Abstract
Purpose
To test the therapeutic efficacy of AZM, a macrolide antibiotic for prolonging murine “high risk” corneal allograft survival.
Methods
Fully MHC mismatched corneas were transplanted from C57BL/6 donors to BALB/c recipients with suture-induced vascularized “high risk” corneal beds. Recipient mice were either not treated or treated with topical AZM, oral AZM, or both. Evaluation of graft vascularization and clarity was performed in masked fashion. Lymph nodes were excised and analyzed for CD4, FoxP3, and CD44 by flow cytometry; and for T cell priming by proliferation and cytokine production in mixed lymphocyte cultures. Corneal whole mounts were evaluated by confocal microscopy.
Results
The incidence of graft rejection in the control group (81.8%) was significantly reduced by AZM treatment (18.2% topical, 21.7% oral, 33.3% topical + oral), although corneal vascularization was not affected by treatment. The frequency of corneas that retained complete clarity following transplantation was higher in the AZM treated groups. Reduced graft rejection in the AZM treated groups was not associated with a reduced allospecific T cell response or increased frequency of T regulatory cells.
Conclusions
AZM is effective in prolonging survival of “high risk” corneal allografts by an as yet undefined mechanism that does not appear to involve modulation of corneal neovascularization or allo-specific T cell priming.
Keywords: Cornea, Keratoplasty, Azythromycin
INTRODUCTION
Corneal allotransplantation is the main treatment to rescue vision that is lost due to cornea scarring and opacification [1]. Penetrating keratoplasty (full thickness corneal transplantation) is the oldest and most common form of human solid tissue transplantation. The success of penetrating keratoplasty is high, with 5 year survival rates as high as 90% [2]. This is due in part to the immune privileged characteristics of the cornea. Routine corneal transplants, where the donor cornea is grafted into an avascular non-inflamed eye require only local immuno-suppression, most commonly corticosteroids. In contrast vascularized or high risk corneal transplant prognosis is poor, with rejection rates approaching 70% even with maximal local and systemic immune suppression. Strikingly these rejection rates are even higher than those seen with living donor kidney transplantation where five year survival rates are above 80% [3]. Management of high risk corneal transplants has until recently represented a slowly evolving and controversial field in ophthalmology.
Most investigators define a “high risk” cornea transplant recipient as one with a previously failed corneal graft, or a cornea with vascularization in at least 2 of 4 quadrants [4]. Recent studies from multiple laboratories have shown that corneal neo-vascularization disrupts the immune privilege of naïve corneas [5–10]. A common model of high risk corneal transplantation involves grafting to corneal beds that are vascularized by placement of a suture in the central cornea prior to transplantation [11]. In this model, corneal allograft rejection is an immunological process that is mediated mainly by allospecific CD4+ T cells [12–17]. Immunosuppresive agents such as glucocorticoids that are employed to combat graft rejection in high risk corneal transplantation are associated with systemic immune suppression and with ocular complications such as glaucoma and cataract. Thus it is desirable to identify drugs capable of inhibiting corneal allograft rejection without major side effects including systemic immune suppression and ocular complications.
The macrolide family of antibiotics possess anti-inflammatory properties that are independent of their bactericidal or bacteria-static actions. Roxithromycin, clarithromycin, and azithromycin (AZM) are marcolide antibiotics with known anti-inflammatory actions when administered systemically [18]. AZM has been shown to significantly increase IL-10 expression by T cells without inhibiting IL-2 production, whereas clarithromycin treatment inhibited IL-2 production without elevating IL-10 [19, 20]. Given the central role of IL-2 in generation of adaptive immunity, clarithromycin would have systemic immunosuppressive effects, whereas AZM might inhibit inflammation with less of an effect on systemic immunity [21–23]. Additionally, an ophthalmic formulation of AZM is already approved by the FDA for the treatment of bacterial conjunctivitis (AzaSite, 1.5% azithromycin solution in DuraSite, Inspire Pharmaceuticals Inc. Durham, NC).
Azasite has been shown to directly reduce inflammation in the cornea following a thermal cautery. Specifically topical administration of Azasite to injured corneas caused mild and short term reductions in the size of the inflammatory infiltrate and resulted in a shift to an anti-inflammatory cytokine profile [24]. It has also been demonstrated that oral or injected AZM treatment reduces the production of inflammatory cytokines and chemoattractants such as CXCL1 and CXCL8 that are important players in corneal inflammation [25–27]. Thus we hypothesized that AZM treatment could increase graft survival by suppressing the local inflammation without systemic immune suppression.
To test this hypothesis we compared the incidence of corneal allograft rejection on suture induced high-risk graft beds of mice treated daily with topical, oral, or topical and oral AZM. We found that all AZM treatment regimes significantly reduced the incidence of allograft rejection without altering the systemic allospecific T cell response. Moreover, even corneas that ultimately rejected exhibited a delayed onset of opacity when treated with topical AZM.
MATERIALS AND METHODS
Animals
C57BL/6 and BALB/c mice from Jackson Laboratories (Bar Harbor, Maine) were housed in the Animal Resource Facility at the University of Pittsburgh Medical Center. The use of animals was in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and all procedures were approved by the University of Pittsburgh Institutional Animal Care and Use committee.
Induction of Corneal Vascularization: High Risk Model
Corneal neovascularization was induced in graft recipients by placing two full thickness 11-0 nylon sutures (Sharppoint, Houston, TX) in the central cornea of female BALB/c mice. Corneal allografts were placed 1 month later when 75% of the recipient corneal beds exhibited vascularization.
Orthotopic Corneal Transplantation
Allogeneic orthotopic corneal transplantations where performed as previously described [6]. Briefly, the central 2 mm of donor corneas from C57BL/6 mice were excised and secured in recipient high risk graft beds of BALB/c mice with eight interrupted 11-0 nylon sutures (Sharppoint, Vangaurd, Houston, TX). Grafts where evaluated twice a week by a masked scorer using slitlamp biomicroscopy. Mice with flat anterior chambers, ulceration, or other complication associated with surgical failure were excluded from the experiments. Transplanted mice either received no treatment, or received topical AzaSite (1 drop per day on the transplanted cornea), oral AZM (0.25mg in 10μl of PBS administered by mouth every day), or both treatments beginning on the day of transplantation and continuing throughout the follow-up period.
Scoring of Allografts
Transplanted corneas were scored at least twice a week in masked fashion by two investigators with a high degree of consensus. Opacification was scored based on the following parameters: 0=clear graft, 0.5=small isolated imperfections or regions of translucence in the graft, 1=minimal translucence or haziness extending over the entire graft with complete visualization of the iris and pupil, 1.5= translucence or haziness extending over the entire graft and/or regional opacity in a minority of the graft, 2= stromal opacity over the majority of the graft with visualization of the pupil margin and the iris structures, 2.5=stromal opacity extending over the majority of the graft with only small regional translucence allowing very limited and strongly obscured visualization of the iris, 3=complete stromal opacity with no visualization of the iris structures, 3.5=complete stromal opacity associated with a swollen or misshapen eye, 4= destruction of the graft, a loss of graft integrity or graft perforation. Rejection was defined by a score of ≥3 on two consecutive readings (no mice were allowed to progress to a score of 4 in this study). [32] [37] [38]. Neovacularization was also quantified, but was not used as a basis of rejection. Each quadrant was given a score ranging from 1–4 where 1=neovascularization present at the limbus, 2= neovascularization to the graft-host interface but not invading the graft, 3= neovascularization extending into the graft, 4 neovascularization extending into the central cornea. The four quadrants where added and a score ranging from 0 to 16 was given at each time point.
Immunohistochemical Analysis of Inflammatory Infiltration of corneal allografts
Complete corneas, including the graft and the recipient tissue were harvested from mice at 25 days post transplantation. Four incisions were made in the graft bed so that cornea could be effectively mounted on a slide for imaging. The Corneas were washed in PBS+4% FBS and fixed for two hours in BD Cytofix/Cytoperm. The corneas were then blocked with anti-FC receptor and stained with anti-mouse CD45 PE, anti-mouse CD90.2 APC and DAPI. Confocal images were acquired on a Nikon (Tokyo, Japan) A-1 confocal microscope using a 20x oil (0.85NA) and were analyzed with MetaMorph 7.7 software (Sunnyvale, CA).
Flow Cytometric Analysis of T lymphocytes in Lymph Nodes, Spleen, and Peripheral Blood of transplant recipients
At post transplant days 7 and 25, mice were anesthetized with 2.5 mg of ketamine and 0.25 mg xylazine and blood was collected by cardiac puncture and mixed with an equivalent volume of heparin in PBS (1000units/ml, Sigma St. Louis MO). The mice were then euthanized by cervical dislocation and their spleens and lymph nodes were dispersed into single cell suspensions by forcing the tissue through 40μM sterile nylon cell strainer. Following red blood cells lysis, the cells were incubated for 20 minutes at 4 C with combinations of the following fluorochrome-conjugated antibodies: anti-CD4 (clone RM4-5), anti-CD8α (clone 53-6.7), anti-CD25 (clone PC61), anti-CD44 (clone IM7), and anti-CD45 (clone 30-F11), all from BD Pharmigen. All sample were fixed in either 0.4% paraformaldhyde in PBS (Sigma, St. Louis MO) for surface staining only, or in eBioscience Fixation/Permabilization buffer for combination surface and intracellular staining. Intracellular staining was carried out by washing fixed cells once in eBioscience permablization buffer and then incubating the cells with anti-FOXP3 conjugated to phycoerythrin (PE) in permablization buffer for 20 minutes. Flow cytometry was accomplished with a FACSAria machine, and data were analyzed with FlowJo software (Tree Star Inc. Ashland, OR).
Mixed Lymphocyte Reactions
Spleen cells from BALB/c transplant recipient mice were stained with violet proliferation dye (Invitrogen, Eugene, OR) as per manufactures instructions. Splenocytes from C57Bl/6 or BALB/c mice were exposed to three thousand rads of gamma radiation and used as stimulator cells. The labeled graft recipient splenocytes were then incubated in duplicate cultures with an equal number of C57Bl/6 stimulator cells (experimental group), BALB/c stimulator cells (negative control), or BALB/c stimulator cells plus 0.1 μg/ml of anti-CD3 (Clone 145-2C11 BD Pharmigen) as a positive control. After three days in culture the supernatants and the cells were harvested. The cells were stained for surface markers as described above and cell viability was determined by using FarRed viability (Invitrogen Eugene, OR) stain to distinguish viable cells from dead cells via flow cytometry. Proliferation was measured by dye dilution using FlowJo software.
The supernanants were analyzed by sandwich enzyme linked immunosorbent assay (ELISA) for interferon gamma (IFN-γ). Briefly, Immulon 4HBX high binding polystyrene ELSIA plates (Thermo-Fischer Rochester, NY) were coated with anti-IFN-γ (clone R4-6A2) over night at 4°C, washed with PBS + 0.05% tween 20 (Sigma, St. Louis MO), and the wells were blocked for 1 hour with PBS + 0.05% tween and 1% bovine serum albumin (Sigma, St. Louis MO). The wells were washed and then loaded with either 100μls of either diluted supernatant from the lymphocyte cultures, or of various concentrations of a purified mouse IFN-γ standard. The plates were incubated overnight at 4°C, the wells were washed and loaded with biotin conjugated anti-IFN-γ (clone XMG1.2, BD Pharmigen). After 1 hour at room temperature the wells were washed, loaded with strepaviden conjugated to horseradish peroxidase, and incubated for 30 minutes at room temperature. The wells were washed and loaded with 100μls TMB peroxidase substrate (Fitzgerald Industries International Acton, MA). Color development was stopped by addition of 100μLs of 1N H2S04, and absorbance was read at 450nm using a SpectraMax plus plate reader (Molecular Devices, Sunnyvale CA).
Statistical Analysis
Corneal transplant survival in treated and untreated mice was plotted and significance determined by a log rank test (GraphPad Software Inc, La Jolla, Ca). The difference in the percent of clear or rejecting corneas at day 25 post-transplant was assessed by Chi-square analysis. All other values were compared by a student T-test or a one way analysis of variance and Tukey’s post test. The p values are noted in figure legends when a significant difference was observed.
RESULTS
Azithromycin Treatment Prolongs High Risk Corneal Allograft Survival
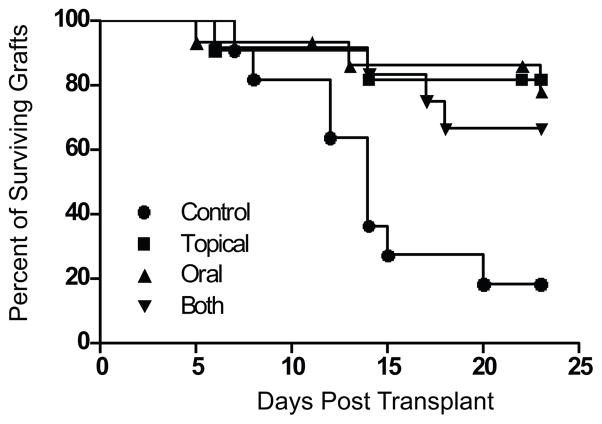
The efficacy of AZM in prolonging high risk corneal allograft survival was determined in fully MHC mismatched (C57B6 to BALB/c) grafts. Recipients were either left untreated or treated daily with topical, oral, or both topical and oral AZM. Corneal allografts where scored as described (Materials and Methods) and the graft was consider rejected upon two opacity scores of 3 or higher. AZM treatment by all three routes significantly reduced the rate and incidence of allograft rejection, and no significant differences were observed in graft survival among AZM treatment groups (Fig. 1).
Figure 1. Azithromycin treatment significantly reduces the rejection rate of corneal allografts in high risked Balb/c mice.
Balb/c mice with suture-induced high-risk corneal beds received corneal grafts from MHC miss-matched C57Bl/6 mice. The recipient mice were either left untreated (● n=11), received daily doses of 1% AZM solution directly on the eye (■ n=14), received daily oral doses of 250μg of AZM in PBS (▲ n=14) or received both topical and oral treatment daily (▼ n=12). Rejection was based on two consecutive opacity scores of 3 as assessed by a masked observer. The differences in rejection rate between the control group and the AZM treatment groups were significant (p=0.02) when assessed by a log rank test. The incidence of rejection was significantly decreased in treated mice when compared to the control group (p<0.05). No significant differences were observed among treatment groups in rate or frequency of graft rejection.
Not all grafts developed corneal opacity and not all grafts that developed a corneal opacity rejected within 25 days of transplant. At day 25 post-transplant the frequencies of clear corneal grafts (opacity score 0–0.5) among the treatment groups were as follows: 9% of controls; 33% of topical AZM treated; 43% of oral AZM treated; and 54% of topical plus oral AZM treated. Chi Square analysis revealed no significant differences among the treated groups, but did reveal a significant difference in clarity of the control group compared to the double treated group (p= 0.02).
Azithromycin Delays the Development of Opacity but Does Not Prevent Neovascularization in Corneal Allografts
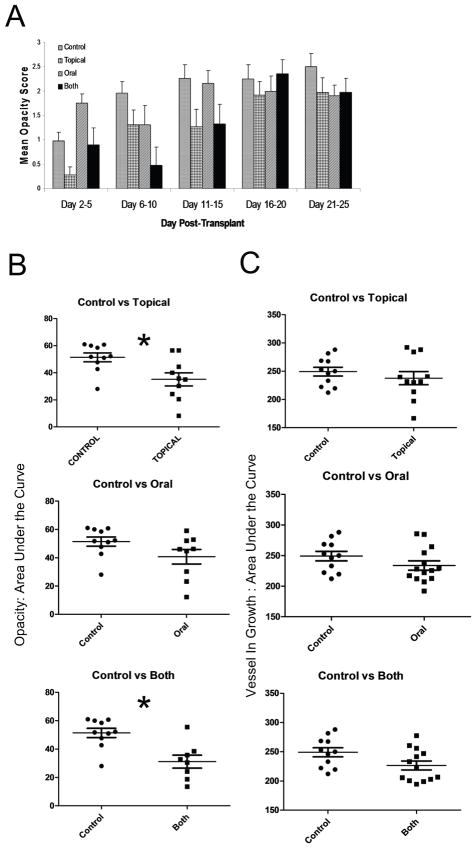
While some of the accepted corneal transplants remained clear through the 25 day observation period, others developed varying degrees of opacity. We observed a delay in the onset of opacity in mice that received topical AZM (Fig. 2A) resulting in a significant reduction in the area under the curve of the mean opacity scores (Fig. 2B).
Figure 2. Topical treatment significantly reduces graft opacity but not vessel in growth.
Balb/c mice with suture-induced high-risk corneal beds received corneal grafts from MHC miss-matched C57Bl/6 mice. The recipient mice were either left untreated, received daily doses of 1% AZM solution topically, received daily oral doses of 250μg of AZM in PBS or received both topical and oral treatment daily. Mice were examined twice a week following transplantation and opacity was scored on a scale of 0–3. The mean ± SEM opacity score (excluding mice that failed to develop opacity) was recorded over time (A). Corneal opacity scores (B) and area of blood vessel in growth (C) were plotted over time for each mouse and reduced to a single value in the scatter plots by calculating the area under the curve (AUC). Each group was compared to the control group by a Student t Test * p< 0.05.
The reduced opacity scores in mice receiving topical AZM was not associated with a significant reduction in vascularization of the graft (Fig. 2C). We did note a trend toward reduced vascularization in mice receiving combined oral and topical AZM treatment, but the difference did not achieve statistical significance at p<0.05. These findings are in agreement with a previous study in which topical Azasite reduced inflammation, but failed to influence neovascularization in corneas with cautery induced thermal injury [24].
Treatment with Azithromycin Changes the Inflammatory Infiltrate in Corneal Allografts
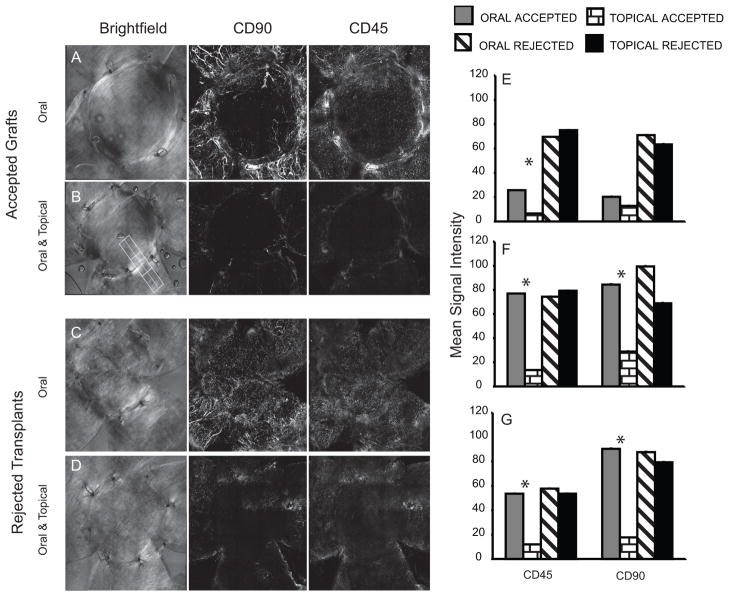
At 25 days post-transplant corneas were harvested, fixed and stained for CD45 and CD90 (a pan T cell marker). The boundary between the grafted tissue and the recipient bed is visible in the bright field images in the left hand panels (Fig. 3A–D. We expected AZM treatment to prevent infiltration of immune effectors cells into the graft. Interestingly, we observed a significantly larger leukocyte infiltrate in accepted corneas (though not in rejected corneas) of mice receiving only oral AZM (Fig. 3A & E–G) compared to mice that received topical and oral treatment (Fig. 3B & E–G). The reduced leukocytic infiltrate in topical + oral treated group was observed in the corneal bed, graft interface, and within the allograft (Fig. 3)
Figure 3. Effect of AZM on leukocytic infiltrate.
The figure illustrates representative corneas of mice treated with oral + topical AZM (B&D) or oral AZM only (A&C). Bright field images (left panel) illustrate the corneal bed and graft. Confocal maximum projection z-stack images illustrate localization of T cells (CD90, middle panel) and bone marrow derived cells (CD45, right panel). Accepted corneas with opacity scores of 0–0.5 at the time of sacrifice (A&B) and rejected corneas scores of 2.5–3 at the time of sacrifice. (C&D). The mean staining intensity within the graft (E), graft bed (G), and at the interface (F) was determined by drawing multiple contiguous regions (such as the figure drawn in the 3B) in the maximum projection z-stack images of corneas with equivalent opacity scores (clear or rejected). The numerous regions were analyzed in order to cover the majority of the corneas. The bar graph represents the mean ± SEM intensity in multiple corneas. The staining intensity for CD45 in all three regions of the cornea was significantly reduced (p<0.05) by topical AZM treatment and CD90 was significantly reduced by topical treatment compared to oral AZM in both the bed and the interface.
Azithromycin Does not Significantly Alter the Treg Population Following Transplantation
Increased levels of FoxP3 expression on CD4+ T cells in the draining lymph node (DLN) positively correlates with cornea graft survival [28]. Therefore, we determined if AZM treatment influenced the frequency of FoxP3+ CD4+ Tregs in the DLN of allograft recipients or their level of FoxP3 expression. Cells were obtained from the DLN 7 and 25 days after placement of corneal allografts and stained for CD4, CD44, and FoxP3. Flow cytometric analysis gating on CD4+ T cells revealed no significant differences in the frequency of Tregs (CD4+ FoxP3+) in DLN of AZM treated and control allograft recipients at 7 days post transplant (data not shown) or 25 days post transplant (Figs. 4A&B). There were also no significant group differences in the relative MFI of FoxP3 expression (Fig. 4C). Surprisingly, we observed an increased ratio of CD4 effector cells (CD4+ CD44high FoxP3−) to Tregs (CD4+ FoxP3+) in the AZM treated groups, though the difference achieved statistical significance only in the topical AZM group (Fig. 4D).
Figure 4. Azithromycin treatment did not significantly affect the Treg phenotype.
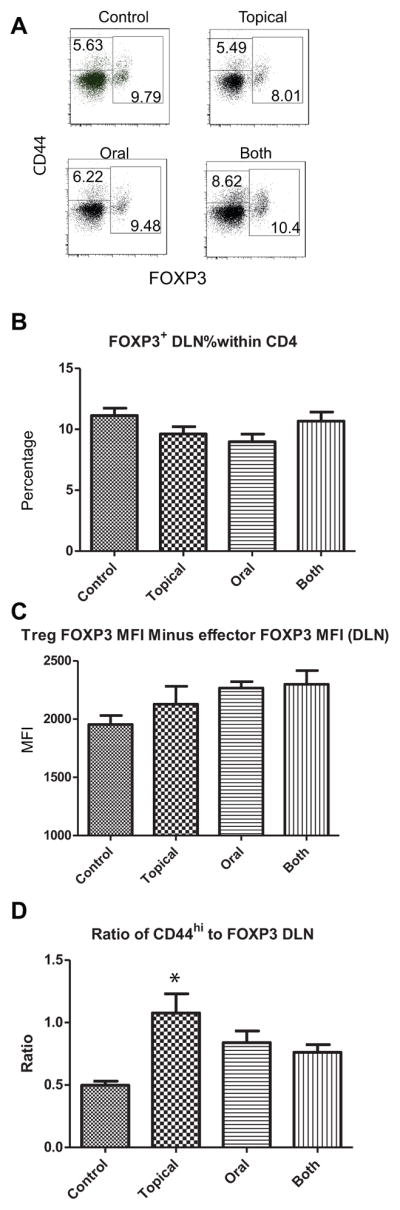
Balb/c mice with suture-induced high-risk corneal beds received corneal grafts from MHC miss-matched C57Bl/6 mice. The recipient mice were either left untreated (control), received daily doses of 1% AZM solution directly on the eye (topical), received daily oral doses of 250μg of AZM in PBS (oral) or received both topical and oral treatment daily (both). Lymph nodes draining the transplanted cornea were excised at 25 days post transplant and stained for CD4, CD44, and FoxP3. Representative flow plots showing CD44 and FoxP3 expression on CD4 T cells (A). Numbers in boxes represent the frequency of effector/memory cells (CD44hi FoxP3−), and Tregs (FoxP3+). Mean ± SEM frequency of FoxP3 cells within the CD4 gate (B). Mean ± SEM relative MFI of FoxP3 expression obtained by subtracting the MFI of the FoxP3 negative population from the MFI of the FoxP3 positive population (C). The mean ± SEM ratio of effector/memory cells to Tregs (D). The ratio was significantly increased (p < 0.05) in mice receiving topical AZM compared to controls as determined by a one-way ANOVA and Tukey’s post test.
Azithromycin Treatment Does Not Alter T cell Function
We next determined if the reduced graft rejection in the AZM treated mice correlated with a reduction in the relative size or functionality of CD4+ effector T cells in the spleen. Splenocytes were obtained from BALB/c graft recipients at 7 and 25 days after transplant of a C57BL/6 allogeneic cornea and stained for CD4, CD44, and FoxP3 and the frequency of CD4+ memory/effector T cells (CD+ CD44high FoxP3−) was determined. A trend toward a lower frequency of CD4+ effector T cells was observed in AZM treated groups at 7 days post transplant (Fig. 5A), but differences did not achieve statistical significance. No group differences in frequency of CD4+ effector T cells was observed in spleens at 25 days post transplant (Fig. 5B). Splenocytes of BALB/c mice obtained 25 days after placement of corneal allografts were stained with violet proliferation dye, and stimulated for 3 days with plate bound anti-CD3, or with allogeneic (C57BL/6) or syngeneic (BALB/c) splenocytes. Splenocytes from BALB/c allograft recipient mice exhibited increased proliferation and IFN-γ production in response to stimulation with allogeneic C57BL/6 stimulator cells relative to those stimulated with syngeneic BALB/c stimulator cells (data not shown). However, the levels of IFN-γ production (Figs. 5C&D) and proliferation (Figs. 5E&F) were not significantly affected by any of the AZM treatment regimens.
Figure 5. T cell effector function.
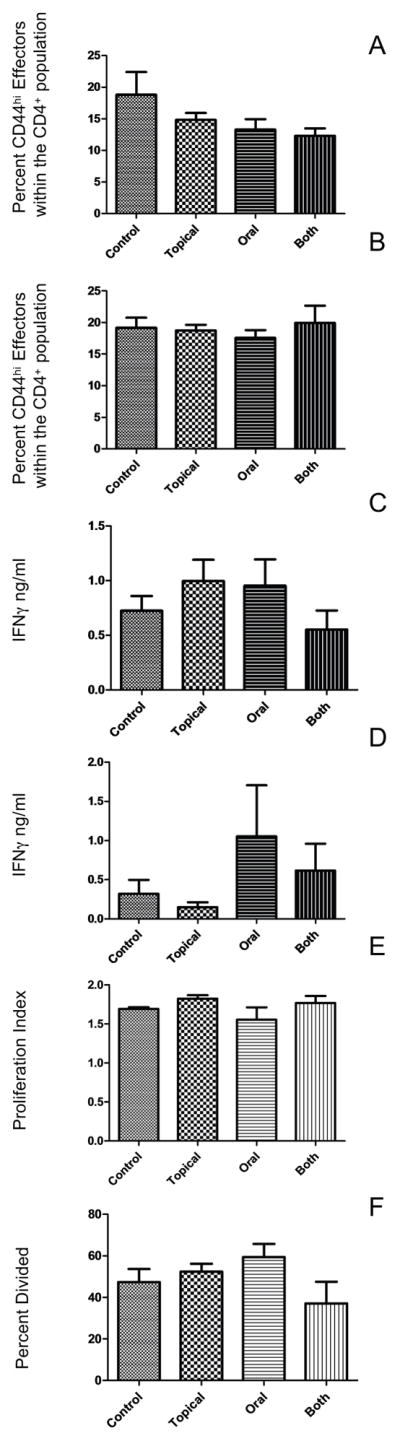
The percent of CD44hiFOXP3− cells within the CD4+ splenocyte population 7-days (A) and 25 days post-transplant (B). Spleen cells from mice 25 days post transplant were cultured for 3 days with anti-CD3 or irradiated B6 splenocytes (C–D). The amount IFNγ produced was determined by ELISA. (data is representative of 1 of 2 experiments.) The ability of T cells to proliferate in response to anti-CD4 was also measured and daily AZM treatments did not significantly reduced the functionality of T cells compared to controls (E–F).
Discussion
We have demonstrated that AZM treatment significantly increases the number of grafts that survive a high-risk corneal transplant procedure. All the grafts included in this study had an intact anterior chamber, were not opaque during the first 5 days following surgery, and showed no other signs of surgical failure. Moreover, in our hands syngeneic corneal grafts are not rejected, and allogeneic grafts are not rejected in CD4 T cell-deficient mice (data not shown). Thus our model is consistent with graft rejection and not graft failure. The administration of topical antibiotics is standard practice for corneal transplant recipients, and Azasite is a broad spectrum antibiotic that has been approved for use in treating ocular infections. Therefore, the addition of Azasite to the post-transplant treatment regimen would benefit the patient through its dual antibacterial and anti-inflammatory activity. Furthermore our studies have demonstrated that the doses of AZM that effectively extend corneal graft acceptance do not have obvious side effects or cause a systemic reduction in measured immune functions. In our study T cells in AZM treated mice produced cytokines and proliferated as well as T cells in untreated mice when polyclonally stimulated with anti-CD3 directly ex vivo. Previous studies using higher doses of AZM did show broad effects on immune function [18, 26, 27], but the clinically relevant doses employed in our studies effectively inhibited local inflammation without detectable systemic immune suppression.
The general anti-inflammatory properties of the macrolide family have been studied primarily in models of chronic inflammatory airway diseases associated with bacterial infections [25, 29–31,32–34]. In those studies, AZM was shown to inhibit both neutrophil and T cell migration into inflamed airway tissue. Macrolides have varying effects the production and efficacy of many proinflammatory cytokines such as IL-1, IL-6, IL-8 (CXCL-8), and tumor necrosis factor with AZM appearing to be the mildest anti-inflammatory macrolide in the family [18, 19]. AZM treatment limits the signal strength of chemoattratants such as IL-8 and the production of inflammatory molecules such as GM-CSF that attract neutrophils to the sites of inflammation and are essential factors in graft rejection [35–38]. Thus, AZM likely functions at least in part by inhibiting leukocytic infiltration into corneal allografts.
All three routes of AZM administration (topical corneal, oral, and combined) significantly reduced the incidence of graft rejection. However, the parameter defining rejection permits a significant amount of opacity to form in a non-rejected cornea. When the degree of opacity in rejected and non-rejected corneas was compared two interesting observations emerged. First, at day 25 post-transplant there was a higher frequency of clear corneas (opacity score of 0–0.5) in the treated groups compared to the untreated controls, although the difference was statistically significant only in the group receiving oral plus topical AZM. Thus, AZM not only reduced the frequency of rejection, but increased clarity in corneas that did not reject. The second observation was that topical AZM treatment reduced the opacity that typically occurs in the first few days following transplant. This finding is consistent with a previous study demonstrating that topical AZM inhibited an innate inflammatory response induced in the cornea by thermal cautery [24]. By reducing this early opacity, topical Azasite might reduce or delay the activation of alloreactive CD4+ T cells and their infiltration into the corneal graft. A delay in the expansion of CD4 effector/memory cells would be consistent with our observations of a reduced T effector/memory population at day 7 post-transplant (Fig. 5), but enlarged population at day 25 (Fig. 4).
The AZM treatment groups also differed with respect to the magnitude of the corneal infiltrate. The corneas treated with topical AZM showed a reduction in the relative T cell infiltrate compared to mice treated with oral AZM (Fig. 3). The overall CD45 infiltrate in accepted grafts was also reduced in the allograft recipients that were treated with topical AZM when compared to oral AZM treated mice. This is consistent with previous studies demonstrating that AZM can reduce the chemokines, cytokines, and adhesion molecules required for leukocytic extravasation from blood [25, 36]. These findings suggest that topical AZM is more effective than oral AZM at inhibiting leukocyte extravasation into grafted corneas, and that the size of the leukocytic infiltrate can vary significantly in corneas with similar opacity scores. Despite the reduced incidence of corneal graft rejection in AZM treated mice vascularization of the allograft was not significantly reduced. A previous study demonstrated that topical corneal AZM treatment failed to inhibit vascularization following implantation of VEGF-A containing pellets in the cornea [24]. Together with our findings this suggests that AZM is ineffective at blocking corneal vascularization, but does appear to inhibit leukocytic extravasation from corneal vessels.
Although not exhaustive, our studies did not demonstrate an effect of AZM on the generation or function of alloreactive CD4+ T cells, or on the generation of Tregs. There was no difference in the frequency of CD4+ CD44high FoxP3− effector/memory T cells in the spleens of AZM treated or non-treated allograft recipients at 7 or 25 days post grafting. In fact, the AZM treated mice tended to have a higher ratio of CD4+ effector T cells to Tregs than non-treated controls. There was no significant difference in the ability of spleen cells from AZM-treated allograft recipients to produce IFN-γ or proliferate in response to alloantigens. Therefore, it does not appear that AZM treatment markedly affected the generation or function of allospecific CD4+ T cells, though a more comprehensive study including earlier time points after corneal transplantation will be required to firmly establish this point.
The finding that topical AZM treatment is highly effective in reducing the incidence of corneal graft rejection in a high risk mouse model is very encouraging. Since the 1% ophthalmic AZM solution Azasite is now FDA approved for treatment of bacterial or trachomatous conjunctivitis, and administration of topical antibiotics is standard practice for all corneal transplant recipients, it would seem reasonable to extend these studies to humans who are at high risk of corneal graft rejection due to previous inflammatory corneal conditions.
Acknowledgments
Support: The authors acknowledge support from NIH/NEI EY10359 (RLH), P30EY08098(RLH), an unrestricted grant from Research To Prevent Blindness, New York, NY, and the Eye and Ear Foundation Pittsburgh, PA.
Bibliography
- 1.Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin Experiment Ophthalmol. 2005;33(6):642–57. doi: 10.1111/j.1442-9071.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 2.Price FWWW, Jr, Collins KS, Marks RG. Five-year corneal graft survival. A large, single-center patient cohort. Archives of Ophthalmology. 1993;111(6):799–805. doi: 10.1001/archopht.1993.01090060087029. [DOI] [PubMed] [Google Scholar]
- 3.Port FK, Merion RM, Goodrich NP, Wolfe RA. Recent trends and results for organ donation and transplantation in the United States, 2005. Am J Transplant. 2006;6(5 Pt 2):1095–100. doi: 10.1111/j.1600-6143.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 4.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110(10):1392–403. [PubMed] [Google Scholar]
- 5.Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126(1):71–7. doi: 10.1001/archopht.126.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Amescua G, Collings F, Sidani A, et al. Effect of CXCL-1/KC production in high risk vascularized corneal allografts on T cell recruitment and graft rejection. Transplantation. 2008;85(4):615–25. doi: 10.1097/TP.0b013e3181636d9d. [DOI] [PubMed] [Google Scholar]
- 7.Dana MR. Angiogenesis and lymphangiogenesis-implications for corneal immunity. Semin Ophthalmol. 2006;21(1):19–22. doi: 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10(8):813–5. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 9.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22(3):273–81. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37(12):2485–94. [PubMed] [Google Scholar]
- 11.Niederkorn JY. High-risk corneal allografts and why they lose their immune privilege. Curr Opin Allergy Clin Immunol. 10(5):493–7. doi: 10.1097/ACI.0b013e32833dfa11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol. 2001;167(4):1891–9. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 13.Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67(12):1503–8. doi: 10.1097/00007890-199906270-00001. [DOI] [PubMed] [Google Scholar]
- 14.Sano Y, Ksander BR, Streilein JW. Murine orthotopic corneal transplantation in high-risk eyes. Rejection is dictated primarily by weak rather than strong alloantigens. Invest Ophthalmol Vis Sci. 1997;38(6):1130–8. [PubMed] [Google Scholar]
- 15.Sano Y, Ksander BR, Streilein JW. Analysis of primed donor-specific T cells in recipient mice bearing orthotopic corneal allografts. Transplantation. 2000;70(9):1302–10. doi: 10.1097/00007890-200011150-00007. [DOI] [PubMed] [Google Scholar]
- 16.Sonoda Y, Sano Y, Ksander B, Streilein JW. Characterization of cell-mediated immune responses elicited by orthotopic corneal allografts in mice. Invest Ophthalmol Vis Sci. 1995;36(2):427–34. [PubMed] [Google Scholar]
- 17.Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999;40(11):2614–21. [PubMed] [Google Scholar]
- 18.Scaglione F, Rossoni G. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J Antimicrob Chemother. 1998;41(Suppl B):47–50. doi: 10.1093/jac/41.suppl_2.47. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama K, Shirai R, Mukae H, et al. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin Exp Immunol. 2007;147(3):540–6. doi: 10.1111/j.1365-2249.2007.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy BS, Sundareshan V, Cory TJ, Hayes D, Jr, Anstead MI, Feola DJ. Azithromycin alters macrophage phenotype. J Antimicrob Chemother. 2008;61(3):554–60. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 21.Whalen JD, Thomson AW, Lu L, Robbins PD, Evans CH. Viral IL-10 gene transfer inhibits DTH responses to soluble antigens: evidence for involvement of genetically modified dendritic cells and macrophages. Mol Ther. 2001;4(6):543–50. doi: 10.1006/mthe.2001.0492. [DOI] [PubMed] [Google Scholar]
- 22.Perrin GQ, Johnson HM, Subramaniam PS. Mechanism of interleukin-10 inhibition of T-helper cell activation by superantigen at the level of the cell cycle. Blood. 1999;93(1):208–16. [PubMed] [Google Scholar]
- 23.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147(8):2713–6. [PubMed] [Google Scholar]
- 24.Sadrai Z, Hajrasouliha AR, Chauhan S, Saban DR, Dastjerdi MH, Dana R. Effect of topical azithromycin on corneal innate immune responses. Invest Ophthalmol Vis Sci. 52(5):2525–31. doi: 10.1167/iovs.10-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai WC, Rodriguez ML, Young KS, et al. Azithromycin blocks neutrophil recruitment in Pseudomonas endobronchial infection. Am J Respir Crit Care Med. 2004;170(12):1331–9. doi: 10.1164/rccm.200402-200OC. [DOI] [PubMed] [Google Scholar]
- 26.Ivetic Tkalcevic V, Bosnjak B, Hrvacic B, et al. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur J Pharmacol. 2006;539(1–2):131–8. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 27.Ianaro A, Ialenti A, Maffia P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292(1):156–63. [PubMed] [Google Scholar]
- 28.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–53. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labro MT. Cellular and molecular effects of macrolides on leukocyte function. Curr Pharm Des. 2004;10(25):3067–80. doi: 10.2174/1381612043383403. [DOI] [PubMed] [Google Scholar]
- 30.Tamaoki J. The effects of macrolides on inflammatory cells. Chest. 2004;125(2 Suppl):41S–50S. doi: 10.1378/chest.125.2_suppl.41s. quiz 51S. [DOI] [PubMed] [Google Scholar]
- 31.Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117(Suppl 9A):5S–11S. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Kusano S, Kadota J, Kohno S, et al. Effect of roxithromycin on peripheral neutrophil adhesion molecules in patients with chronic lower respiratory tract disease. Respiration. 1995;62(4):217–22. doi: 10.1159/000196450. [DOI] [PubMed] [Google Scholar]
- 33.Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;156(1):266–71. doi: 10.1164/ajrccm.156.1.9612065. [DOI] [PubMed] [Google Scholar]
- 34.Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun. 1995;210(3):781–6. doi: 10.1006/bbrc.1995.1727. [DOI] [PubMed] [Google Scholar]
- 35.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56(5):559–64. [PubMed] [Google Scholar]
- 36.Shinkai M, Foster GH, Rubin BK. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(1):L75–85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- 37.Strieter RM, Kasahara K, Allen RM, et al. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992;141(2):397–407. [PMC free article] [PubMed] [Google Scholar]
- 38.Bosnar M, Bosnjak B, Cuzic S, et al. Azithromycin and clarithromycin inhibit lipopolysaccharide-induced murine pulmonary neutrophilia mainly through effects on macrophage-derived granulocyte-macrophage colony-stimulating factor and interleukin-1beta. J Pharmacol Exp Ther. 2009;331(1):104–13. doi: 10.1124/jpet.109.155838. [DOI] [PubMed] [Google Scholar]