Introduction
Obesity is a global health issue, with 315 million adults are classified as obese, defined as a body mass index (BMI) of ≥ 30 kg/m2.1, 2 Both children3 and the elderly4 are susceptible to obesity. Significant progress in the medical management of the metabolic symptoms related with obesity, has increased the lifespan of the obese individual.5 There is a tradeoff with longevity in the aging obese person, as the musculoskeletal system must bear the burden of carrying excessive weight over the person’s lifespan. As BMI values increase, joint pain symptoms and severity increase.6 Joint pain may reflect the underlying pathological process of osteoarthritis (OA). For every 5kg weight gain, there is a commensurate 36% increased risk for developing OA.1 In obese individuals, pain is most prevalent in the load-bearing joints including the lower limb and the low back,6, 7 but can manifest in upper extremity joints, hand and digits,8 thoracic spine and neck. In addition, cadaveric studies have revealed that obesity is related to greater knee OA severity than in normal weight individuals.9 Also, obesity is associated with faster OA progression than normal weight. Pain-related physical incapacitation worsens obesity, subsequent gait abnormalities and muscle weakness.10 Importantly, pain may mediate obesity-induced impairment of physical functioning and deterioration of health-related quality of life.11, 12 Weight loss sets in motion a cascade of events that can prevent OA onset or combat existing OA symptoms and disability. These events include reduction of mechanical and biological stressors. This article will review the newest evidence of the relationship between obesity and OA, and the effect of weight loss on the prevention and treatment of OA.
Obesity-Specific Mechanisms of OA Pathophysiology
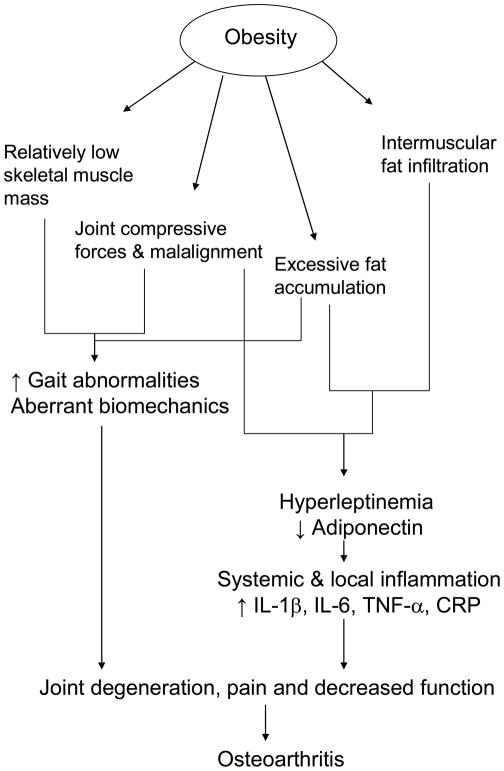
While there are numerous pathways that contribute to OA onset, obesity-specific mechanisms include relative loss of muscle mass and strength over time, mechanical stress and systemic inflammation. Excessive adipose tissue compresses load-bearing joints and creates an inflammatory environment within tissues and joints. Figure 1 briefly summarizes the proposed obesity-related mechanisms underlying OA. Obesity induces abnormal joint loads and leads to adverse changes in the composition, structure and properties of articular cartilage. With increased body weight, both muscle mass and fat mass increase; yet the volume of muscle mass remains relatively low and inadequate to match the loads placed upon it. When strength is normalized for body mass, obese persons have lower muscle strength than normal weight counterparts, including the quadricep13 and lumbar14 muscle groups. Obese people attempt to compensate for muscle weakness and instability by altering gait patterns and adopting different body transfer patterns to move excessive weight. With inadequate lower limb strength, less absorption of the impact forces on weight bearing joints occurs. Repetitive forces damage articular cartilage. Joint misalignment in the load bearing joints may occur with increased body segment girths, altered posture, skeletal muscle strength imbalance or weakness of muscles that control joint motion.15 In obesity, skeletal muscle becomes laden with intramuscular fat, and this fat is associated with elevated systemic levels of proinflammatory biomarkers. As obesity worsens, these biomarkers induce a feed-forward process of muscle catabolism and loss of strength.16 Over time, the cumulative effects of excessive body fat, and mechanical loading and aberrant joint motion, contribute to the OA pathophysiology and onset of inflammation and pain.17
Figure 1.
Potential obesity related pathways that contribute to osteoarthritis.
Low grade systemic inflammation is now considered a hallmark of obesity and manifests as elevations in interleukins (IL) 1β, IL-6, tumor necrosis factor (TNF-α) and the acute phase reactant C-reactive protein (CRP).16 These biomarkers might link obesity with the onset and progression of OA. In severe obesity, levels of these proteins are as much as 10-fold higher than those in normal weight.18, 19 Similar to adults, high body mass is related to increased CRP levels and decreased adiponectin levels in children.20 The local inflammation response in the synovial fluid of joints afflicted by OA includes elevations of IL-1β, and skeletal muscle. Systemic levels of IL-1β, IL-6, TNF-α and CRP also rise with the presence of hip or knee OA. Five-year prospective evidence indicates that elevated levels of TNF-α or CRP can predict the progression OA.21, 22 Chronically high IL-6 levels are predictive of knee OA over a ten year period.23 IL-1 protein content of the vastus lateralis is 34% higher while quadricep strength is 40% lower in obese persons with OA compard to those without.24
Inflammation is mediated by the activities of several adipokines such as adiponectin and leptin.25 Leptin modulates food intake by acting on neural pathways in the hypothalamus and brainstem. While the specific mechanisms underlying adipokine action in OA are not fully known, recent evidence suggests that excessive leptin levels may activate cellular pathways that contribute to cartilage breakdown.25 Normally, leptin activates expression of growth factors and production of extracellular matrix in cartilage, and can up-regulate matrix mellatoproteinases and IL-1 both of which contribute to nitric oxide production and subsequent chondrocyte apoptosis and cartilage breakdown.26 Leptin is also found in cartilage and osteophytes in persons with OA. Hyperleptinemia occurs locally in the human osteoarthritic joint. The combined influence of pain and worsening inflammation in untreated obesity likely contributes to an elevated risk for functional impairment in the obese, older adult. Adiponectin is a hormone secreted by adipocytes. Although produced in relatively low concentrations compared to that found in plasma, this hormone could be found in the synovial fluid of osteoarthritic joints likely derived from the infrapatellar fat pad and synovium. Conflicting evidence indicates that adiponectin can be pro-inflammatory (triggering IL-6 and nitric oxide production) or anti-inflammatory (upregulating inhibitors of metalloproteinases).27 But what is clear is that there is a link between dysregulation of adiponectin and OA.
Therefore, the collective and interrelated effects of relatively low muscle mass and accumulation of adipose tissue in obesity, contribute to joint degeneration and OA onset via joint compressive forces and aberrant biomechanics, hyperleptinemia and inflammation.
Functional Disability, Obesity and OA
Obesity and OA collectively increase the incidence of mobility disability.28–40 Activities such as walking, chair rise and stair climb, and timed up-and-go tasks are performed at slower speeds and are more challenging for the obese individual. Cross-sectional data support that as BMI increases by one standard deviation, the times to complete timed-up and go and chair rise tests increase by 5.0% and 6.4%, respectively.30 There is a progressive worsening of function and mobility with an increase in BMI. Gait parameters such as stride length and the average daily number of steps taken decreases by 55% when BMI exceeds 30 kg/m2.40 Of note, lower limb physical function and disability are not affected by adiposity distribution (assessed by 20 meter walk, knee flexion/ extension strength and chair rise time), as demonstrated by a cross sectional study of a group of older adults with either central or gynoid obesity.41
Prospective studies consistently show obesity-related deterioration of walking ability, chair rise and stair climb ability.42 Mechanisms for disability include muscle weakness, increased stiffness and pain. The severity of cartilage defects in obese people with knee OA is moderately associated with stiffness, pain and subjective and objective assessments of disability.43
Kinesiophobia Due to Pain
The definition of OA includes the presence of pain symptoms. As such, pain may be a significant factor contributing to mobility impairment in obese individuals.32, 44 The combined effects of obesity and degenerative joints may induce fear of movement (kinesiophobia), because weight-bearing activities such as walking, climbing stairs, body transfers and activities of daily living cause pain.45 As OA pain worsens over the long term, obese persons disengage from regular weight bearing activities and weight gain is exacerbated. We have recently found that obese persons with low back pain and knee pain rate kinesiophobia higher than non-obese individuals.46, 47 While higher kinesiophobia scores corresponded to higher perceived disability for tasks such as body transfers (chair rise), climbing stairs, jumping and running, these higher scores were surprisingly not associated with worse performance during functional tests such as flexibility, range of motion and muscle strength. These findings suggest that functional impairment in OA may be partly regulated by fear and perceived inability to perform certain tasks. Catastrophizing about pain is associated with severity of pain in obese patients with knee OA.48, 49 Both catastrophic thought patterns and somatization may foster hyervigilance to OA pain, and lead to avoidance of physical activity.50 This psychosocial component is commonly overlooked when developing plans of care for the obese patient with OA pain.
Weight loss reduces joint pain and increases physical function. Randomized controlled trials show that knee OA pain reduction is associated with increased mobility and physical function.51–54 As weight loss occurs, the compressive forces through the loading bearing joints such as the knee are dramatically reduced by almost fourfold.55 A reduction of body weight can attenuate the painful symptoms and likely reduces the fear of movement. In obese adults, achieving ~5% loss of body weight will relieve some joint pain, but a loss of at least 10% of body weight is associated with moderate to large clinical improvements in joint pain.56 The management of OA pain with weight loss extends past pain reduction, and has powerfully positive ramifications for increased physical capability and independence, increased participation in home and community activities, and overall quality of life.
Weight Loss and Treatment of OA in the Obese Adult
Several options for weight loss exist, ranging from medications, to exercise and dietary modification, and bariatric surgery. The “right choice” of treatment for the obese patient should be tailored to meet the individual needs. Depending on the severity of obesity and OA, creative staging of interventions for progressive weight loss in OA may be implemented to minimize pain symptoms and kinesiphobia.
Weight Loss Medications
Medications may be used alone or in concert with other interventions to induce weight loss. Two FDA approved medications to treat obesity are Orlistat (Xenical; Hoffman LaRoche Pharmaceuticals Company) and Sibutramine (Meridia; Abbott Laboratories). Orlistat is a gastric and pancreatic lipase inhibitor which decreases fat absorption in intestines by roughly 30%.57 Meta analysis revealed that 120 mg of Orlistat (three times daily) elicits ≥ 5% weight loss in 33% of patients.58 Numerous studies have supported the efficacy of Sibutramine when administered for 6–24 months.59 Thirty four % of patients achieved a minimum of 5% loss of body weight, and 15% of patients lost 10% or more of body weight over the course of one year. An important finding is that medications may be more effective when coupled with exercise and diet. For example, when Sibutramine is used in conjunction with a lifestyle modification intervention (ie, exercise and diet), weight loss is greater than that achieved with medication or the intervention alone.60
Exercise and Diet
This review will focus on the randomized controlled trials (RCT) of exercise interventions in the older demographic. Published RCTs have examined weight loss and functional effects after aerobic exercise and resistance exercise programs, multimodal exercise programs, and multimodal training with or without caloric restriction. Several RCTs were identified that included resistance exercise (RX) and/or aerobic exercise (AX) (Table 1). RX features the use resistance exercise machines, strengthening exercise using body weight and home-based strengthening exercise. AX typically involves sustained large muscle activity such as walking, climbing stairs, stationary cycling, or aquatic aerobic exercise,61, 62,63–65,66 Multimodal training consists of a variety of aerobic, resistive and flexibility components during a single session. Multimodal activity programs have been implemented for durations lasting three months to one year.67–69 Often, the multimodal activity programs are coupled with dietary changes as part of a comprehensive lifestyle overhaul.
Table 1.
Randomized controlled trials (RCTs) of exercise interventions to treat osteoarthritis (OA) symptoms in obese adults.
| Author | Population | Exercise Program | Weight change | OA related Outcomes |
|---|---|---|---|---|
| Focht et al.71 (N=316) | ≥60 years | ADAPT; 18 months Four study groups |
Exercise + Diet had greatest ↓ in OA pain and greatest ↓ stair climb time and 6 min walk distance compared with the remaining groups at 18 months (p<0.05) | |
| 1. Exercise (3 days/ wk RX 2 sets, 12 reps of leg exercises, 15 min AX at 50–75% Heart rate reserve) | NR | |||
| 2. Diet (5% loss of body weight using group sessions) | NR | |||
| 3. Exercise + Diet | NR | |||
| 4. Control group | NR | |||
| Lim et al.66 (N=75) | ≥50 years | 2 months Three study groups |
WOMAC scores ↓ by 13.8 and 9.9 points in the aquatic and conditioning groups compared to 2.7 in the control group (p<0.05). Knee pain intensity ↓ by 25% and 14% in the aquatic and land based exercise groups; pain interference ↓ by 33% and 19%, but ↑ by 6% in the control group (p<0.05). | |
| 1. Aquatic exercise (40 min/ 3 × wk; 65% max HR) | 1.1 kg | |||
| 2. Land based conditioning exercise Leg extensor exercise (40 min/ 3 × wk; at 60% 1 RM) | 0.96 kg | |||
| 3. Control (home based exercise for legs) | 0.47 kg | |||
| Messier et al.51 (N=24) | ≥60 years | 6 months Two study groups Exercise vs Diet+Exercise |
The exercise and Diet+Exercise groups had 32–40% and 15–46% ↓ ambulation and transfer knee pain, respectively. | |
| 1. Exercise (3X/wk, 20min walking, 20–30 min RX; 10–12 reps of 7 exercises) | 1.8 kg | |||
| 2. Exercise + Diet (group sessions met 1 hr/wk) | 8.5 kg | |||
| Messier et al.54(N=316) | ≥60 years | ADAPT; 18 months Four study groups |
WOMAC pain scores ↓ the most in the Exercise + Diet group compared to all remaining groups (−2.2 points vs. −0.40 to −1.23 points; p<0.05). WOMAC physical function scores ↑ most in the Exercise+Diet and Diet only groups compared with Exercise and control groups (24% and 18% vs. 12% and 13%; p<0.05). No significant changes were observed in lateral and medial knee joint space width. |
|
| 1. Exercise (3 days/ wk RX 2 sets, 12 reps of leg exercises, 15 min AX at 50–75% Heart rate reserve) | 3.5 kg | |||
| 2. Diet (5% loss of body weight using group sessions) | 4.6 kg | |||
| 3. Exercise + Diet | 5.2 kg | |||
| 4. Healthy lifestyle (control) | 1.1 kg | |||
| Miller et al.70 (N=87) | 69.7 ±0.6 yr | PAIBCT; 6 months Two study groups |
WOMAC sum scores ↓ 11.2 and 1.7 points in the weight loss and weight stable groups. WOMAC Pain subscores ↓ by 35% in the weight loss group and ↑ by 1.6% in the weight stable group; function subscores ↑ 35% and 6% in the weight loss and stable groups. Knee stiffness scores were lower in the weight loss group by month 6 (all p<0.05). Change scores in body weight were correlated with changes in WOMAC sum, pain, and function scores (r values 0.307–0.346; all p<0.05). |
|
| 1. Weight loss group Aerobic (walking, cycling;50–85% HHR) Strength (leg extension, leg curl, heel raise ands step ups; 2 sets × 12 reps); meal replacements, structured menus, educational component; controls received lectures on health topics 3 × week (45 min each session) | 8.3 kg | |||
| 2. weight stable group Bimonthly meetings to discuss health topics |
0.1 kg | |||
| Schlenk et al.69 (N=26) | 63.2 ± 9.8 yr | 6 months; Two study groups |
WOMAC physical function subscores ↓ from 22.5 to 18.9 and from 23.6 to 21.6 in the exercise and control groups, respectively. (no significant difference between groups) | |
| 1. Exercise (walking, lower limb targeted exercises) | NR | |||
| 2. Control group | NR |
NR = not reported; AX = aerobic exercise; RX = resistance exercise
WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index
ADAPT = Arthritis, Diet, and Activity Promotion Trial; PAIBCT = Physical activity, Inflammation and Body Composition Trial
Among these RCTs, several have focused on the obese knee OA population and are presented in Table 1.51, 54, 66, 69–71 Training periods ranged from 2 to 6 months with a frequency of 2 to 3 times a week, and follow-ups up to 18 months. AX intensities required to elicit favorable functional changes included an intensity of 50–85% heart rate reserve for land based exercise54, 70, 71 or 65% of maximal heart rate in aquatic exercise.66 The intensity of performing RX exercises varied among studies, ranging from using own the weight of the limb segment, body weight or cuff weight as the resistance,71 or use of dumbbells.51 Even simple home-based exercise studies indicate efficacy in reducing OA pain in this population; studies have featured quadricep contractions and functional tasks (e.g., rising from a chair) for up to 24 months.72 Compared with education control and diet groups, the exercise group achieved a 30% reduction in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores and improvement in WOMAC functional scores.
Benefits of exercise for OA symptoms can include reduction of body weight. When exercise is coupled with diet, greater weight loss can occur. Compressive forces on the joint are significantly reduced in proportion with the degree of weight loss.73 Comparative studies show that multimodal exercise induces a 3.7% loss of body weight, whereas the diet only and diet+multimodal exercise result in 4.9% and 5.7% losses of body weight by six months.54 Knee OA pain can be reduced with exercise or combined intervention, but the largest pain reduction (30.3%) was related with the greatest weight loss.54 Pain improvement during the exercise intervention is the strongest contributing factor in explaining the association between exercise adherence and decreases in self-reported disability.74
The value of exercise for OA in the obese patient is that it can be used to treat the disease and help prevent or delay the onset of the disease. Ideally, the incorporation of AX to stimulate caloric expenditure and RX to strengthen the musculature supporting the joints provides a well-rounded program to treat OA symptoms. Exercise can be applied to this population at any disease stage to help provide pain relief, strengthen muscles that surround the arthritic joint, and help control or reduce body weight, the latter being the main modifiable factor underlying OA. To help overcome kinesiophobia, exercise may need to be supervised initially and periodically thereafter to help ensure that activity is performed at the appropriate training stimulus and not compromised because of fear. Importantly, exercise interventions can be cost effective to treat knee OA in obese adults. Pain severity and functional outcomes such as walking performance and stair climb demonstrate the greatest improvements after exercise alone than after diet programs or exercise+diet interventions.75
Bariatric Surgery
For many obese patients, meaningful weight loss is difficult as lifestyle changes are unappealing and long-term adherence is typically low. Bariatric surgery can elicit massive weight loss when post-surgical instructions are followed. Common surgical techniques include laparascopic adjustable gastric banding, sleeve gastrectomy and vertical banded gastroplasty. Joint pain can be attenuated or abolished in morbidly obese persons with pain in the hip,76, 77 knee, 77–80 ankle,79, 80 spine,77, 79–83 neck,79 shoulder,79 elbow wrist and hand,79 and knee, ankle and foot pain.84 Although there are methodological inconsistencies in the measurement follow-up times for joint pain between and within studies, the most common post-operative time point was approximately two years. Reductions in BMI values ranged from 6.2–14.7 kg/m2 and this corresponded with a resolution of knee and back pain in 5–100% of patients, while pain severity was reduced in 31–94% depending on the joint and study.85
Knee
Several studies have demonstrated that among patients with knee OA pain, bariatric procedures can predictably provide relief. Dramatic reductions in pain can occur as quickly as three months post-surgery.77 Studies reported 9%86 to 76%76 less prevalence in knee pain by the final study time point as reflected by self-report surveys of joint pain and severity. Average and median WOMAC pain scores for knee pain were reduced by 51%,79 and 66%, 86 respectively, at follow-up. Another study showed that Knee Society pain subscores are reduced by 14.8% while the function subscores improved by almost the same percent after surgery.78 A mechanism of pain relief underlying these collective findings may be knee joint space widening with weight loss. For example, Abu-Abeid et al. found that when BMI was reduced by an average of 6.3 kg/m2 after bariatric surgery, joint space widened form 4.6mm to 5.25mm.78 These intriguing findings show that independent of physical activity level or muscle strength, knee pain related disability could be improved with weight loss alone. Relief from pain may facilitate re-engagement of the individual into regular exercise or activities that were previously unattainable.
Low Back
The lumbar spine is the most researched “joint area” in bariatric populations. In one study, back pain was followed in morbidly obese patients undergoing vertical banded gastroplasty and non-obese counterparts for two years 82. BMI was reduced by 14.3 kg/m2, and improvements occurred in all pain and disability assessments (Visual analogue scale for pain, Oswestry Disability Index, Roland-Morris Disability Questionnaire and the Waddell Disability Index). Uncontrolled studies have revealed that the frequency of back pain was reduced in 83% of patients,79 and lumbar back pain symptoms were reduced in 82–90% patients after 6–22 months.76, 79 In obese persons with chronic debilitating axial back pain the severity of back pain symptoms was reduced by 44% after bariatric surgery.81 Pain relief was also associated with lower ODI scores.
Other Joint Pain
Weight loss after bariatric surgery may not impact hip OA pain as much as that experienced by other load-bearing joints. While some data indicate that hip pain can improve,76 most studies do not support favorable changes in hip pain after surgery. Lateral and medial hip pain symptoms were not significantly reduced by one year,79 and the presence of hip OA pain was not different in bariatric patients at two or six years after surgery.80 Even if obesity increases the vertical loading stressors and compressive forces with weight bearing activity, the positioning of the femoral head in the acetabulum may not be affected with increased weight as much as other load bearing joints.87 Limited data indicate that the frequency of foot pain is reduced by 42% to 95% after a bariatric surgery procedure.76, 79 While hand OA pain symptoms moderately decreased after bariatric surgery, shoulder pain did not decrease with palpation or range of motion.79 The lack of OA pain relief in the shoulder may be due to the low baseline prevalence of shoulder OA or high error within the small sample sizes to detect a surgery related change.
Potential Mechanisms Underlying Relief from OA Symptoms
Weight loss with medications, exercise (with or without diet) and bariatric surgery can favorably alter the mechanical and biochemical profiles of obese adults with OA. Mechanical stress can be reduced as shown by a lowering of maximal knee compressive forces relative to magnitude of weight loss. 73 Surgical weight loss can also substantially lower joint compressive forces, which may increase the joint space width.78 Reductions in the central deposition of fat on the abdomen and in the girths of lower limb segments may facilitate normalization of joint alignment. The collective benefits of lower joint loading and joint realignment would attenuate cartilage stress and silence one trigger of local joint inflammation. Weight loss reduces the synthesis of IL-688,89and TNF-α and increases the production of anti-inflammatory cytokines (IL-10) by subcutaneous adipose. A loss of body fat attenuates systemic levels of inflammatory cytokines such as IL-6 by 25–30%.18 Leptin and CRP levels also decrease with weight loss.88,89 Irrespective of the method of weight loss, suppression of the proinflammatory cytokines can occur. These biochemical changes would complement the mechanical benefits of weight loss to reduce OA symptoms.
Prevention
Identification of effective treatments to prevent OA in obese younger populations is lacking. This is partly due to the challenges of long-term prospective research, and the lack of control in documenting processes that may influence OA onset. However, we surmise that the participation in regular physical activity and weight management may be critical in avoiding early onset of OA or increased risk of the disease. Some advocates suggest a screening process that begins in adolescence, in which family history is reviewed. If there is a positive family history, the individual can be counseled by the health care team on prevention techniques including strengthening exercise, (eg, leg raises, weight bearing exercise, strengthening exercise [quadriceps, hamstrings]), endurance exercise and judicious use of resistance exercise.90 Guidelines to achieve or maintain a healthy weight can include dietary recommendations, healthful living and management of musculoskeletal pain. Successful disease prevention programs include the family, and therefore OA risk may be decreased if the entire family adopts healthy behaviors and loses excessive weight. From the physiological perspective, the OA related states of chronic inflammation and elevated mechanical stress on the joints may be curtailed or avoided if preventative measures are put into place during adolescence. Inflammation is improved with interventions that induce a 5% weight loss, regardless of the type or duration of the intervention.20 The adage ‘an ounce of prevention is worth a pound of cure’ may be directly applicable to the obese person at risk for OA; for every reduction in weight, there is a decrease in the risk of OA onset.
Conclusion
Obesity induces several pathways that predispose an individual to symptomatic OA. Growing evidence indicates that irrespective of weight loss method, reduction of body fat can reduce the mechanical and biochemical stressors that contribute to joint degeneration. A variety of methods can be used treat OA including medications, exercise (with or without diet) and bariatric surgery. Prevention of OA may be achieved in part through screening of children at risk for OA, and education of the whole family to increase the chance of long-term success of disease prevention.
Acknowledgments
Dr. Heather Vincent has NIH grant funding including AR057552-01A1, AR059786, and has been supported by the US Bone and Joint Decade Scholar Program. Dr. Robert Hurley has NIH grant funding, AR057552-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. American Journal of Orthopedics. 2008;37(3):148–151. [PubMed] [Google Scholar]
- 2.Caterson ID, Gill TP. Obesity: epidemiology and possible prevention. Practice in Research in Clinical Endocrinology and Metabolism. 2002;16(4):595–610. doi: 10.1053/beem.2002.0228. [DOI] [PubMed] [Google Scholar]
- 3.Fried M. Bariatric surgery in paediatrics--when and how? International Journal of Pediatric Obesity. 2008;3(Suppl 2):15–19. doi: 10.1080/17477160802404640. [DOI] [PubMed] [Google Scholar]
- 4.Salihu HM, Bonnema SM, Alio AP. Obesity: What is an elderly population growing into? Maturitas. 2009;63(1):7–12. doi: 10.1016/j.maturitas.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298(17):2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- 6.Andersen RE, Crespo CJ, Bartlett SJ, Bathon JM, Fontaine KR. Relationship between body weight gain and significant knee, hip, and back pain in older Americans. Obesity Research. 2003;11(10):1159–1162. doi: 10.1038/oby.2003.159. [DOI] [PubMed] [Google Scholar]
- 7.Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. Journal of Pain. 2007;8(5):430–436. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Haara MM, Heliövaara M, Kröger H, et al. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. Journal of Bone and Joint Surgery, American volume. 2004;86-A(7):1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Muehleman C, Margulis A, Bae WC, Masuda K. Relationship between knee and ankle degeneration in a population of organ donors. BMC Medicine. 2010;8:48. doi: 10.1186/1741-7015-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nebel MB, Sims EL, Keefe FJ, et al. The relationship of self-reported pain and functional impairment to gait mechanics in overweight and obese persons with knee osteoarthritis. Archives of Physical Medicine and Rehabilitation. 2009;90(11):1874–1879. doi: 10.1016/j.apmr.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo M, Allison DB, Faith MS, Zhu S, Fontaine KR. Obesity and quality of life: mediating effects of pain and comorbidities. Obesity Research. 2003;11(2):209–216. doi: 10.1038/oby.2003.33. [DOI] [PubMed] [Google Scholar]
- 12.Anandacoomarasamy A, Caterson ID, Leibman S, et al. Influence of BMI on health-related quality of life: Comparison between an obese adult cohort and age-matched population norms. Obesity (Silver Spring) 2009 Apri; doi: 10.1038/oby.2009.121. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Miyatake N, Fujii M, Nishikawa H, et al. Clinical evaluation of muscle strength in 20–79-years-old obese Japanese. Diabetes Research & Clinical Practice. 2000;48(1):15–21. doi: 10.1016/s0168-8227(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 14.Vincent KR, Braith RW, Vincent HK. Influence of resistance exercise on lumbar strength in older, overweight adults. Archives of Physical Medicine and Rehabilitation. 2006;87(3):383–389. doi: 10.1016/j.apmr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Hinman RS, Hunt MA, Creaby MW, Wrigley TV, McManus FJ, Bennell KL. Hip muscle weakness in individuals with medial knee osteoarthritis. Arthritis Care Research. 2010;62(8):1190–1193. doi: 10.1002/acr.20199. [DOI] [PubMed] [Google Scholar]
- 16.Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. Journal of Applied Physiology. 2007;102(3):919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Current Opinion in Rheumatology. 2010;22(5):533–537. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compher C, Badellino KO. Obesity and inflammation: Lessons from bariatric surgery. Journal of Parenteral and Enteral Nutrition. 2008;32(6):645–647. doi: 10.1177/0148607108326070. [DOI] [PubMed] [Google Scholar]
- 19.Messier SP. Obesity and osteoarthritis: disease genesis and nonpharmacologic weight management. Rheumatic Diseases Clinics of North America. 2008;34(3):713–729. doi: 10.1016/j.rdc.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam CS, Clément K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obesity Reviews. 2010;11(2):118–126. doi: 10.1111/j.1467-789X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 21.Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Annals of Rheumatic Diseases. 2000;59(1):71–74. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector TD, Hart DJ, Nandra D, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis and Rheumatism. 1997;40(4):723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 23.Livshits G, Zhai G, Hart DJ, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis and Rheumatism. 2009;60(7):2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levinger I, Levinger P, Trenerry MK, et al. Increased inflammatory cytokine expression in the vastus lateralis of patients with knee osteoarthritis. Arthritis and Rheumatism. 2011;63(5):1343–1348. doi: 10.1002/art.30287. [DOI] [PubMed] [Google Scholar]
- 25.McNulty AL, Miller MR, O’Connor SK, Farshid G. The effects of adipokines on cartilage ad meniscus metabolism. Connective Tissue Research. 2011:1–11. [Google Scholar]
- 26.Lago R, Gómez R, Lago F, Gómez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cellular Immunology. 2008;252(1–2):139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Hu PF, Bao JP, Wu LD. The emerging role of adipokines in osteoarthritis: a narrative review. Molecular Biology Reports. 2011;(38):873–878. 2. doi: 10.1007/s11033-010-0179-y. [DOI] [PubMed] [Google Scholar]
- 28.Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. International Journal of Obesity. 2006;30(2):364–373. doi: 10.1038/sj.ijo.0803130. [DOI] [PubMed] [Google Scholar]
- 29.Apovian CM, Frey CM, Wood GC, Rogers JZ, Still CD, Jensen GL. Body mass index and physical function in older women. Obesity Research. 2002;10(8):740–747. doi: 10.1038/oby.2002.101. [DOI] [PubMed] [Google Scholar]
- 30.Davis JW, Ross PD, Preston SD, Nevitt MC, Wasnich RD. Strength, physical activity, and body mass index: relationship to performance-based measures and activities of daily living among older Japanese women in Hawaii. Journal of the American Geriatrics Society. 1998;46(3):274–279. doi: 10.1111/j.1532-5415.1998.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 31.Hemmingsson E, Ekelund U. Is the association between physical activity and body mass index obesity dependent? International Journal of Obesity. 2007;31(4):663–668. doi: 10.1038/sj.ijo.0803458. [DOI] [PubMed] [Google Scholar]
- 32.Lamb SE, Guralnik JM, Buchner DM, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women’s Health and Aging Study. Annals of Rheumatic Disorders. 2000;59(5):331–337. doi: 10.1136/ard.59.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang IA, Guralnik JM, Melzer D. Physical activity in middle-aged adults reduces risks of functional impairment independent of its effect on weight. Journal of the American Geriatrics Society. 2007;55(11):1836–1841. doi: 10.1111/j.1532-5415.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 34.Okoro CA, Zhong Y, Ford ES, Balluz LS, Strine TW, Mokdad AH. Association between the metabolic syndrome and its components and gait speed among U.S. adults aged 50 years and older: a cross-sectional analysis. BMC Public Health. 2006;14(6):282. doi: 10.1186/1471-2458-6-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stenholm S, Sainio P, Rantanen T, Alanen E, Koskinen S. Effect of co-morbidity on the association of high body mass index with walking limitation among men and women aged 55 years and older. Aging Clinical and Experimental Research. 2007;19(4):277–283. doi: 10.1007/BF03324702. [DOI] [PubMed] [Google Scholar]
- 36.Stenholm S, Rantanen T, Alanen E, Reunanen A, Sainio P, Koskinen S. Obesity history as a predictor of walking limitation at old age. Obesity (Silver Spring) 2007;15(4):929–938. doi: 10.1038/oby.2007.583. [DOI] [PubMed] [Google Scholar]
- 37.Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. American Journal of Epidemiology. 2002;156(2):110–121. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 38.Valentine RJ, Misic MM, Rosengren KS, Woods JA, Evans EM. Sex impacts the relation between body composition and physical function in older adults. Menopause. 2009;16(3):518–523. doi: 10.1097/gme.0b013e31818c931f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity (Silver Spring) 2007;15(7):1886–1894. doi: 10.1038/oby.2007.223. [DOI] [PubMed] [Google Scholar]
- 40.Yamakawa K, Tsai CK, Haig AJ, Miner JA, Harris MJ. Relationship between ambulation and obesity in older persons with and without low back pain. International Journal of Obesity. 2004;28(1):137–143. doi: 10.1038/sj.ijo.0802478. [DOI] [PubMed] [Google Scholar]
- 41.Foster NA, Segal NA, Clearfield JS, et al. Central versus lower body obesity distribution and the association with lower limb physical function and disability. PMR. 2010;2(12):1119–1126. doi: 10.1016/j.pmrj.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obesity Reviews. 2010 Jan 6; doi: 10.1111/j.1467-789X.2009.00703.x. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 43.Anandacoomarasamy A, Smith G, Leibman S, et al. Cartilage defects are associated with physical disability in obese adults. Rheumatology (Oxford) 2009;48(10):1290–1293. doi: 10.1093/rheumatology/kep246. [DOI] [PubMed] [Google Scholar]
- 44.Weaver GD, Kuo YF, Raji MA, et al. Pain and disability in older Mexican-American adults. Journal of the American Geriatrics Society. 2009;57(6):992–999. doi: 10.1111/j.1532-5415.2009.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gooberman-Hill R, Woolhead G, Mackichan F, Ayis S, Williams S, Dieppe P. Assessing chronic joint pain: lessons from a focus group study. Arthritis and Rheumatism. 2007;57(4):666–671. doi: 10.1002/art.22681. [DOI] [PubMed] [Google Scholar]
- 46.Vincent HK, Lamb KM, Day TI, Tillman SM, Vincent KR, George SZ. Morbid obesity is associated with fear of movement and lower quality of life in patients with knee pain-related diagnoses. PM&R. 2010;2(8):713–722. doi: 10.1016/j.pmrj.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Vincent HK, Omli MR, Day TI, Hodges M, Vincent KR, George S. Fear of movement, quality of life and self-reported disability in obese patients with chronic lumbar pain. Pain Medicine. 2010;12(1):154–164. doi: 10.1111/j.1526-4637.2010.01011.x. [DOI] [PubMed] [Google Scholar]
- 48.Somers TJ, Keefe FJ, Carson JW, Pells JJ, Lacaille L. Pain catastrophizing in borderline morbidly obese and morbidly obese individuals with osteoarthritic knee pain. Pain Research and Management. 2008;13(5):401–406. doi: 10.1155/2008/652453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somers TJ, Keefe FJ, Pells JJ, et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: relationships to pain and disability. Journal of Pain Symptom Management. 2009;37(5):863–872. doi: 10.1016/j.jpainsymman.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. European Journal of Pain. 2004;8(5):495–502. doi: 10.1016/j.ejpain.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Messier SP, Loeser RF, Mitchell MN, et al. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. Journal of the American Geriatrics Society. 2000;48(9):1062–1072. doi: 10.1111/j.1532-5415.2000.tb04781.x. [DOI] [PubMed] [Google Scholar]
- 52.Toda Y, Toda T, Takemura S, Wada T, Morimoto T, Ogawa R. Change in body fat, but not body weight or metabolic correlates of obesity, is related to symptomatic relief of obese patients with knee osteoarthritis after a weight control program. Journal of Rheumatology. 1998;25:2181–2186. [PubMed] [Google Scholar]
- 53.Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis and Cartilage. 2005;13:20–27. doi: 10.1016/j.joca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 54.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis and Rheumatology. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 55.Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis and Rheumatology. 2005;52(7):2026–2032. doi: 10.1002/art.21139. [DOI] [PubMed] [Google Scholar]
- 56.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Annals of Rhematic Diseases. 2007;66(4):433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drew BS, Dixon AF, Dixon JB. Obesity management: Update on orlistat. Vascular Health and Risk Management. 2007;3(6):817–821. [PMC free article] [PubMed] [Google Scholar]
- 58.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Systematic Reviews. 2003;4:CD004094. doi: 10.1002/14651858.CD004094. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Annals of Internal Medicine. 2005;142(7):532–346. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 60.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. New England Journal of Medicine. 2005;353(20):2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 61.Vincent HK, Bourguignon C, Vincent KR. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity (Silver Spring) 2006;14(11):1921–1930. doi: 10.1038/oby.2006.224. [DOI] [PubMed] [Google Scholar]
- 62.Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. European Journal of Applied Physiology. 2010;109(3):517–525. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- 63.Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause. 2009;16(1):66–72. doi: 10.1097/gme.0b013e31817dacf7. [DOI] [PubMed] [Google Scholar]
- 64.Bouchard DR, Soucy L, Sénéchal M, Dionne IJ, Brochu M. Changes in objective and self-reported measures of physical capacity after an intervention in obese older women. Journal of Women and Aging. 2010;22(1):34–36. doi: 10.1080/08952840903489011. [DOI] [PubMed] [Google Scholar]
- 65.Davison KK, Ford ES, Cogswell ME, Dietz WH. Percentage of body fat and body mass index are associated with mobility limitations in people aged 70 and older from NHANES III. Journal of the American Geriatrics Society. 2002;50(11):1802–1809. doi: 10.1046/j.1532-5415.2002.50508.x. [DOI] [PubMed] [Google Scholar]
- 66.Lim JY, Tchai E, Jang SN. Effectiveness of aquatic exercise for obese patients with knee osteoarthritis: a randomized controlled trial. PMR. 2010;2(8):723–731. doi: 10.1016/j.pmrj.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Grant S, Todd K, Aitchison TC, Kelly P, Stoddart D. The effects of a 12-week group exercise programme on physiological and psychological variables and function in overweight women. Public Health. 2004;118(1):31–42. doi: 10.1016/S0033-3506(03)00131-8. [DOI] [PubMed] [Google Scholar]
- 68.Manini TM, Newman AB, Fielding R, et al. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010;18(6):1168–1175. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlenk EA, Lias JL, Sereika SM, Dunbar-Jacob J, Kwoh CK. Improving physical activity and function in overweight and obese older adults with osteoarthritis of the knee: a feasibility study. Rehabilitation Nursing. 2011;36(1):32–42. doi: 10.1002/j.2048-7940.2011.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity (Silver Spring) 2006;14(7):1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 71.Focht BC, Rejeski WJ, Ambrosius WT, Katula JA, Messier SP. Exercise, self-efficacy, and mobility performance in overweight and obese older adults with knee osteoarthritis. Arthritis and Rheumatism. 2005;53(5):659–665. doi: 10.1002/art.21466. [DOI] [PubMed] [Google Scholar]
- 72.Jenkinson CM, Doherty M, Avery AJ, et al. Effects of dietary intervention and quadriceps strengthening exercises on pain and function in overweight people with knee pain: randomised controlled trial. British Medical Journal. 2009;339:b3170. doi: 10.1136/bmj.b3170. 3110.1136/bmj.b3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Messier SP, Legault C, Loeser RF, et al. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis and Cartilage. 2011;19(3):272–280. doi: 10.1016/j.joca.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Gool CH, Penninx BW, Kempen GI, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis and Rheumatology. 2005;53(1):24–32. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- 75.Sevick MA, Miller GD, Loeser RF, Williamson JD, Messier SP. Cost-effectiveness of exercise and diet in overweight and obese adults with knee osteoarthritis. Medicine & Science in Sports & Exercise. 2009;41(6):1167–1174. doi: 10.1249/MSS.0b013e318197ece7. [DOI] [PubMed] [Google Scholar]
- 76.McGoey BV, Deitel M, Saplys RJ, Kliman ME. Effect of weight loss on musculoskeletal pain in the morbidly obese. Journal of Bone and Joint Surgery, British volume. 1990;72(2):322–323. doi: 10.1302/0301-620X.72B2.2138158. [DOI] [PubMed] [Google Scholar]
- 77.Josbeno DA, Jakicic JM, Hergenroeder A, Eid GM. Physical activity and physical function changes in obese individuals after gastric bypass surgery. Surgery for Obesity and Related Diseases. 2008 doi: 10.1016/j.soard.2008.08.003. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 78.Abu-Abeid S, Wishnitzer N, Szold A, Liebergall M, Manor O. The influence of surgically-induced weight loss on the knee joint. Obesity Surgery. 2005;15(10):1437–1442. doi: 10.1381/096089205774859281. [DOI] [PubMed] [Google Scholar]
- 79.Hooper MM, Stellato TA, Hallowell PT, Seitz BA, Moskowitz RW. Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery. International Journal of Obesity (London) 2007;31(1):114–120. doi: 10.1038/sj.ijo.0803349. [DOI] [PubMed] [Google Scholar]
- 80.Peltonen M, Lindroos AK, Torgerson JS. Musculoskeletal pain in the obese: a comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003;104(3):549–557. doi: 10.1016/S0304-3959(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 81.Khoueir P, Black MH, Crookes PF, Kaufman HS, Katkhouda N, Wang MY. Prospective assessment of axial back pain symptoms before and after bariatric weight reduction surgery. Spine. 2009;9(6):454–463. doi: 10.1016/j.spinee.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Melissas J, Kontakis G, Volakakis E, Tsepetis T, Alegakis A, Hadjipavlou A. The effect of surgical weight reduction on functional status in morbidly obese patients with low back pain. Obesity Surgery. 2005;15(3):378–381. doi: 10.1381/0960892053576703. [DOI] [PubMed] [Google Scholar]
- 83.Melissas J, Volakakis E, Hadjipavlou A. Low-back pain in morbidly obese patients and the effect of weight loss following surgery. Obesity Surgery. 2003;13(3):389–393. doi: 10.1381/096089203765887714. [DOI] [PubMed] [Google Scholar]
- 84.Dixon JB, Dixon ME, O’Brien PE. Quality of life after lap-band placement: influence of time, weight loss, and comorbidities. Obesity Research. 2001;9(11):713–721. doi: 10.1038/oby.2001.96. [DOI] [PubMed] [Google Scholar]
- 85.Vincent HK, Ben-David K, Cendan J, Vincent KR, Lamb KM, Stevenson A. Effects of bariatric surgery on joint pain: a review of emerging evidence. Surgery in Obesity and Related Disorders. 2010;6(4):451–460. doi: 10.1016/j.soard.2010.03.284. [DOI] [PubMed] [Google Scholar]
- 86.Korenkov M, Shah S, Sauerland S, Duenschede F, Junginger T. Impact of laparoscopic adjustable gastric banding on obesity co-morbidities in the medium- and long-term. Obesity Surgery. 2007;17(5):679–683. doi: 10.1007/s11695-007-9118-y. [DOI] [PubMed] [Google Scholar]
- 87.Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obesity Reviews. 2006;7(3):239–250. doi: 10.1111/j.1467-789X.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 88.Reed JL, De Souza MJ, Williams NI. Effects of exercise combined with caloric restriction on inflammatory cytokines. Applied Physiology Nutrition and Metabolism. 2010;35(5):573–582. doi: 10.1139/H10-046. [DOI] [PubMed] [Google Scholar]
- 89.Moschen AR, Molnar C, Geiger S, et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor α expression. Hepatology. 2010;59:1259–1264. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 90.Nicholson S, Dickman K, Maradiegue A. Reducing premature osteoarthritis in the adolescent through appropriate screening. Journal of Pediatric Nursing. 2009;24(1):69–74. doi: 10.1016/j.pedn.2008.03.009. [DOI] [PubMed] [Google Scholar]