Abstract
While loss of the protein Lyst causes abnormal lysosomes in patients with Chediak-Higashi Syndrome, the contribution of Lyst to lysosome biology is not known. Previously we found that the Dictyostelium ortholog of Lyst, LvsB, is a cytosolic protein that associates with lysosomes and post-lysosomes to prevent their inappropriate fusion. Here we provide three lines of evidence that indicate that LvsB contributes to lysosome function by antagonizing the function of DdRab14, a protein that promotes homotypic fusion among lysosomes. (1) Instead of restricting DdRab14 to lysosomes, cells that lack LvsB expand DdRab14 localization to include post-lysosomes. (2) Expression of activated DdRab14 phenocopies the loss of LvsB, causing inappropriate heterotypic fusion between lysosomes and post-lysosomes and their subsequent enlargement. (3) Conversely, expression of inactivated DdRab14 suppresses the phenotype of LvsB null cells and restores their lysosomal size and segregation from post-lysosomes. Our data suggest a scenario where LvsB binds to late lysosomes and promotes the inactivation of DdRab14. This inactivation allows the lysosomes to mature into post-lysosomes for eventual secretion. We propose that human Lyst may function similarly to regulate Rab-dependent fusion of lysosomal compartments.
INTRODUCTION
The endolysosomal system is a complex collection of pleiomorphic organelles that traffic a wide range of molecules and receive input from multiple sources including the TGN, phagocytosis and endocytosis (1). To accomplish their function, endolysosomal vesicles must control their composition by undergoing multiple fusion and fission events. In this way, one molecule internalized by endocytosis may eventually reach the lysosome while another one may be recycled back to the plasma membrane. To achieve proper sorting of different cargo molecules, the fusion between different compartments of this system must be precisely regulated. Thus, it is not surprising that a large number and diversity of regulatory proteins have been identified in different compartments of the endolysosomal system, including Rabs, SNAREs, HOPS, etc (2, 3). A major challenge in this field is understanding how these and other components collaborate to accomplish the tightly regulated sorting necessary for the elaborate functions of the endolysosomal system.
The importance of the endolysosomal system is evinced by the severe hereditary diseases that are caused by defects in its regulation. Many lysosomal storage diseases have been identified that impinge on important regulatory mechanisms (4). Among them, Chediak-Higashi syndrome has been a difficult case to dissect in detail. The gene affected in patients with this disorder was identified as one encoding a 430KDa protein named LYST (lysosomal trafficking regulator) whose function remains unknown (5). Cells from these patients contain grossly enlarged lysosomes that fail to function properly and lead to defects in skin pigmentation, blood clotting and immune defense. To date, the intracellular localization of LYST is not known and no binding partner has been identified in vivo. Thus, it has not been possible to postulate any mechanism by which LYST may regulate lysosomal size and function.
We have shown that Dictyostelium LvsB protein is the ortholog of human LYST and, like LYST, is also required for the proper function of the lysosome (6). Loss of LvsB results in the enlargement of acidic lysosomal compartments and causes secretory defects (7, 8). These observations suggest that the Dictyostelium LvsB-null mutant represents an excellent single-cell model system for the study of the cellular defects that cause Chediak-Higashi Syndrome.
The endolysosomal system of Dictyostelium consists of multiple compartments that rapidly process endocytosed materials and excrete indigestible substances. Endocytic and phagocytic vesicles are quickly acidified and receive lysosomal enzymes to digest their contents. The acidic lysosomal vesicles subsequently mature into post-lysosomes, neutral secretory vesicles that are destined for exocytosis (9, 10). Consequently, the Dictyostelium lysosome is not a terminal organelle as in most mammalian cells, but is most similar to the secretory lysosomes of specialized mammalian cells (11).
Previously, we showed that LvsB localizes on late lysosomes and post-lysosomes. Moreover, in LvsB-null cells lysosomes fuse inappropriately with post-lysosomes; a rare occurrence in wild type cells (6). A consequence of the inappropriate fusion between compartments is that the maturation of secretory competent post-lysosomes is delayed (12). These results suggested that the function of LvsB (and of LYST) is to act as a negative regulator of vesicle fusion and that the enlarged lysosome phenotype of Chediak-Higashi Syndrome patients could result from uncontrolled lysosomal fusion.
To better understand how LvsB controls vesicle fusion events it is important to determine whether LvsB interacts with any of the known components that promote vesicle fusion. In mammalian cells the Rab family of GTPases plays a major role in the regulation of vesicular trafficking. Rabs have been implicated in the control of both fusion and fission events that are required for the proper maturation of endosomes as well as phagocytic compartments (13). This Rab mediated control of vesicular trafficking seems to be conserved across many species including Dictyostelium. Currently, 54 putative Rab related GTPase genes have been identified in Dictyostelium. Characterization studies have shown that the diversity of these proteins extends to their functional capacities along many membrane trafficking pathways. Similar to the complexity of mammalian cells, a number of Dictyostelium Rab proteins including Rab21, Rab7, and Rab14 serve to regulate distinct maturation steps along the endocytic and/or phagocytic pathways (14–18). Interestingly, studies of the Dictyostelium small GTPase DdRab14 showed that activation of DdRab14 induced a phenotype reminiscent of that shown in LvsB-null cells (15, 16, 18). Dictyostelium DdRab14 is found on lysosomes and on the membranes of contractile vacuoles. Expression of constitutively active DdRab14 (DdRab14Q67L) enhanced the fusion of lysosomal vesicles leading to the formation of enlarged lysosomes. Similarly, activation of DdRab14 caused phagosomes to fuse together, forming large vesicles containing multiple engulfed bacteria (18), a phenotype shared with LvsB null cells (6). These studies suggested that LvsB and DdRab14 may control similar fusion events along the endolysosomal system. However, this possibility has not been examined as the studies of DdRab14 focused solely on the early homotypic fusion of lysosomes whereas our LvsB studies centered on the control of heterotypic fusion between lysosomes and post-lysosomes.
In the present study we provide evidence that LvsB acts as an antagonist to the fusion-promoting activity of the GTPase DdRab14. We also show that the function of LvsB is restricted to the regulation of fusion between lysosomes and post-lysosomes and not of other compartments. These results provide a mechanistic framework to understand the function of LvsB, and by extension, of human LYST.
RESULTS
Wild-type DdRab14 mislocalizes on post-lysosomes in LvsB-null cells
To explore a possible functional interaction between DdRab14 and LvsB, we initially determined whether the localization of wild-type DdRab14 was affected by the loss of LvsB. GFP-DdRab14, a previously characterized reporter of endogenous DdRab14 localization in Dictyostelium (18), was expressed in wild-type and LvsB-null cells. The expression of wild-type GFP-DdRab14 did not alter the size or morphology of endolysosomal vesicles in wild-type or LvsB-null cells (data not shown). In both cell lines GFP-DdRab14 brightly labeled the membranes of the contractile vacuole (Figure 1A–B). This organelle was readily identified by its dynamic contractile behavior in time-lapse movies (data not shown). The presence of DdRab14 on acidic endolysosomal vesicles has been previously demonstrated using immunoelectron microscopy and cell fractionation experiments (15). To determine whether DdRab14 is also localized on the neutral post-lysosomal compartment we stained cells with antibodies against vacuolin, a flotillin-like protein that associates primarily with the post-lysosome (9). We found that wild-type cells contained few vesicles labeled by both GFP-DdRab14 and vacuolin (5.8% of vesicles counted, n=86) (Figure 1C–C″). In contrast, LvsB-null cells contained many large vesicles that were clearly labeled by both GFP-DdRab14 and vacuolin (60% of vesicles counted, n=40) (Figure 1D–D″). Thus LvsB null cells, which suffer from inappropriate lysosomal fusion, also exhibit abnormal colocalization of lysosomal (DdRab14) and post-lysosomal (vacuolin) markers.
Figure 1. Loss of LvsB induces mislocalization of wild-type Rab14 on post-lysosomes but does not affect its localization on the contractile vacuole.
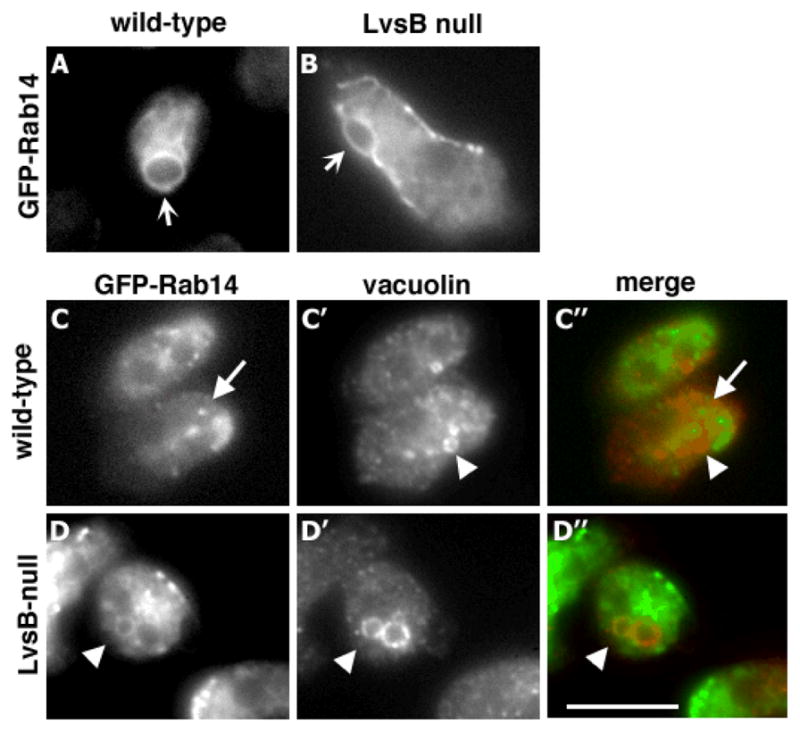
Wild-type and LvsB-null cells were transfected with wild-type GFP-Rab14. The localization of wild-type GFP-Rab14 on the contractile vacuole was indistinguishable in wild-type (A) and LvsB-null cells (B) (arrows). Contractile vacuoles were identified using time-lapse microscopy by their characteristic expanding and contracting behavior (data not shown). Cells expressing GFP-Rab14 were also stained with anti-vacuolin antibody to visualize the post-lysosomal compartment. In wild type cells (C), Rab14 was detected in small vesicles (arrow) that did not colocalize with vacuolin (C′) (arrowhead). In contrast, LvsB null cells contained large vesicles that were labeled by both GFP-Rab14 (D) and vacuolin (D′) (arrowheads). This cell also shows an adjacent vacuolin-labeled post-lysosome that did not contain GFP-Rab14. Merged images are shown in C″ and D″. Focal planes in C & D were different from those in A & B to avoid imaging the contractile vacuoles. Bar,10 μm.
Activation of DdRab14 mimics the phenotype of LvsB-null cells
The abnormal colocalization of DdRab14 and vacuolin in LvsB-null cells may be due to one of two possible scenarios. First, since lysosomes fuse with post-lysosomes in LvsB null cells, it is possible that DdRab14 on the lysosomal membrane is passively mixed with post-lysosomal markers when such heterotypic fusion events occur. Alternatively, the activity of DdRab14, which promotes fusion among lysosomes (18), may be antagonized by LvsB when late lysosomes become post-lysosomes. In this case, loss of LvsB would allow DdRab14 to remain active on a lysosome as it matures into a post-lysosome and would then promote heterotypic fusion between post-lysosomes with earlier compartments. A major difference between these two models is that in one, DdRab14 activity is not responsible for the heterotypic fusion while in the other, DdRab14 is the cause of heterotypic fusion.
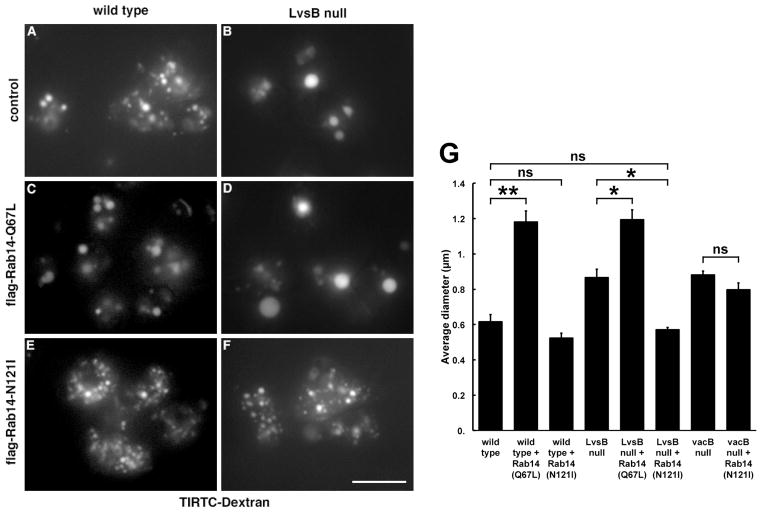
To distinguish between these models we compared the effect of introducing constitutively active DdRab14-(Q67L) into wild-type and LvsB-null cells. We expressed flag-tagged DdRab14-(Q67L) in both cell lines and evaluated the morphology of their entire endolysosomal system by incubating cells with TRITC-dextran for 1 hour (Figure 2A–D). Western blot analysis demonstrated that the flag-DdRab14-(Q67L) protein was equally expressed in both cell lines (data not shown). As reported previously, relative to wild type control cells, LvsB-null cells contained fewer but greatly enlarged dextran-labeled vesicles (Figure 2A, B and G) (6, 8). Similarly, the expression of flag-DdRab14-(Q67L) in wild-type cells caused the enlargement of dextran-labeled vesicles (Figure 2C, G). The expression of DdRab14-(Q67L) in LvsB-null cells caused additional enlargement of their dextran-labeled vesicles to the same diameter as those in wild-type cells expressing DdRab14-(Q67L) (Figure 2D, G).
Figure 2. Expression of mutant forms of Rab14 alters the endolysosomal morphology of wild-type and LvsB-null cells.
Wild-type and LvsB-null cells were transfected with the plasmid for expression of constitutively active flag-Rab14-(Q67L) or constitutively inactive flag-Rab14-(N121I). (A–F) The endosomal morphology was evaluated by incubating with TRITC-dextran for 1 hour. Untransfected wild-type and LvsB-null cells were used as controls. (G) The size of >30 vesicles/experiment were measured from micrographs similar to those shown in A–F and plotted as the mean +/− SEM (n=3). Statistical significance by two-tailed Student’s t-test is indicated among relevant pairs (ns, not significant, P>0.05; *, P< 0.05; **, P< 0.01). Expression of the active form of Rab14-(Q67L) in wild-type cells (C), resulted in the accumulation of significantly enlarged vesicles. Expression of the active form of Rab14-(Q67L) in the LvsB-null cells (D) also caused a significant enlargement of endosomes compared to the vesicles in the LvsB-null untransfected cell line (B). The expression of inactive Rab14-(N121I) did not cause a significant decrease in the size of labeled endosomes in wild-type cells (E). In contrast, Rab14-(N121I) greatly decreased the size of endosomes in LvsB-null cells (F) to a size similar to those found in wild-type cells (A). (G) The size of Dextran labeled endosomes were also measured in vacuolin B mutant cells with or without expression of Rab14-(N121I). Vacuolin B mutant cells contain enlarged endosomes similar to those seen in LvsB null cells. Expression of Rab14-(N121I) in vacuolin B-null cells did not reduce the size of their endosomes to a wild-type size. Thus, while activation of Rab14 in wild-type cells mimics the phenotype of LvsB-null cells, the inactivation of Rab14 suppresses the phenotype of LvsB-null cells but not that of vacuolin B-null cells. These results suggest an antagonistic relationship between LvsB and Rab14 in controlling endolysosomal vesicle size. Bar, 10μm
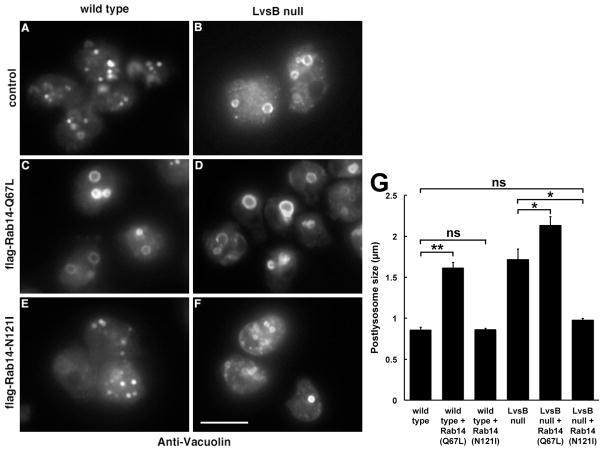
We next determined the effect of DdRab14-(Q67L) expression on the vacuolin-labeled post-lysosome. LvsB null cells contained enlarged vacuolin-labeled vesicles compared to those found in wild type cells (Figure 3A, B, and G). Again, DdRab14-(Q67L) expression caused an enlargement of post-lysosomes in wild type cells to a size comparable to those in LvsB null cells (Figure 3C, G), and a further enlargement in LvsB null cells (Figure 3D, G). Thus, expression of constitutively active DdRab14-(Q67L) in wild type cells seems to replicate the effect of loss of LvsB function suggesting an active role for DdRab14 in promoting heterotypic fusion in LvsB-null cells.
Figure 3. Expression of mutant forms of Rab14 alters the size of vacuolin-labeled post-lysosomes in wild-type and LvsB-null cells.
(A–F) Wild-type and LvsB-null cells expressing mutant forms of Rab14 as in Figure 2 were fixed and stained with antibodies against vacuolin B. (G) The size of >30 vesicles/experiment were measured and plotted as the mean +/− SEM (n=3). Statistical significance by two-tailed Student’s t-test is indicated among relevant pairs (ns, not significant, P>0.05; *, P< 0.05; **, P< 0.01). Expression of the active form of Rab14-(Q67L) caused the enlargement of post-lysosomes in wild-type cells (C) so that they resembled those in LvsB-null cells (B). Active Rab14-(Q67L) slightly increased the size of post-lysosomes in LvsB-null cells (D), compared to those in non-expressing cells (B). On the other hand, while expression of the inactive form of Rab14-(N121I) in wild-type cells did not significantly alter the size of their post-lysosomes (E), its expression drastically reduced the size of post-lysosomes of LvsB-null cells (F) to a size similar to wild-type control (A). Bar, 10μm
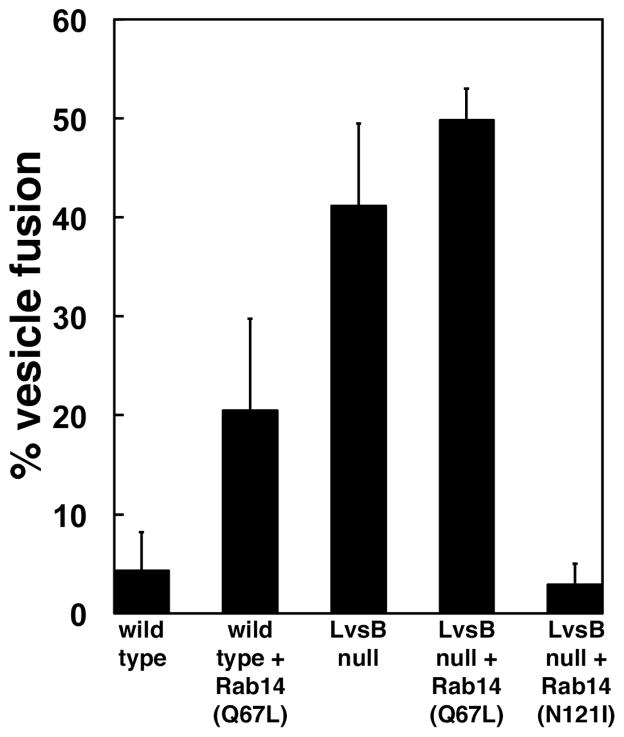
To explore this phenotypic similarity in more detail we tested the effect of active DdRab14-(Q67L) expression on the rates of heterotypic fusion between lysosomes and post-lysosomes (Figure 4). We used an in vivo fusion assay of vesicles labeled by endocytosis of two differently labeled dextrans (6). A 30 minute chase period allows the first dextran pulse to reach the post-lysosomal compartment before the second dextran pulse is internalized by the cells. In wild type cells the early and late compartments remained distinct and, as a result, did not display significant colocalization between the two dextran markers (4.3%, n=310) (Figure 4 and Supplemental Figure 1A). In contrast, the two markers colocalized to a great extent in LvsB null cells (41.2%, n=136) indicating inappropriate heterotypic fusion between early and late labeled vesicles (Figure 4 and Supplemental Figure 1C). Remarkably, the expression of active DdRab14 (Q67L) caused a fivefold increase in the percentage of heterotypic fusion in wild-type cells (20.5%, n=241) but only a slight increase in LvsB-null cells (49.8%, n=130) (Figure 4 and Supplemental Figure 1B and D).
Figure 4. Expression of mutant forms of Rab14 influences the fusion of early and late endocytic vesicles in wild-type and LvsB-null cells.
Cells were given a pulse of FITC dextran followed by a 30 minute chase and then a second pulse with TRITC dextran. The fusion of endosomal vesicles was determined by colocalization of the two differently labeled dextrans as shown in Supplemental Figure 1. The fraction of vesicles containing both fluid phase markers were quantified in two independent experiments and shown as the mean +/− range. Expression of the active form of Rab14-(Q67L) caused a five-fold increase in the heterotypic fusion in wild-type cells but not in LvsB-null cells. Significantly, the expression of the inactive form of Rab14-(N121I) suppressed the heterotypic fusion shown in LvsB-null cells.
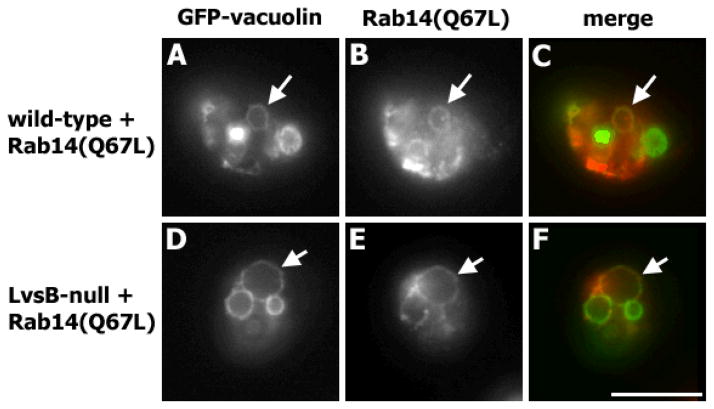
Lastly, we determined whether the active DdRab14-(Q67L) protein remained associated with post-lysosomes, thereby affecting heterotypic fusion. We visualized the localization of active DdRab14-(Q67L) in cells co-expressing flag-DdRab14-(Q67L) and GFP-vacuolin B. Active DdRab14-(Q67L) localized mainly on the contractile vacuole membranes in both wild-type and LvsB-null cells (data not shown). Unlike wild-type DdRab14, we found that active DdRab14-(Q67L) colocalized with vacuolin on post-lysosomes of wild type cells (Figure 5, A–C). This localization could account for the increase in heterotypic fusion in wild type cells expressing active DdRab14-(Q67L). As expected, in LvsB-null cells the active form of DdRab14-(Q67L) was also found on post-lysosomes (Figure 5, D–F).
Figure 5. The active form of Rab14-(Q67L) associates with post-lysosomes in wild-type and LvsB-null cells.
Wild-type and LvsB-null cells were transfected with GFP-Vacuolin B and flag-Rab14-(Q67L) and stained with anti-flag monoclonal antibody. Active Rab14-(Q67L) colocalized with vacuolin B in both wild-type (A – C) and LvsB-null (D – F) cells (arrows). Bar, 10μm.
Taken together, these results show that enhanced activation of DdRab14 promotes heterotypic fusion between early and late endolysosomal compartments leading to the formation of grossly enlarged hybrid vesicles, a phenotype identical to that observed in LvsB null cells.
Inactivation of DdRab14 suppresses the lysosomal defect of LvsB-null cells
Our data suggested that the phenotype of LvsB null cells could be caused by the inability to inactivate DdRab14 at the appropriate time during the maturation of lysosomes into post-lysosomes. If this scenario is correct, then we would expect that lowering DdRab14 activity should compensate for the defect of LvsB null cells. To test this idea we examined the effect of expressing constitutively inactive DdRab14-(N121I) on the phenotype of wild type and LvsB-null cells. Western blot analysis confirmed that the flag-DdRab14-(N121I) protein was expressed at similar levels in both cell lines and staining with anti-flag monoclonal antibodies showed that the protein had the expected cytosolic localization (data not shown). We then determined the morphology of dextran-labeled vesicles and vacuolin-stained post-lysosomes in these cell lines.
Remarkably, expression of inactive DdRab14-(N121I) was able to completely suppress the phenotype of LvsB null cells. Both the morphology of their dextran labeled vesicles (Figure 2F, G) and the size of their vacuolin-labeled post-lysosomes (Figure 3F, G) were similar to those observed in wild type cells (Figure 2A, G and 3A, G). Furthermore, expression of inactive DdRab14-(N121I) in LvsB null cells caused a dramatic reduction in their rates of heterotypic fusion to only 2.9% (n=326), close to that observed in wild-type controls (Figure 4 and Supplemental Figure 1E). In contrast, expression of DdRab14-(N121I) caused only a small change in the size of dextran-labeled vesicles in wild-type cells (Figure 2E, G), and did not seem to affect their post-lysosome size (Figure 3E, G). Therefore, the reduction of DdRab14 activity in LvsB null cells restored normal size and segregation of their lysosomes and post-lysosomes.
The phenotype of LvsB-null cells is not suppressed by changes in DdRab7 activity
While our results show that LvsB antagonizes the function of Rab14, it is possible that LvsB has a broad role in regulating the activity of Rab GTPases along the endocytic pathway. Of the Dictyostelium Rab GTPases that have been characterized, Rab7 emerged as the best candidate for investigating this possibility. Dictyostelium Rab7 is known to localize to lysosomes, post-lysosomes, and phagosomes (17). This localization pattern is similar to that of LvsB, and we previously showed that Rab7 colocalizes with LvsB on post-lysosomal vesicles (6). In contrast to DdRab14, expression of constitutively active DdRab7-(Q67L) causes a reduction in the size of acidic vesicles by promoting the retrograde traffic from late lysosomes to earlier compartments (17). Consistent with this, dominant negative DdRab7-(T22N) blocks retrograde traffic and causes an enlargement of acidic lysosomal compartments (17). This enlargement of acidic compartments is reminiscent of both the LvsB null, and dominant active Rab14 phenotypes, though the reported causal defect is different (Harris, 2002a & 2002b). Based on these localization and phenotypic similarities, we wanted to test the possibility of a functional interaction between Rab7 and LvsB. Therefore, we compared the effect of activating and inactivating mutations of DdRab7 on the phenotype of LvsB null cells and contrasted those with our previous observations using DdRab14 mutants. Figure 6A shows the size of dextran-labeled vesicles in LvsB null cells expressing the different Rab mutant proteins. In contrast to the suppression of the LvsB null phenotype caused by inactive DdRab14-(N121I), neither active DdRab7-(Q67L) nor inactive DdRab7-(T22N) reduced the size of dextran labeled vesicles in these cells. As expected, impairing retrograde traffic with inactive DdRab7-(T22N) caused a further enlargement of the dextran-labeled vesicles in LvsB null cells. Importantly, the enhancement of retrograde traffic with DdRab7-(Q67L) did not suppress the large endosomal phenotype of LvsB null cells. This suggests that the functional interaction between LvsB and Rab14 is specific to these two proteins.
Figure 6. Changes in DdRab7 activity do not suppress the defects of LvsB-null cells.
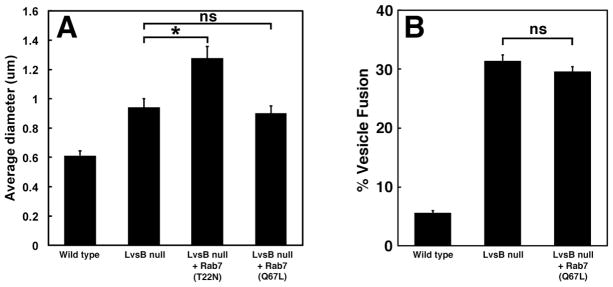
(A) Mutant forms of DdRab7 do not suppress the enlarged endosome phenotype of LvsB-null cells. LvsB-null cells expressing mutant forms of DdRab7 were labeled with TRITC-dextran as in Figure 2. The size of >30 vesicles/experiment were measured and plotted as the mean +/− SEM of at least three experiments (ns, not significant, P>0.05; *, P< 0.05). In contrast to the suppression of the LvsB null phenotype by the inactive form of Rab14-(N121I) (Figure 2), neither activating nor inactivating mutants of DdRab7 caused any reduction in the size of LvsB null endosomes. (B) Expression of active DdRab7 does not suppress the abnormal fusion of early and late endocytic vesicles in LvsB null cells. The fusion of early and late endosomal vesicles was determined by colocalization of two differently labeled dextrans as described in Figure 4 and plotted as the mean +/− SEM as in (A). Expression of the active form of DdRab7-(Q67L) did not alter the high rate of heterotypic fusion in LvsB-null cells.
These results support the model that, in contrast to DdRab14 and LvsB, DdRab7 does not control the fusion between lysosomes and post-lysosomes. Since the active and inactive mutations of Rab7 produce effects opposite to those of the corresponding Rab14 mutations we wanted to ask if the dominant active form of DdRab7-(Q67L) could suppress the inappropriate fusion phenotype of LvsB null cells. Indeed, using the in vivo fusion assay we found that expression of DdRab7-(Q67L) did not suppress the high rate of heterotypic vesicle fusion in LvsB null cells (Figure 6B).
Taken together our data indicates that the functional interaction between LvsB and DdRab14 seems specific to these two proteins and does not extend to additional GTPases like DdRab7.
Inactivation of DdRab14 does not suppress the post-lysosomal defects of Vacuolin-null cells
The suppression of the LvsB null phenotype by expression of inactive DdRab14-(N121I) is consistent with our hypothesis that LvsB acts as an antagonist to DdRab14. However, it is also possible that expression of DdRab14-(N121I) causes a general block of membrane fusion that would reduce the size of all endolysosomal vesicles in LvsB null cells. To distinguish between these possibilities we determined the effect of inactive DdRab14-(N121I) expression in vacuolin B-null cells. Vacuolin B mutant cells display an enlarged post-lysosomal morphology similar to that observed in LvsB null cells (10). However, unlike LvsB null cells, the enlarged post-lysosome phenotype of vacuolin B null cells arises from a defect in the exocytosis of post-lysosomes. We found that expression of DdRab14-(N121I) caused only a minimal reduction in the size of dextran-labeled vesicles in vacuolin B null cells (Figure 2G). This decrease in size was of similar magnitude to that caused by DdRab14-(N121I) expression in wild type cells and quite different from its effect in LvsB null cells. Hence, expression of DdRab14-(N121I) specifically suppresses the phenotype of LvsB null cells supporting the notion that LvsB is an antagonist of DdRab14.
The observation that DdRab14-(N121I) did not suppress the phenotype of vacuolin B null cells implies that the enlarged post-lysosomes in these cells do not experience heterotypic fusion as it occurs in LvsB null cells. Indeed, using the two-dextran fusion assay we found that heterotypic fusion occurs with a low frequency in vacuolin B null cells (4.9%) similar to wild type cells (3.3%). In addition, staining with lysotracker, a probe that accumulates in acidic compartments, showed that vacuolin B null cells contain small acidic lysosomes similar to those in wild type cells (Supplemental Figure 2). Therefore, the enlargement of post-lysosomes in vacuolin B and LvsB null mutants arise by different mechanisms. In vacuolin B null cells they arise by a defect in exocytosis and in LvsB null cells they arise by a DdRab14-mediated pathway.
LvsB does not control fusion of post-lysosomes with early endosomes or with the contractile vacuole
While we have shown that LvsB inhibits fusion between lysosomes and post-lysosomes, it is possible that LvsB also controls fusion of post-lysosomes with other compartments. This possibility is particularly relevant for early endosomes and the contractile vacuole since they also contain DdRab14 (15, 16, 18).
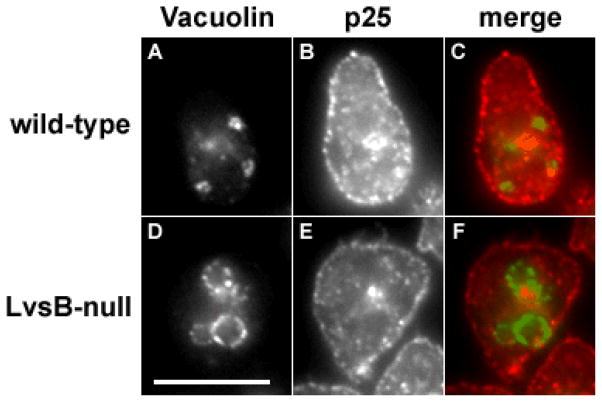
To test the possibility that post-lysosomes fuse with early endosomes in LvsB null cells we tested the localization of the integral membrane protein p25, a marker for the plasma membrane and early endosomes. The protein p25 is internalized together with endocytic cargo in early endosomes, and shortly retrieved to a recycling compartment before recycling back to the plasma membrane (19). Accordingly, p25 is absent from lysosomes and post-lysosomes in wild type cells. We immunolocalized p25 in wild type and LvsB-null cells expressing GFP-Vacuolin B (Figure 7). In both cell lines p25 localized normally to the plasma membrane and the recycling compartment and did not colocalize with vacuolin B. This result suggests that early endosomes do not fuse inappropriately with post-lysosomes in the absence of LvsB.
Figure 7. Early endocytic traffic is not impaired in the absence of LvsB.
Control (A – C) and LvsB-null cells (D –F) expressing GFP-Vacuolin B were fixed and stained with antibodies against p25. p25 is internalized together with endocytic cargo in early endosomes, then retrieved to a recycling compartment before final recycling back to the plasma membrane (19). In control cells, p25 localized on the plasma membrane and the juxtanuclear recycling compartment and did not colocalize with vacuolin on post-lysosomes. Similarly, p25 did not colocalize with vacuolin in the LvsB-null cells. This result suggests that early endosomes and post-lysosomes did not fuse in the absence of LvsB. Bar, 10μm.
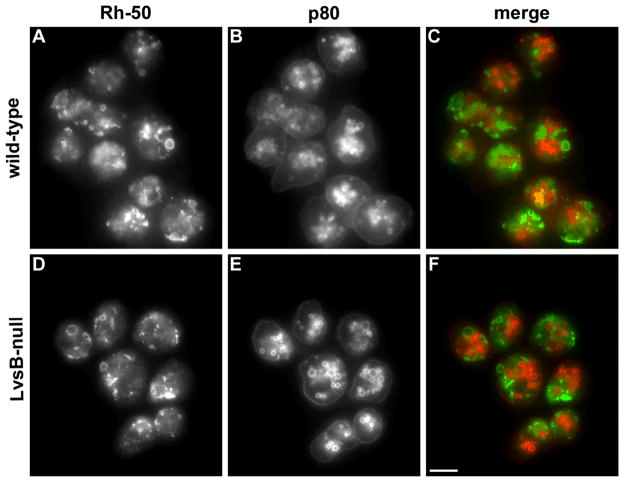
We also explored whether LvsB may have a role in restricting the fusion of endolysosomal vesicles with the membranes of the contractile vacuole. The Dictyostelium contractile vacuole is a dynamic osmoregulatory organelle composed of a reticular network of tubules and bladders. As mentioned before, DdRab14 localizes prominently on the membranes of the contractile vacuole and could potentially regulate traffic between lysosomes and the CV. In fact, the contractile vacuole and the lysosomal compartment share several other markers including golvesin (20), the vacuolar-ATPase (21) and SNARE proteins (22). We determined the distribution of Rh50, a resident integral membrane protein of the contractile vacuole, and p80, an integral membrane protein found on lysosomes and post-lysosomes in our different cell lines. We did not find any colocalization of these markers in either wild type or LvsB-null cells, indicating that the two compartments remained distinct in both cell types (Figure 8). We also found that expression of constitutively active DdRab14-(Q67L) in wild-type cells did not induce colocalization of Rh50 and p80, demonstrating that the post-lysosome and the contractile vacuole did not fuse (data not shown).
Figure 8. The absence of LvsB does not cause inappropriate fusion between endosomes and the contractile vacuole.
Control and LvsB-null cells were fixed and stained with antibodies against Rh50, a contractile vacuole marker, and p80, a marker for lysosomes and post-lysosomes (36, 37). In both wild-type (A, B) and LvsB-null cells (C, D), Rh50 is distributed normally on the membranes of the contractile vacuole and p80 distributed normally on the plasma membrane and endocytic vesicles. Thus, the lack of LvsB did not cause inappropriate fusion between endosomes and the contractile vacuole. Bar, 10 μm.
DISCUSSION
While the protein Lyst, defective in patients with Chediak Higashi Syndrome, has been implicated in lysosomal trafficking, the mechanism by which it contributes to lysosomal function is not known. Previous studies indicated that the Dictyostelium Lyst orthologue, LvsB, is a negative regulator of heterotypic fusion, repressing the fusion of lysosomes with post-lysosomes (6).
We have shown here three lines of evidence that suggest LvsB acts as a functional antagonist for the GTPase DdRab14. First, DdRab14 is mislocalized to post-lysosomes in LvsB null cells. Second, activation of DdRab14 in wild type cells causes the same heterotypic fusion phenotype observed in LvsB null cells. Third, inactivation of DdRab14 suppresses the mutant phenotype of LvsB null cells. In addition, we have shown that the function of LvsB is restricted to controlling the fusion between lysosomes and post-lysosomes since loss of LvsB does not affect other cellular compartments.
While it may be possible to reinterpret some of our observations as the result of defects in lysosomal fission we believe that the sum of studies published thus far best support a role for LvsB in controlling lysosomal fusion. This includes an increase in the fusion of phagosomes containing bacteria or plastic beads (8), mixing of dextran-labeled endosomes after 2 or 30 minute chase times (6, 8), and the presence of both lysosomal and postlysosomal markers on vesicles in LvsB null cells (6, 8). Comparison of the phenotype of LvsB null cells with that of mutants in fission events such as AP3-null cells (19) may provide further insight into the role of LvsB in lysosomal function.
Our studies were prompted by previous reports that Dictyostelium DdRab14 is localized on lysosomes and promotes the fusion of lysosomes when activated. We have extended those studies by showing that DdRab14 is absent from post-lysosomes since it does not colocalize with the post-lysosomal marker vacuolin. These results indicate that DdRab14 is present exclusively during the lysosomal stage consistent with a role in promoting homotypic fusion among lysosomes (18). In order for a lysosome to mature into a post-lysosome it has to cease fusion with other lysosomes to allow for the removal of proton pumps and lysosomal hydrolases (23). Those pumps and enzymes are then recycled by delivery to newly internalized endosomes. We propose that the inactivation and removal of DdRab14 from lysosomes is necessary to allow them to mature into post-lysosomes.
We showed previously that the localization of LvsB on the lysosome coincides precisely with this transition phase to a post-lysosome (6). Consequently, LvsB arrives on the lysosome at the right time to antagonize DdRab14 and allow post-lysosomal maturation. Here we have shown that in LvsB-null cells DdRab14 colocalizes with the post-lysosomal marker vacuolin. One interpretation of this result is that in the absence of LvsB, DdRab14 is not antagonized and remains on the lysosome as it begins to mature into a post-lysosome. The presence of active DdRab14 on the post-lysosome would then allow it to fuse inappropriately with an earlier lysosome. Consistent with this interpretation we found that constitutive activation of DdRab14 in wild type cells causes a phenotype indistinguishable from that of LvsB null cells, including an increase in heterotypic fusion, enlargement of post-lysosomes, and the presence of DdRab14(Q67L) on post-lysosomes. Expression of DdRab14(Q67L) in LvsB null cells increases further the activity of DdRab14 in these cells causing a concomitant increase in post-lysosome size.
In support of this model we also showed that inactivation of DdRab14 by overexpression of dominant negative DdRab14 blocks heterotypic fusion and therefore suppresses the phenotype of LvsB null cells. This effect is specific to DdRab14 since alterations in DdRab7 activity did not suppress the LvsB null phenotype. Importantly, the dominant negative DdRab14 construct does not affect the size of post-lysosomes in wild-type cells or in vacuolin-null cells since, according to our model, DdRab14 does not contribute to the regulation of post-lysosomal function. Since inactivation of DdRab14 seems crucial for the maturation of lysosomes into post-lysosomes it would imply that a RabGAP protein may also be important for this process. The Dictyostelium genome encodes 25 putative RabGAP proteins that have not been characterized in detail. It will be interesting to determine whether one of these proteins is involved in the interaction between DdRab14 and LvsB shown here.
While DdRab14 is found on lysosomes, it is most prominently observed on the contractile vacuole (15, 16, 18). In addition to DdRab14, lysosomes and contractile vacuoles also share other proteins, including SNAREs and proton pumps. These similarities suggest that vesicle traffic may occur between these compartments (22, 24). In fact, it has been suggested that the vacuolar proton pumps are delivered to the endosomal pathway through the contractile vacuole system (25). More importantly, another study showed that contractile vacuole markers can traffic to the contractile vacuole membranes via early endosomes and the recycling compartment (26). Hence, it would seem possible that LvsB may also be involved in restricting the fusion of post-lysosomes with these compartments. However, we have shown that this is not the case. We did not detect any fusion of post-lysosomes with contractile vacuole membranes or with early/recycling endosomes in the LvsB null cell line. These results further support that LvsB functions specifically in regulating fusion between lysosomes and post-lysosomes.
While the distribution of Lyst has not been determined in any metazoan it seems likely that, like LvsB, Lyst may be localized on lysosomes and lysosome-related organelles. Some of the tissues most drastically affected in patients with Chediak Higashi Syndrome are those that form lysosome-related organelles, such as melanosomes and secretory lysosomes. These organelles undergo a process of maturation that may be analogous to the maturation of the post-lysosome in Dictyostelium. For example, secretory lysosomes mature from lysosomes upon stimulation of cytotoxic T lymphocytes. In lymphocytes from CHS patients the initial formation of lysosomes is normal but subsequent fusion of lysosomes is abnormally high in vivo causing the enlargement of secretory lysosomes (27). A later study using mast cells and pancreatic acinar cells also indicated a role for Lyst in regulating the fusion of secretory vesicles (28). However, a recent study in fibroblasts and macrophages demonstrated that a defect in lysosomal fission was responsible for lysosomal enlargement in CHS mutant cells (29). Clearly, we are far from understanding the molecular function of Lyst and related proteins. It is possible that the different results from these papers are due to the different cell types used. It is also possible that Lyst plays a role in both fusion and fission events and that the different assays used in these studies highlight one role over the other. To resolve this question it may be necessary to use these different assays to compare the phenotype of Lyst mutants with that of cells defective in known components of fusion and fission machineries.
Based on our results we hypothesize that, in analogy with Dictyostelium LvsB, mammalian Lyst may regulate lysosomal fusion by antagonizing the activity of a lysosomal Rab protein. This antagonist function may be a general mechanism of action for other Lyst-related proteins. Lyst and LvsB are members of the BEACH family of proteins with representatives in all eukaryotes. Among them, the Drosophila BEACH protein Bchs antagonizes the activity of Rab11 in membrane trafficking during development (30). In Dictyostelium, a functional link was observed between the BEACH protein LvsA and the contractile vacuole GTPase, Rab8a, (31). In addition, the nematode BEACH protein SEL-2 is a negative regulator of membrane traffic during Notch signaling (32). However, the mechanism by which a BEACH protein, like LvsB, could antagonize the activity of a specific Rab, like DdRab14, has yet to be discovered. An analysis of proteins co-precipitating with TAP-tagged LvsB did not reveal any potential RabGAP or other regulator of membrane traffic (33). Unfortunately, none of the BEACH-related proteins have yet been identified in any of the interactome databases from various organisms. A yeast 2-hybrid screen with human Lyst protein did identify a large number of potential interacting proteins, but none of them have been confirmed in physiological conditions (34). Importantly, none of those potential interacting proteins could explain the antagonism with Rab proteins. We believe that understanding the mechanism of action of LvsB/Lyst is important not just for a better understanding of the Chediak-Higashi Syndrome, but for the discovery of novel mechanisms of membrane traffic regulation.
MATERIALS & METHODS
Strains and culture
Dictyostelium discoideum cells were grown on Petri dishes at 18°C in axenic HL-5 medium supplemented with 0.6% penicillin-streptomycin (GIBCO BLR, Gaithersburg, MD). Mutant or transfected cell lines were grown in medium supplemented with 5 μg/ml blasticidin (Calbiochem, EMD Biosciences, Inc. La Jolla, CA) or 10 μg/ml G418 (Geneticin, Gibco, BRL, Grand Island, NY, USA). Wild type strain NC4A2 was used as control and for the expression of the different constructs used here. LvsB-null cell line (clone B1B11) was derived from NC4A2 (8). Vacuolin B-null cell line was derived from AX2 and was a gift from Dr. Markus Maniak (Universitaet Kassel, Germany) (10).
Expression plasmids and antibodies
The GFP-vacuolin B vector was kindly provided by Dr. Maniak (Universitaet Kassel, Germany) (10). The wild-type GFP-Rab14 and mutant GFP-Rab7Q67L as well as the mutant DdRab14Q67L, DdRab14N121I, and DdRab7T22N template vectors were kindly provided by Dr. Cardelli (LSU Med. Ctr., LA) (14,1997 paper) (17, 18). We then constructed the pTXflag-DdRab14Q67L and pTXflag- DdRab14N121I vectors by amplifying the corresponding coding regions and cloning them into the pTX-flag expression vector (35) at the BamHI and XbaI sites. Similarly, we amplified the coding regions of wild-type DdRab7 and DdRab7T22N and cloned them into the pTX-GFP expression vector at the BamHI and XbaI sites.
Anti-vacuolin monoclonal antibody 264-79-2 (postlysosomes) was a kind gift from Dr. Maniak (10). Monoclonal anti-Flag antibody (M2) was purchased from Sigma-Aldrich, St. Louis, MO. Monoclonal antibodies against p25 (H72) (early endosomes), p80 (H161) (lysosomes and postlysosomes) and polyclonal anti-RH50 (contractile vacuole) were a kind gift from Dr. Pierre Cosson (Centre Medical Universitaire de Geneve, Switzerland) (36, 37).
Endosome Labeling
Visualization of the endolysosomal system of the different cell lines was done by live microscopy of labeled cells. Cells (1×106 cells/ml) were allowed to adhere to well chambers (Nalge-Nunc Int., Rochester, NY) and were incubated in low fluorescence medium (http://dictybase.org/techniques/media/lowflo_medium.html) for 1 hour at room temperature. Cells were then incubated for 1 hour with 1mg/ml TRITC-dextran (mw 64kDa; Sigma- Aldrich Inc. St Louis, MO, USA) diluted in low fluorescence medium. Cells were washed twice with the low fluorescence medium and were imaged immediately on an inverted Nikon fluorescence microscope.
Immunolocalization and cell imaging
Cells (2×106 cells/ml) were allowed to attach on coverslips for 15 minutes at 18 °C and washed briefly with PDF buffer (2mM KCl, 1.1 mM K2HPO4, 1.32 mM KH2P04, 0.1mM CaCl2, 0.25mM MgSO4, pH 6.7) and then overlaid with a thin layer of 2% PCR agarose (BioRad, Hercules, California). Cells were then fixed with 1% formaldehyde in methanol for 5 minutes at −20°C followed by a wash with phosphate-buffered saline (PBS), rinsed briefly with distilled water and mounted on microscope slides with mounting media (MOWIOL, Calbiochem, EMD Biosciences Inc. La Jolla, CA). The slides were allowed to dry in the dark and analyzed.
For immunolocalization studies, primary antibody was added to the fixed cells and incubated for 1 hour at 37°C in the dark. Cells were washed four times with PBS and incubated with Texas-Red conjugated goat-anti mouse antibody or Texas-Red conjugated goat anti-rabbit or FITC conjugated goat anti-mouse (30μg/ml; Molecular Probes, Eugene, OR) for 1 hour at 37°C in the dark. Cells were washed four times with PBS, rinsed briefly in ddH2O and mounted on microscope slides as described above.
Cells were imaged on an inverted Nikon Microscope TE200 (Nikon Instruments, Dallas, TX, USA). GFP, Texas Red and DAPI filters were used. Images were acquired on a Photometrics cooled CCD camera and processed using Metamorph software. Vesicle sizes were measured using ImageJ software.
Endosome fusion assay using two fluid phase markers
Cells (1×106 cells/ml) were allowed to adhere to well chambers and were incubated in low fluorescence medium, as described above. Cells were labeled with a 5 minute pulse of 4mg/ml FITC-dextran (mw 77kDa; Sigma- Aldrich Inc. St Louis, MO, USA) diluted in low florescence medium. Cells were then briefly rinsed two times in low fluorescence medium followed by a chase for 30 minutes. Subsequently cells were labeled with a 5 minute pulse of 2mg/ml TRITC-dextran diluted in low fluorescence medium, washed two times in low fluorescence media and visualized immediately. Images of living cells in different fields were taken continuously for 10 minutes in both the GFP and Texas Red Filters. Images were compared to determine the fraction of vesicles that contained only one label (red or green) compared with those that contained both labels (yellow) as a result of fusion of two different labeled vesicles.
Supplementary Material
Acknowledgments
We would like to thank the members of the De Lozanne and O’Halloran labs for their comments and help throughout the development of this project. We extend our thanks to Dr. James Cardelli (Louisiana State University Health Sciences Center) for providing the DdRab14 and DdRab7 constructs and to Dr. Markus Maniak (Universitaet Kassel, Germany) for providing Vacuolin B mutant cells, and anti-vacuolin antibodies, and to Pierre Cosson (Centre Medical Universitaire de Geneve, Switzerland) for anti-p25, anti-p80 and anti-Rh50 antibodies. This research was supported by grants from the National Institutes of Health (GM48745 and GM)
References
- 1.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 2.Yu IM, Hughson FM. Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol. 2010;26:137–156. doi: 10.1146/annurev.cellbio.042308.113327. [DOI] [PubMed] [Google Scholar]
- 3.Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 4.Ward DM, Griffiths GM, Stinchcombe JC, Kaplan J. Analysis of the lysosomal storage disease Chediak-Higashi Syndrome. Traffic. 2000;1(11):816–822. doi: 10.1034/j.1600-0854.2000.011102.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Curr Opin Hematol. 2008;15(1):22–29. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
- 6.Kypri E, Schmauch C, Maniak M, De Lozanne A. The BEACH protein LvsB is localized on lysosomes and postlysosomes and limits their fusion with early endosomes. Traffic. 2007;8:774–783. doi: 10.1111/j.1600-0854.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 7.Cornillon S, Dubois A, Brückert F, Lefkir Y, Marchetti A, Benghezal M, De Lozanne A, Letourneur F, Cosson P. Two members of the beige/CHS (BEACH) family are involved at different stages in the organization of the endocytic pathway in Dictyostelium. J Cell Sci. 2002;115:737–744. doi: 10.1242/jcs.115.4.737. [DOI] [PubMed] [Google Scholar]
- 8.Harris E, Wang N, Wu W-l, Weatherford A, De Lozanne A, Cardelli J. Dictyostelium LvsB Mutants Model the Lysosomal Defects Associated with Chediak-Higashi Syndrome. Mol Biol Cell. 2002;13:656–669. doi: 10.1091/mbc.01-09-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauchenberger R, Hacker U, Murphy J, Niewohner J, Maniak M. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr Biol. 1997;7(3):215–218. doi: 10.1016/s0960-9822(97)70093-9. [DOI] [PubMed] [Google Scholar]
- 10.Jenne N, Rauchenberger R, Hacker U, Kast T, Maniak M. Targeted gene disruption reveals a role for vacuolin B in the late endocytic pathway and exocytosis. J Cell Sci. 1998;111:61–70. doi: 10.1242/jcs.111.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3(2):122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- 12.Charette SJ, Cosson P. A LYST/beige homolog is involved in biogenesis of Dictyostelium secretory lysosomes. J Cell Sci. 2007;120(Pt 14):2338–2343. doi: 10.1242/jcs.009001. [DOI] [PubMed] [Google Scholar]
- 13.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurana T, Brzostowski JA, Kimmel AR. A Rab21/LIM-only/CH-LIM complex regulates phagocytosis via both activating and inhibitory mechanisms. Embo J. 2005;24(13):2254–2264. doi: 10.1038/sj.emboj.7600716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush J, Nolta K, Rodriguez-Paris J, Kaufmann N, O’Halloran T, Ruscetti T, Temesvari L, Steck T, Cardelli J. A Rab4-like GTPase in Dictyostelium discoideum colocalizes with V-H(+)-ATPases in reticular membranes of the contractile vacuole complex and in lysosomes. J Cell Sci. 1994;107(Pt 10):2801–2812. doi: 10.1242/jcs.107.10.2801. [DOI] [PubMed] [Google Scholar]
- 16.Bush J, Temesvari L, Rodriguez-Paris J, Buczynski G, Cardelli J. A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol Biol Cell. 1996;7(10):1623–1638. doi: 10.1091/mbc.7.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buczynski G, Bush J, Zhang L, Rodriguez-Paris J, Cardelli J. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol Biol Cell. 1997;8(7):1343–1360. doi: 10.1091/mbc.8.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris E, Cardelli J. RabD, a Dictyostelium Rab14-related GTPase, regulates phagocytosis and homotypic phagosome and lysosome fusion. J Cell Sci. 2002;115(Pt 18):3703–3713. doi: 10.1242/jcs.00050. [DOI] [PubMed] [Google Scholar]
- 19.Charette SJ, Mercanti V, Letourneur F, Bennett N, Cosson P. A role for adaptor protein-3 complex in the organization of the endocytic pathway in dictyostelium. Traffic. 2006;7(11):1528–1538. doi: 10.1111/j.1600-0854.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider N, Schwartz JM, Kohler J, Becker M, Schwarz H, Gerisch G. Golvesin-GFP fusions as distinct markers for Golgi and post-Golgi vesicles in Dictyostelium cells. Biol Cell. 2000;92(7):495–511. doi: 10.1016/s0248-4900(00)01102-3. [DOI] [PubMed] [Google Scholar]
- 21.Temesvari LA, Rodriguez-Paris JM, Bush JM, Zhang L, Cardelli JA. Involvement of the vacuolar proton-translocating ATPase in multiple steps of the endo-lysosomal system and in the contractile vacuole system of Dictyostelium discoideum. J Cell Sci. 1996;109(Pt 6):1479–1495. doi: 10.1242/jcs.109.6.1479. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Stavrou I, Bersuker K, Brady RJ, De Lozanne A, O’Halloran TJ. AP180-mediated trafficking of Vamp7B limits homotypic fusion of Dictyostelium contractile vacuoles. Mol Biol Cell. 2009;20(20):4278–4288. doi: 10.1091/mbc.E09-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniak M. Fusion and fission events in the endocytic pathway of Dictyostelium. Traffic. 2003;4(1):1–5. doi: 10.1034/j.1600-0854.2003.40101.x. [DOI] [PubMed] [Google Scholar]
- 24.Clarke M, Kohler J, Arana Q, Liu T, Heuser J, Gerisch G. Dynamics of the vacuolar H(+)-ATPase in the contractile vacuole complex and the endosomal pathway of Dictyostelium cells. J Cell Sci. 2002;115(Pt 14):2893–2905. doi: 10.1242/jcs.115.14.2893. [DOI] [PubMed] [Google Scholar]
- 25.Padh H, Lavasa M, Steck TL. Endosomes are acidified by association with discrete proton-pumping vacuoles in Dictyostelium. J Biol Chem. 1991;266(9):5514–5520. [PubMed] [Google Scholar]
- 26.Mercanti V, Blanc C, Lefkir Y, Cosson P, Letourneur F. Acidic clusters target transmembrane proteins to the contractile vacuole in Dictyostelium cells. J Cell Sci. 2006;119(Pt 5):837–845. doi: 10.1242/jcs.02808. [DOI] [PubMed] [Google Scholar]
- 27.Stinchcombe JC, Page LJ, Griffiths GM. Secretory lysosome biogenesis in cytotoxic T lymphocytes from normal and Chediak Higashi syndrome patients. Traffic. 2000;1(5):435–444. doi: 10.1034/j.1600-0854.2000.010508.x. [DOI] [PubMed] [Google Scholar]
- 28.Hammel I, Lagunoff D, Galli SJ. Regulation of secretory granule size by the precise generation and fusion of unit granules. J Cell Mol Med. 2010;14(7):1904–1916. doi: 10.1111/j.1582-4934.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durchfort N, Verhoef S, Vaughn MB, Shrestha R, Adam D, Kaplan J, Ward DM. The enlarged lysosomes in beige j cells result from decreased lysosome fission and not increased lysosome fusion. Traffic. 2012;13(1):108–119. doi: 10.1111/j.1600-0854.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khodosh R, Augsburger A, Schwarz TL, Garrity PA. Bchs, a BEACH domain protein, antagonizes Rab11 in synapse morphogenesis and other developmental events. Development. 2006;133(23):4655–4665. doi: 10.1242/dev.02650. [DOI] [PubMed] [Google Scholar]
- 31.Essid M, Gopaldass N, Yoshida K, Merrifield C, Soldati T. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Mol Biol Cell. 2012;23(7):1267–1282. doi: 10.1091/mbc.E11-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza N, Vallier LG, Fares H, Greenwald I. SEL-2, the C. elegans neurobeachin/LRBA homolog, is a negative regulator of lin-12/Notch activity and affects endosomal traffic in polarized epithelial cells. Development. 2007;134:691–702. doi: 10.1242/dev.02767. [DOI] [PubMed] [Google Scholar]
- 33.Kypri E. PhD Thesis. Austin: University of Texas at Austin; 2007. Study of LvsB in Dictyostelium discoideum provides insights into the Chediak-Higashi syndrome. [Google Scholar]
- 34.Tchernev VT, Mansfield TA, Giot L, Kumar AM, Nandabalan K, Li Y, Mishra VS, Detter JC, Rothberg JM, Wallace MR, Southwick FS, Kingsmore SF. The Chediak-Higashi protein interacts with SNARE complex and signal transduction proteins. Mol Med. 2002;8(1):56–64. [PMC free article] [PubMed] [Google Scholar]
- 35.Levi S, Polyakov M, Egelhoff TT. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid. 2000;44(3):231–238. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- 36.Ravanel K, de Chassey B, Cornillon S, Benghezal M, Zulianello L, Gebbie L, Letourneur F, Cosson P. Membrane sorting in the endocytic and phagocytic pathway of Dictyostelium discoideum. Eur J Cell Biol. 2001;80(12):754–764. doi: 10.1078/0171-9335-00215. [DOI] [PubMed] [Google Scholar]
- 37.Benghezal M, Gotthardt D, Cornillon S, Cosson P. Localization of the Rh50-like protein to the contractile vacuole in Dictyostelium. Immunogenetics. 2001;52(3–4):284–288. doi: 10.1007/s002510000279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.