RhD- positive male soldiers.
| All | Rh+ | Rh− | ||||||||||||||||
| N | mean | N | mean | N | mean | |||||||||||||
| Toxo− | Toxo+ | Toxo− | Toxo+ | Tau | p | Toxo− | Toxo+ | Toxo− | Toxo+ | Tau | p | Toxo− | Toxo+ | Toxo− | Toxo+ | Tau | p | |
| Age | 337 | 154 | 26.70 | 27.83 | 280 | 125 | 26.77 | 28.26 | 57 | 28 | 26.35 | 26.26 | ||||||
| Total N-70 | 335 | 152 | 18.97 | 16.57 | −0.09 | 0.002 | 278 | 123 | 18.97 | 17.21 | −0.07 | 0.032 | 57 | 28 | 18.96 | 13.50 | −0.23 | 0.002 |
| Anxiety | 335 | 152 | 4.31 | 3.68 | −0.10 | 0.001 | 278 | 123 | 4.28 | 3.74 | −0.09 | 0.009 | 57 | 28 | 4.47 | 3.39 | −0.19 | 0.009 |
| Depression | 335 | 152 | 2.18 | 1.80 | −0.07 | 0.018 | 278 | 123 | 2.16 | 1.86 | −0.05 | 0.102 | 57 | 28 | 2.26 | 1.50 | −0.18 | 0.016 |
| Phobia | 335 | 152 | 2.87 | 2.50 | −0.07 | 0.024 | 278 | 123 | 2.86 | 2.57 | −0.06 | 0.096 | 57 | 28 | 2.95 | 2.14 | −0.13 | 0.069 |
| Hysteria | 335 | 152 | 2.87 | 2.34 | −0.11 | 0.000 | 278 | 123 | 2.91 | 2.42 | −0.10 | 0.002 | 57 | 28 | 2.68 | 2.00 | −0.12 | 0.095 |
| Hypochondria | 335 | 152 | 2.51 | 2.24 | −0.06 | 0.062 | 278 | 123 | 2.54 | 2.36 | −0.03 | 0.295 | 57 | 28 | 2.40 | 1.71 | −0.16 | 0.031 |
| Vegetative lability | 335 | 152 | 3.15 | 2.71 | −0.08 | 0.013 | 278 | 123 | 3.18 | 2.82 | −0.06 | 0.089 | 57 | 28 | 3.00 | 2.18 | −0.16 | 0.033 |
| Psychasteny | 335 | 152 | 2.13 | 1.91 | −0.05 | 0.074 | 278 | 123 | 2.14 | 2.10 | −0.02 | 0.566 | 57 | 28 | 2.11 | 1.00 | −0.24 | 0.001 |
| Neuroticism | 314 | 143 | 69.06 | 65.15 | −0.07 | 0.025 | 259 | 117 | 68.86 | 66.49 | −0.04 | 0.253 | 55 | 25 | 69.98 | 59.32 | −0.22 | 0.005 |
| Extroversion | 314 | 143 | 116.84 | 116.70 | 0.00 | 0.887 | 259 | 117 | 116.79 | 116.83 | 0.01 | 0.678 | 55 | 25 | 117.11 | 116.80 | −0.00 | 0.957 |
| Openness | 314 | 143 | 101.81 | 102.40 | −0.00 | 0.943 | 259 | 117 | 102.89 | 101.41 | −0.03 | 0.393 | 55 | 25 | 100.47 | 106.88 | 0.130 | 0.089 |
| Agreeableness | 318 | 144 | 122.42 | 123.45 | 0.03 | 0.265 | 259 | 117 | 121.80 | 123.28 | 0.05 | 0.174 | 55 | 25 | 125.35 | 123.68 | −0.03 | 0.689 |
| Conscientiousness | 316 | 144 | 128.81 | 130.07 | 0.03 | 0.265 | 259 | 117 | 128.73 | 130.12 | 0.04 | 0.272 | 55 | 25 | 129.18 | 130.00 | 0.03 | 0.745 |
| Row WMT | 314 | 143 | 23.19 | 23.04 | −0.01 | 0.695 | 278 | 123 | 23.38 | 22.72 | −0.07 | 0.044 | 57 | 28 | 22.28 | 24.46 | 0.24 | 0.001 |
| IQ WMT | 312 | 142 | 101.49 | 101.09 | −0.01 | 0.712 | 278 | 123 | 101.95 | 100.37 | −0.06 | 0.083 | 57 | 28 | 99.25 | 104.54 | 0.21 | 0.004 |
| Row Otis | 311 | 141 | 14.53 | 14.12 | −0.03 | 0.368 | 273 | 117 | 14.75 | 13.86 | −0.07 | 0.027 | 53 | 27 | 13.38 | 15.41 | 0.21 | 0.007 |
| IQ Otis | 309 | 140 | 101.97 | 100.78 | −0.02 | 0.595 | 273 | 117 | 102.62 | 100.14 | −0.06 | 0.099 | 53 | 27 | 98.60 | 104.30 | 0.18 | 0.017 |
Tau shows effect size and sign, p shows statistical significance measured with partial Kendall tests. Significant results (p<0.05, two-sided test) are printed in bold. Toxoplasma-free and Toxoplasma-infected subjects are coded with 0 and 1, respectively. Therefore, negative Tau means lower test score in Toxoplasma infected subjects. Formal correction for multiple (51) tests was not performed. Theoretically, 2–3 of 51 tests presented in this table should provide false positive results.
Table 2. Descriptive statistics and results of testing differences in personality traits and intelligence between RhD-negative and RhD-positive Toxoplasma-infected and Toxoplasma-free male soldiers.
| All | Toxo− | Toxo+ | ||||||||||||||||
| N | mean | N | mean | N | mean | |||||||||||||
| RhD− | RhD+ | RhD− | RhD+ | Tau | p | RhD− | RhD+ | RhD− | RhD+ | Tau | p | RhD− | RhD+ | RhD− | RhD+ | Tau | p | |
| Age | 87 | 415 | 26.36 | 27.26 | 57 | 280 | 26.35 | 26.77 | 28 | 125 | 26.26 | 28.26 | ||||||
| Total N-70 | 87 | 411 | 16.97 | 18.36 | 0.05 | 0.108 | 57 | 278 | 18.96 | 18.97 | 0.00 | 0.980 | 28 | 123 | 13.50 | 17.21 | 0.12 | 0.027 |
| Anxiety | 87 | 411 | 4.08 | 4.11 | 0.00 | 0.890 | 57 | 278 | 4.47 | 4.28 | −0.03 | 0.440 | 28 | 123 | 3.39 | 3.74 | 0.06 | 0.303 |
| Depression | 87 | 411 | 1.97 | 2.04 | 0.00 | 0.869 | 57 | 278 | 2.26 | 2.16 | −0.04 | 0.291 | 28 | 123 | 1.50 | 1.86 | 0.05 | 0.401 |
| Phobia | 87 | 411 | 2.66 | 2.75 | 0.03 | 0.377 | 57 | 278 | 2.95 | 2.86 | 0.00 | 0.979 | 28 | 123 | 2.14 | 2.57 | 0.07 | 0.189 |
| Hysteria | 87 | 411 | 2.44 | 2.74 | 0.06 | 0.057 | 57 | 278 | 2.68 | 2.91 | 0.05 | 0.194 | 28 | 123 | 2.00 | 2.42 | 0.07 | 0.204 |
| Hypochondria | 87 | 411 | 2.18 | 2.46 | 0.05 | 0.108 | 57 | 278 | 2.40 | 2.54 | 0.02 | 0.565 | 28 | 123 | 1.71 | 2.36 | 0.12 | 0.029 |
| Vegetative lability | 87 | 411 | 2.68 | 3.08 | 0.06 | 0.050 | 57 | 278 | 3.00 | 3.18 | 0.02 | 0.666 | 28 | 123 | 2.18 | 2.82 | 0.13 | 0.021 |
| Psychasteny | 87 | 411 | 1.70 | 2.13 | 0.09 | 0.003 | 57 | 278 | 2.11 | 2.14 | 0.02 | 0.655 | 28 | 123 | 1.00 | 2.10 | 0.21 | <0.001 |
| Neuroticism | 82 | 386 | 57.90 | 59.16 | 0.03 | 0.314 | 55 | 259 | 60.75 | 59.72 | −0.02 | 0.639 | 25 | 117 | 51.92 | 58.01 | 0.13 | 0.018 |
| Extroversion | 82 | 386 | 95.21 | 95.52 | 0.01 | 0.827 | 55 | 259 | 95.20 | 95.52 | −0.01 | 0.843 | 25 | 117 | 94.84 | 95.44 | 0.05 | 0.344 |
| Openness | 82 | 386 | 82.70 | 82.22 | −0.01 | 0.814 | 55 | 259 | 81.24 | 82.51 | 0.02 | 0.600 | 25 | 117 | 86.28 | 81.80 | −0.06 | 0.270 |
| Agreeableness | 82 | 386 | 104.63 | 102.00 | −0.06 | 0.045 | 55 | 259 | 105.45 | 101.64 | −0.08 | 0.027 | 25 | 117 | 103.08 | 103.03 | −0.01 | 0.838 |
| Conscientiousness | 82 | 386 | 109.18 | 108.84 | −0.01 | 0.763 | 55 | 259 | 108.75 | 108.31 | −0.01 | 0.795 | 25 | 117 | 109.72 | 109.65 | −0.01 | 0.905 |
| Row WMT | 87 | 411 | 22.94 | 23.20 | 0.03 | 0.362 | 57 | 278 | 22.28 | 23.38 | 0.09 | 0.010 | 28 | 123 | 24.46 | 22.72 | −0.15 | 0.005 |
| IQ WMT | 87 | 411 | 100.79 | 101.55 | 0.03 | 0.347 | 57 | 278 | 99.25 | 101.95 | 0.08 | 0.027 | 28 | 123 | 104.54 | 100.37 | −0.13 | 0.016 |
| Row Otis | 82 | 399 | 13.93 | 14.45 | 0.05 | 0.094 | 53 | 273 | 13.38 | 14.75 | 0.12 | 0.002 | 27 | 117 | 15.41 | 13.86 | −0.12 | 0.030 |
| IQ Otis | 82 | 399 | 99.99 | 101.76 | 0.05 | 0.137 | 53 | 273 | 98.60 | 102.62 | 0.10 | 0.007 | 27 | 117 | 104.30 | 100.14 | −0.11 | 0.047 |
Tau shows effect size and sign, p shows statistical significance measured with partial Kendall. RhD-negative and RhD-positive subjects are coded with 0 and 1, respectively. Therefore, negative Tau means lower test score in RhD-positive subjects. Formal correction for multiple (51) tests was not performed. Theoretically, 2–3 of 51 tests presented in this table should provide false positive results.
Figure 1. Differences in nonverbal (WMT) and verbal (OTIS) intelligence between Toxoplasma-infected and Toxoplasma-free RhD-positive and RhD-negative subjects.
The graph shows arithmetic means, standard errors (whiskers), medians, and 25% and 75% quartiles. The presented values differ from raw data listed in Tables 1 and 2 because the intelligence has been controlled for the age of men, that is, the intelligence has been computed for covariate (age) as its mean.
The same analyses (partial Kendall correlations with age as a covariate) was performed for the independent binary variable RhD phenotype, in the whole population and separately in the Toxoplasma-infected and Toxoplasma-free subjects, see Table 2. Significant association of RhD phenotype with the total N-70 score, hypochondria, vegetative lability, psychasteny, and the NEO-PI-R neuroticism were observed only in Toxoplasma-infected subjects. However, the association of RhD phenotype with nonverbal and verbal intelligence was detected also in Toxoplasma-free subjects, suggesting that not only the protective effect of RhD positivity against consequences of toxoplasmosis but also the main effect of RhD phenotype (or its protective effect against some unknown third factor) probably played a role in the observed associations between RhD phenotype and various personality traits.
The partial Kendall correlation test can control for one confounding variable only. To study the effect of interactions and several potential confounding variables we performed General Linear Model analyses with independent variables age, toxoplasmosis, RhD phenotype, ABO phenotype and RhD phenotype-toxoplasmosis and ABO phenotype-toxoplasmosis interactions. The analyses showed significant effect of RhD phenotype-toxoplasmosis interaction on psychasteny and IQ and no significant effects of ABO phenotype or ABO phenotype interaction (Tab. 3).
Table 3. Results of testing the effects of age, toxoplasmosis, RhD phenotype, ABO phenotype, and RhD-toxoplasmosis and ABO-toxoplasmosis interaction on personality traits and intelligence.
| age | ABO | RhD | Toxo | ABO-Toxo | RhD-Toxo | |
| Total N-70 | 0.819 | 0.677 | 0.076 | 0.034 | 0.807 | 0.149 |
| Anxiety | 0.999 | 0.637 | 0.541 | 0.032 | 0.578 | 0.395 |
| Depression | 0.467 | 0.476 | 0.513 | 0.119 | 0.798 | 0.392 |
| Phobia | 0.410 | 0.674 | 0.353 | 0.121 | 0.907 | 0.289 |
| Hysteria | 0.674 | 0.992 | 0.237 | 0.089 | 0.677 | 0.560 |
| Hypochondria | 0.522 | 0.168 | 0.016 | 0.174 | 0.378 | 0.280 |
| Vegetative lability | 0.647 | 0.765 | 0.188 | 0.168 | 0.685 | 0.470 |
| Psychasteny | 0.287 | 0.552 | 0.936 | 0.148 | 0.163 | 0.019 |
| Neuroticism | 0.443 | 0.846 | 0.200 | 0.052 | 0.791 | 0.164 |
| Extroversion | 0.825 | 0.793 | 0.964 | 0.671 | 0.336 | 0.999 |
| Openness | 0.618 | 0.483 | 0.471 | 0.549 | 0.562 | 0.144 |
| Agreeableness | 0.447 | 0.844 | 0.357 | 0.493 | 0.848 | 0.264 |
| Conscientiousness | 0.863 | 0.351 | 0.903 | 0.746 | 0.562 | 0.760 |
| Row WMT | 0.308 | 0.243 | 0.651 | 0.203 | 0.794 | 0.010 |
| IQ WMT | 0.422 | 0.237 | 0.806 | 0.317 | 0.943 | 0.037 |
| Row Otis | 0.167 | 0.611 | 0.783 | 0.081 | 0.132 | 0.003 |
| IQ Otis | 0.287 | 0.551 | 0.936 | 0.148 | 0.163 | 0.019 |
The table shows p-values of particular GLM tests. Significant results (p<0.05, two-sided test) are printed in bold. Formal correction for multiple tests was not performed. Theoretically, about one false positive result should be present in each column.
Discussion
Soldiers with and without latent Toxoplasma infection differ in several personality traits. Generally, the infected subjects expressed lower levels of potentially pathognomic factors measured with the N-70 questionnaire and of neuroticism tested with the NEO-PI-R (Big Five model). The RhD-positive, Toxoplasma-infected subjects express lower while RhD-negative, Toxoplasma-infected subjects express higher verbal and nonverbal intelligence than their Toxoplasma-free peers. The observed Toxoplasma-associated differences in personality traits, including intelligence were always larger in RhD-negative than in RhD-positive subjects.
The GLM analysis showed that the effect of RhD-toxoplasmosis interaction on intelligence is highly significant. This analysis also showed a significant effect RhD-toxoplasmosis interaction on psychasteny. It must be reminded, however, that this effect is non-significant after the correction for multiple statistical tests. The GLM also showed absence of main effects of RhD phenotype and toxoplasmosis (after correction for multiple tests), which contrasted with results of partial Kendall correlation tests. The lower power of parametric tests for ordinal data with asymmetric distribution as well as the presence of several other independent variables and their interactions in more complex GLM models could be responsible for this difference between results of parametric and nonparametric tests. GLM analysis models also showed absence of effect of ABO phenotype and its interaction on personality and intelligence. Absence of any effect of ABO phenotype contrasted with existence of numerous effects of RhD phenotype – see the Table 3, confirming the special role of RhD proteins in human physiology.
Association between Toxoplasma infection and human personality factors were studied thoroughly in the past 20 years. About 10 published studies have demonstrated associations of toxoplasmosis with human personality traits mostly using Cattell's 16PF and Cloninger's TCI questionnaires; for review, see [26], [43], [44]. Only one study, showing positive association of toxoplasmosis with extroversion and its negative association with conscientiousness, used the NEO-PI-R questionnaire [45]. A correlation study has also shown that the difference in the prevalence of latent toxoplasmosis between the general populations of particular countries can explain a significant portion of the variance in aggregate neuroticism among populations [46].
Surprisingly, the results obtained in the present study performed on military personnel differed from those observed earlier on university students. For example, Toxoplasma-infected and Toxoplasma-free soldiers expressed no differences in extroversion or conscientiousness and Toxoplasma-infected and Toxoplasma-free students expressed no difference in neuroticism. Moreover, the results of the correlation study comparing the prevalence of latent toxoplasmosis with aggregate neuroticism in the general populations of particular countries suggest that Toxoplasma-infected subjects have higher rather than lower neuroticism [46]. It was also suspicious that infected soldiers expressed lower and not higher levels of psychopathognomic traits measured with the N-70 questionnaire. Our present hypothesis is that Toxoplasma-infected soldiers express stronger tendency to mask any negative property when responding to questions in questionnaires. Several studies have shown a lower superego strength (Cattell's factor G) and higher suspiciousness (Cattell's factor L) in Toxoplasma-infected men. The testing of soldiers in the current study was a part of their entrance examination for a voluntary (and well-paid) participation in an international military mission and (in contrast with university students or blood donors tested in the previous anonymous studies) the subjects were objectively motivated to mask their negative (e.g. the pathognomic) and to accentuate their positive properties. It is urgently needed to confirm our results in an anonymous research study where the motivation for intentional distortion of data is lower.
Existence of the interaction between toxoplasmosis, RhD phenotype and human behaviour has been confirmed in four studies. Two of them have shown resistance of RhD-positive subjects, especially the RhD-positive heterozygotes, to impairment of reaction times after Toxoplasma infection [27], [28] and one prospective study performed on 3900 military drivers has found an increased risk of traffic accidents in Toxoplasma-infected, RhD-negative subjects [30]. The fourth study has reported opposite relation of toxoplasmosis with Cattell's ego strength, praxernia, and ergic tension and Cloninger's cooperativeness in RhD-positive and RhD-negative blood donors [29]. The latter study also indicates that RhD phenotype might play an important role not only in the toxoplasmosis-associated differences but also in the age-associated differences in specific personality traits.. Another recent study shows that RhD phenotype could also play a role in correlations of age and smoking with psychomotor performance, intelligence and health of draftees [47]. The results of the current study are in an agreement with the already published data. The correlations of toxoplasmosis with personality of soldiers (reflected by the absolute values of Kendall tau shown in Tables 1 and 2) were always much stronger in RhD-negative than RhD-positive subjects, see Table 1. Moreover, the higher verbal and nonverbal intelligence of RhD-positive Toxoplasma-free subjects than Rh-negative Toxoplasma-free soldiers suggests that RhD positivity could protect not only against detrimental effects of latent toxoplasmosis but also against other (still unknown) factors. At the present time, we have no explanation for the opposite relation between RhD phenotype and intelligence in Toxoplasma-infected and Toxoplasma-free subjects. We cannot exclude a possibility that some unknown gene that is in linkage disequilibrium with RHD gene, rather than RHD gene itself, is responsible for the observed phenomena. We cannot even exclude a possibility that the observed phenomena are caused by some unknown confounding variables that co-vary with RhD phenotype and also other observed variables, namely risk of Toxoplasma infection and human personality and intelligence. However, the present data could explain the controversial results concerning the existence (and direction) of the correlation between latent toxoplasmosis on intelligence [48], [49].
The mechanism responsible for physiological and behavioural effects of RhD phenotype is unknown. The RhD molecule is part of a molecular complex (RhAG) on the membrane of red cells [50], [51]. Structural data suggest that the complex is a membrane NH3 or possibly CO2 pump with unknown function [52]–[54]. In RhD-negative subjects, the gene RHD is absent in chromosomes of both maternal and paternal origin due to a large deletion and therefore also the RhD molecule is missing and is probably substituted with another related molecule in the complex [55]. RhD-containing and RhD-free complexes may differ in the specificity, activity and most probable also response to regulation signals. The membrane pump could directly or indirectly influence the partial tension of oxygen and water balance in various tissues, including the brain tissue [56]–[58].
Limitations of the present study
The major limitation of the present study was that the study subjects were objectively motivated to accent positive and to hide negative traits of their personality as their results were to be used as a part of the entrance examination for the participation in a military (peacekeeping) mission. The resulting bias probably cannot influence the result of the intelligence tests; however, it makes it difficult to interpret psychological meanings of the observed relations of toxoplasmosis and RhD phenotype with the personality profile. Many subjects were probably aware about their RhD phenotype; however, nobody was aware either about the hypothesis under study or about their toxoplasmosis status and therefore no systematic bias in the obtained data could be expected.
The second important limitation of the study was the fact that only RhD phenotype and not RhD genotype of the subjects was tested. Results of a previous study suggested that in contrast to RhD-positive heterozygotes, the RhD-positive homozygotes were only transiently protected against some negative effects of toxoplasmosis (namely against prolongation of reaction times) [27]. It is very easy (and cheap) to determine RhD phenotype using the standard agglutination technique. However, a much more sophisticated (and expensive) technique must be used for the determination of RhD genotype. It is also highly probable that a much lower fraction of the soldiers would consent to be involved in a study that would include also DNA analysis. Due to these technical limitations, we compared RhD-negative homozygotes with a mixed population of RhD-positive homozygotes and heterozygotes in all our statistical tests. It is therefore possible that we underestimated the strength of real effects. Only male soldiers were included into the present study. It is critically needed to perform similar study on female subjects in the future because toxoplasmosis usually induces opposite direction shifts in male and female subjects [26], [59].
The third limitation of the present study concerns the fact that the existence of a significant statistical effect does not imply the existence of the real effect of a particular independent variable, e.g. the toxoplasmosis, on a dependent variable, e.g. the intelligence. The observed statistical effect could be caused by an effect of the intelligence on the risk of Toxoplasma infection or even by an effect of some unknown third factor on both intelligence and risk of Toxoplasma infection.
It is highly probable that similar or even stronger associations could exist between infection with other pathogens, e.g. chlamydia, yeasts and herpetic viruses, and behavioural and psychological traits. For example, not only the infection with Toxoplasma but also with human cytomegalovirus is accompanied by decreased Cloninger's personality factor Novelty seeking [60]. Our subjects were not tested for presence of other infectious agents except Toxoplasma and therefore we could not include these potential confounding factors into our models. It must be stressed, however, that the absence of these factors in the models could cause false negative but not false positive results of statistical tests.
Conclusions
The effect of blood groups on personality and intelligence was the subject of many earlier studies. Despite the widespread believe in the existence of such effects in some cultures, e.g. in Japan, rigorous tests usually provided only negative results. It must be reminded, however, that the ABO blood group system rather than the Rhesus factor system was nearly always examined in these studies, see [61]–[64]. Our results imply that in future behavioural studies the attention should be focused not only on the ABO system but also on RhD phenotype and that important confounding variables, especially Toxoplasma infection and smoking [47] should be controlled.
Funding Statement
The authors' work was supported by the Grand Agency of the Czech Republic (Grant No. P303/11/1398) and Charles University of Prague (GAUK 18810, grant UNCE 204004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moore J, Adamo S, Thomas F (2005) Manipulation: expansion of the paradigm. Behav Processes 68: 283–287. [DOI] [PubMed] [Google Scholar]
- 2.Barnard CJ, Behnke JM (1990) Parasitism and Host Behaviour. New York: Taylor and Francis. [Google Scholar]
- 3. Webster JP, McConkey GA (2010) Toxoplasma gondii -altered host behaviour: clues as to mechanism of action. Folia Parasitol 57: 95–104. [DOI] [PubMed] [Google Scholar]
- 4. Webster JP (2007) The effect of Toxoplasma gondii on animal behavior: Playing cat and mouse. Schizophr Bull 33: 752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xiao J, Kannan G, Jones-Brando L, Brannock C, Krasnova IN, et al. (2012) Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience 206: 39–48. [DOI] [PubMed] [Google Scholar]
- 6. Hay J, Aitken PP, Hutchison WM, Graham DI (1983) The effect of congenital and adult-acquired Toxoplasma infections on the motor performance of mice. Ann Trop Med Parasitol 77: 261–277. [DOI] [PubMed] [Google Scholar]
- 7. Hay J, Aitken PP, Arnott MA (1985) The influence of Toxoplasma infection on the spontaneous running activity of mice. Z Parasitenkd 71: 459–462. [DOI] [PubMed] [Google Scholar]
- 8. Hodková H, Kodym P, Flegr J (2007) Poorer results of mice with latent toxoplasmosis in learning tests: impaired learning processes or the novelty discrimination mechanism? Parasitology 134: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 9. Skallová A, Kodym P, Frynta D, Flegr J (2006) The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology 133: 525–535. [DOI] [PubMed] [Google Scholar]
- 10. Hutchison WM, Aitken PP, Wells BW (1980) Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasitol 74: 507–510. [DOI] [PubMed] [Google Scholar]
- 11. Hrdá Š, Votýpka J, Kodym P, Flegr J (2000) Transient nature of Toxoplasma gondii-induced behavioral changes in mice. J Parasitol 86: 657–663. [DOI] [PubMed] [Google Scholar]
- 12. Kannan G, Moldovan K, Xiao JC, Yolken RH, Jones-Brando L, et al. (2010) Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol 57: 151–155. [DOI] [PubMed] [Google Scholar]
- 13. Hay J, Aitken PP, Graham DI (1984) Toxoplasma infection and response to novelty in mice. Z Parasitenkd 70: 575–588. [DOI] [PubMed] [Google Scholar]
- 14. Berdoy M, Webster JP, Macdonald DW (2000) Fatal attraction in rats infected with Toxoplasma gondii . Proc R Soc Biol Sci Ser B 267: 1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM (2007) Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A 104: 6442–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. House PK, Vyas A, Sapolsky R (2011) Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS ONE 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaňková Š, Kodym P, Flegr J (2011) Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol 128: 181–183. [DOI] [PubMed] [Google Scholar]
- 18. Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA (2009) A unique dual activity amino acid hydroxylase in Toxoplasma gondii . PLoS ONE 4: e4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, et al. (2011) The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE 6: e23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Int J Parasitol 30: 1217–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, et al. (2012) Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol Lett 8: 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ling VJ, Lester D, Mortensen PB, Langenberg PW, Postolache TT (2011) Toxoplasma gondii seropositivity and suicide rates in women. J Nerv Ment Dis 199: 440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yazar S, Gur M, Ozdogru I, Yaman O, Oguzhan A, et al. (2006) Anti-Toxoplasma gondii antibodies in patients with chronic heart failure. J Med Microbiol 55: 89–92. [DOI] [PubMed] [Google Scholar]
- 24. Yolken RH, Dickerson FB, Torrey EF (2009) Toxoplasma and schizophrenia. Parasite Immunol 31: 706–715. [DOI] [PubMed] [Google Scholar]
- 25. Flegr J, Lenochová P, Hodný Z, Vondrová M (2011) Fatal attraction phenomenon in humans: cat odour attractiveness increased for Toxoplasma-infected men while decreased for infected women. PLoS Neglect Trop Dis 5: e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flegr J (2010) Influence of latent toxoplasmosis on the phenotype of intermediate hosts. Folia Parasitol 57: 81–87. [DOI] [PubMed] [Google Scholar]
- 27. Novotná M, Havlíček J, Smith AP, Kolbeková P, Skallová A, et al. (2008) Toxoplasma and reaction time: Role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism. Parasitology 135: 1253–1261. [DOI] [PubMed] [Google Scholar]
- 28. Flegr J, Novotná M, Lindová J, Havlíček J (2008) Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women. Neuroendocrinol Lett 29: 475–481. [PubMed] [Google Scholar]
- 29. Flegr J, Novotná M, Fialová A, Kolbeková P, Gašová Z (2010) The influence of RhD phenotype on toxoplasmosis- and age-associated changes in personality profile of blood donors. Folia Parasitol 57: 143–150. [PubMed] [Google Scholar]
- 30. Flegr J, Klose J, Novotná M, Berenreitterová M, Havlíček J (2009) Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis 9: art. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaňková Š, Šulc J, Flegr J (2010) Increased pregnancy weight gain in women with latent toxoplasmosis and RhD-positivity protection against this effect. Parasitology 137: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 32. Flegr J, Hampl R, Černochová D, Preiss M, Bičíkova M, et al. (2012) The relation of cortisol and sex hormone levels to results of psychological, performance, IQ and memory tests in military men and women. Neuroendocrinol Lett 33: 224–235. [PubMed] [Google Scholar]
- 33.Vacíř K (1973) Follow up of decissive processes in time pressure (In Czech: Sledování rozhodovacích procesů v časové tísni) [Doctoral thesis]. Faculty of Philosophy: Charles University. [Google Scholar]
- 34. Hřebíčková M (2002) Internal consistency of the Czech version of the NEO Personality Inventory (NEO-PI-R). Cesk Psychol 46: 521–535. [Google Scholar]
- 35.Formann AK, Piswanger K (1979) Wiener Matrizen-Test. Weinheim: Beltz. [Google Scholar]
- 36.Rasch G (1960) Probabilistic models for some intelligence and attainment tests. Chicago, IL: MESA Press. [Google Scholar]
- 37.Raven JC (1947) Advanced progressive matrices. London: Lewis. [Google Scholar]
- 38.Raven JC (1958) Advanced progressive matrices (2nd ed.). London: Lewis. [Google Scholar]
- 39.Raven JC (1958) Standard progressive matrices. London: Lewis. [Google Scholar]
- 40.Klose J, Černochová D, Král P (2002) Vienna Matrix Test (Vídeňský maticový test). Prague: Testcentrum. [Google Scholar]
- 41.Otis AS (1954) Otis Quick-Scoring Mental Ability Test, New Edition. Tarrytown-on-Hudson, NY: Word Book Co. [Google Scholar]
- 42.Siegel S, Castellan NJ (1988) Nonparametric statistics for the behavioral sciences. New York: McGraw-Hill. xxiii, 399 p. [Google Scholar]
- 43. Webster JP (2001) Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microb Infect 3: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 44. Flegr J (2007) Effects of Toxoplasma on human behavior. Schizophr Bull 33: 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lindová J, Příplatová L, Flegr J (2012) Higher extraversion and lower conscientiousness in humans infected with Toxoplasma . Eur J Person 26: 285–291. [Google Scholar]
- 46. Lafferty KD (2006) Can the common brain parasite, Toxoplasma gondii, influence human culture? Proc R Soc Biol Sci Ser B 273: 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flegr J, Geryk J, Volny J, Klose J, Černochová D (2012) Rhesus factor modulation of effects of smoking and age on psychomotor performance, intelligence, personality profile, and health in Czech soldiers. PLoS ONE 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flegr J, Zitkova S, Kodym P, Frynta D (1996) Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii . Parasitology 113: 49–54. [DOI] [PubMed] [Google Scholar]
- 49. Flegr J, Havlíček J (1999) Changes in the personality profile of young women with latent toxoplasmosis. Folia Parasitol 46: 22–28. [PubMed] [Google Scholar]
- 50. Carritt B, Kemp TJ, Poulter M (1997) Evolution of the human RH (rhesus) blood group genes: A 50 year old prediction (partially) fulfilled. Hum Mol Genet 6: 843–850. [DOI] [PubMed] [Google Scholar]
- 51. Flegel WA (2006) Molecular genetics of RH and its clinical application. Transfus Clin Biol 13: 4–12. [DOI] [PubMed] [Google Scholar]
- 52. Biver S, Scohy S, Szpirer J, Szpirer C, Andre B, et al. (2006) Physiological role of the putative ammonium transporter RhCG in the mouse. Transfus Clin Biol 13: 167–168. [DOI] [PubMed] [Google Scholar]
- 53. Kustu S, Inwood W (2006) Biological gas channels for NH3 and CO2: evidence that Rh (rhesus) proteins are CO2 channels. Transfus Clin Biol 13: 103–110. [DOI] [PubMed] [Google Scholar]
- 54. Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, et al. (2010) Function of human Rh based on structure of RhCG at 2.1 A. Proc Natl Acad Sci U S A 107: 9638–9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wagner FF, Flegel WA (2000) RHD gene deletion occurred in the Rhesus box. Blood 95: 3662–3668. [PubMed] [Google Scholar]
- 56. Prandota J (2004) Possible pathomechanisms of sudden infant death syndrome: key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther 11: 517–546. [DOI] [PubMed] [Google Scholar]
- 57. Prandota J (2010) Migraine associated with patent foramen ovale may be caused by reactivation of cerebral toxoplasmosis triggered by arterial blood oxygen desaturation. Int J Neurosci 120: 81–87. [DOI] [PubMed] [Google Scholar]
- 58.Prandota J (2012) Rhesus-associated glycoprotein (RhAG) phenotype of the red blood cells modulates T. gondii infection-associated psychomotor performance reaction times and changes in the human personality profile. Impaired function of the CO2, AQP1, and AQP4 gas channels may cause hypoxia and thus enhance neuroinflammation in autistic individuals. In: Gemma C, editor. Neuroinflammation: Pathogenesis, Mechanisms and Management. New York: Nova Publishers. [Google Scholar]
- 59. Lindová J, Kuběna AA, Šturcová A, Křivohlavá R, Novotná M, et al. (2010) Pattern of money allocation in experimental games supports the stress hypothesis of gender differences in Toxoplasma gondii-induced behavioural changes. Folia Parasitol 57: 136–142. [PubMed] [Google Scholar]
- 60. Novotná M, Hanušová J, Klose J, Preiss M, Havlíček J, et al. (2005) Probable neuroimmunological link between Toxoplasma and cytomegalovirus infections and personality changes in the human host. BMC Infect Dis 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wiener AS (1965) Blood groups and personality traits. Am J Hum Genet 17: 369. [PMC free article] [PubMed] [Google Scholar]
- 62. Cattell RB (1972) Blood-groups and personality traits. Am J Hum Genet 24: 485. [PMC free article] [PubMed] [Google Scholar]
- 63. Rogers M, Glendon AI (2003) Blood type and personality. Pers Individ Diff 34: 1099–1112. [Google Scholar]
- 64. Wu KH, Lindsted KD, Lee JW (2005) Blood type and the five factors of personality in Asia. Pers Individ Diff 38: 797–808. [Google Scholar]