Abstract
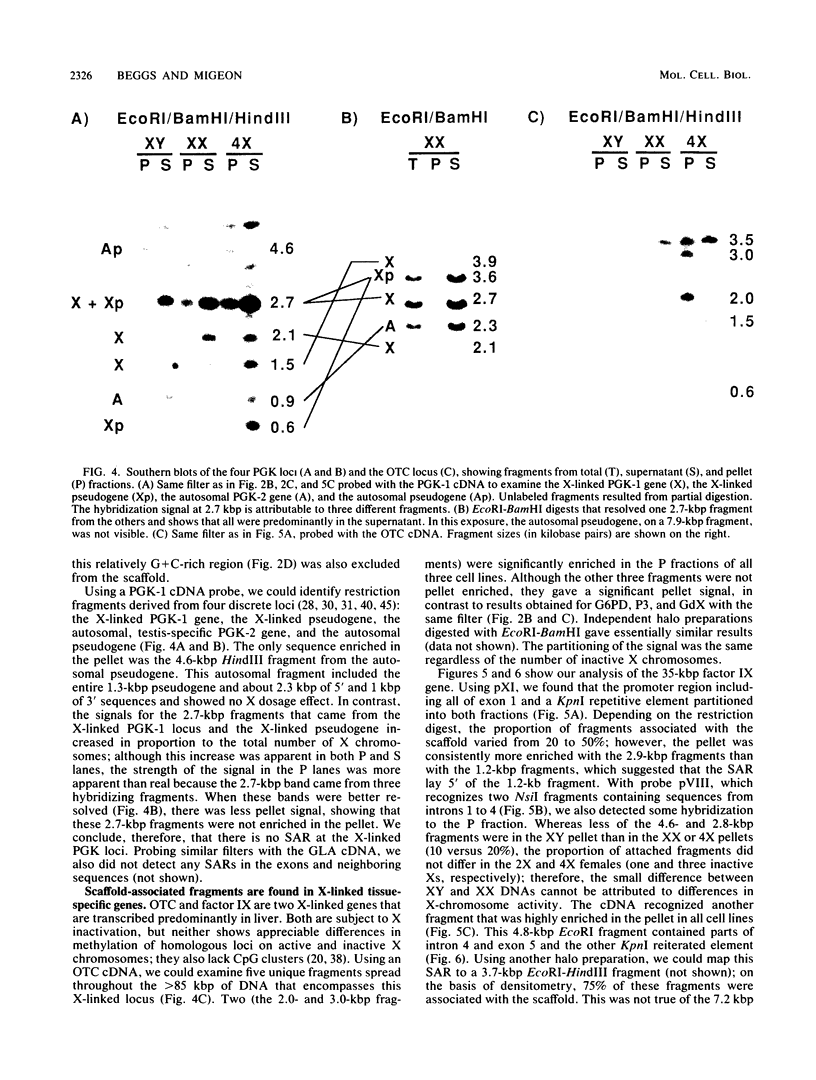
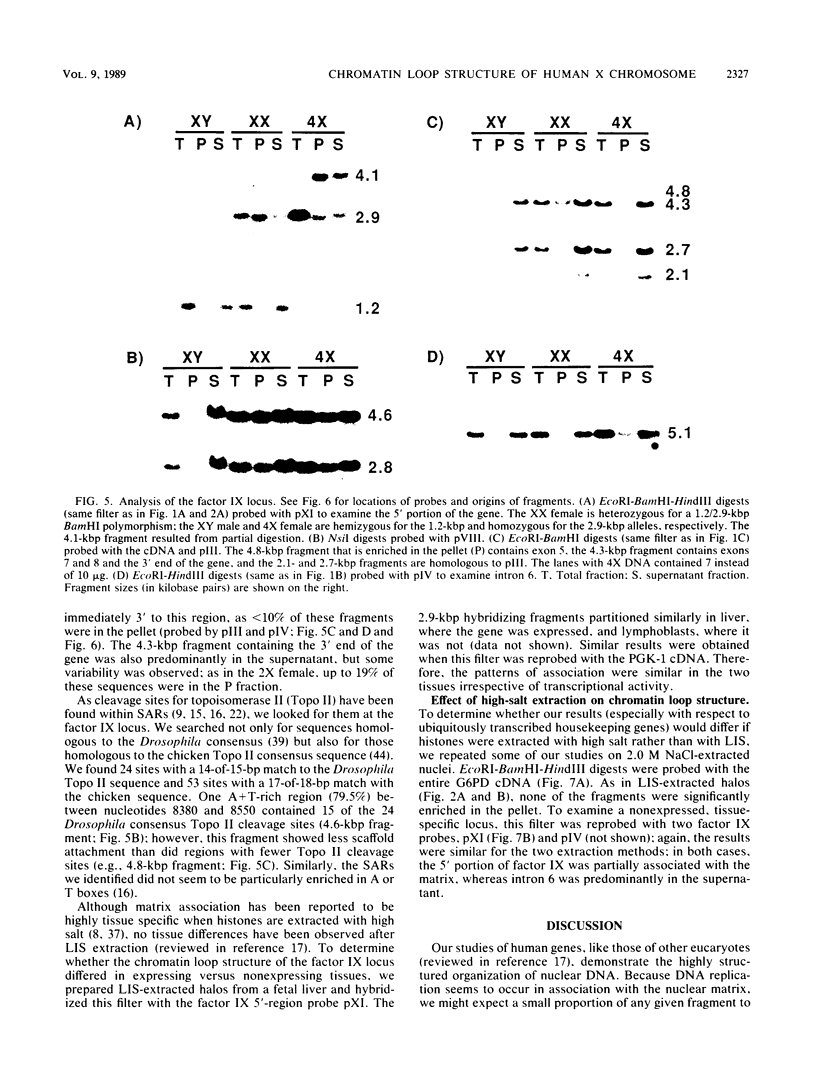
Part of the higher-order structure of chromatin is achieved by constraining DNA in loops ranging in size from 30 to 100 kilobase pairs; these loops have been implicated in defining functional domains and replicons and possibly in facilitating transcription. Because the human active and inactive X chromosomes differ in transcriptional activity and replication, we looked for differences in their chromatin loop structures. Since the islands of CpG-rich DNA at the 5' ends of X-linked housekeeping genes are the regions where functional differences in DNA methylation and nuclease sensitivity are found, we looked for scaffold association of these sequences after extraction of histones with lithium diiodosalicylate. Specifically, we examined the 5' CpG islands within the hypoxanthine phosphoribosyltransferase, glucose 6-phosphate dehydrogenase, P3, GdX, phosphoglycerate kinase type 1, and alpha-galactosidase loci in human lymphoblasts obtained from individuals with 1 to 4 X chromosomes. Although we detected no scaffold-associated regions near these genes, we found several such regions at the ornithine transcarbamylase and blood clotting factor IX loci. Our results suggest that the CpG islands are excluded from the nuclear scaffold and that even though transcriptionally active, housekeeping genes are less likely than X-linked tissue-specific genes to be scaffold associated. In all cases, the pattern of scaffold association was the same for loci on active and inactive X chromosomes.
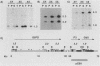
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcalay M., Toniolo D. CpG islands of the X chromosome are gene associated. Nucleic Acids Res. 1988 Oct 25;16(20):9527–9543. doi: 10.1093/nar/16.20.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARR M. L., BERTRAM E. G. A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature. 1949 Apr 30;163(4148):676–676. doi: 10.1038/163676a0. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Chimera J. A., Musich P. R. The association of the interspersed repetitive KpnI sequences with the nuclear matrix. J Biol Chem. 1985 Aug 5;260(16):9373–9379. [PubMed] [Google Scholar]
- Ciejek E. M., Nordstrom J. L., Tsai M. J., O'Malley B. W. Ribonucleic acid precursors are associated with the chick oviduct nuclear matrix. Biochemistry. 1982 Sep 28;21(20):4945–4953. doi: 10.1021/bi00263a018. [DOI] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Mapping sequences in loops of nuclear DNA by their progressive detachment from the nuclear cage. Nucleic Acids Res. 1980 Jul 11;8(13):2895–2906. doi: 10.1093/nar/8.13.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Taggart M. H., Bird A. P. Unmethylated domains in vertebrate DNA. Nucleic Acids Res. 1983 Feb 11;11(3):647–658. doi: 10.1093/nar/11.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen C. R., Hubberman P., Kaslow D. C., Migeon B. R. Comparison of factor IX methylation on human active and inactive X chromosomes: implications for X inactivation and transcription of tissue-specific genes. EMBO J. 1986 Sep;5(9):2223–2229. doi: 10.1002/j.1460-2075.1986.tb04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. E., Briand P., Ionasescu V., Ionasescu G., Williamson R., Brown C., Cavard C., Cathelineau L. Gene for OTC: characterisation and linkage to Duchenne muscular dystrophy. Nucleic Acids Res. 1985 Jan 11;13(1):155–165. doi: 10.1093/nar/13.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. The organisation of chromatin loops: characterization of a scaffold attachment site. EMBO J. 1986 Mar;5(3):511–518. doi: 10.1002/j.1460-2075.1986.tb04240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. A., Gartler S. M. Mammalian X chromosome inactivation: testing the hypothesis of transcriptional control. Somat Cell Mol Genet. 1986 May;12(3):275–280. doi: 10.1007/BF01570786. [DOI] [PubMed] [Google Scholar]
- Hata A., Tsuzuki T., Shimada K., Takiguchi M., Mori M., Matsuda I. Isolation and characterization of the human ornithine transcarbamylase gene: structure of the 5'-end region. J Biochem. 1986 Sep;100(3):717–725. doi: 10.1093/oxfordjournals.jbchem.a121764. [DOI] [PubMed] [Google Scholar]
- Jarman A. P., Higgs D. R. Nuclear scaffold attachment sites in the human globin gene complexes. EMBO J. 1988 Nov;7(11):3337–3344. doi: 10.1002/j.1460-2075.1988.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow D. C., Migeon B. R. DNA methylation stabilizes X chromosome inactivation in eutherians but not in marsupials: evidence for multistep maintenance of mammalian X dosage compensation. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6210–6214. doi: 10.1073/pnas.84.17.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F. Transcribed human ribosomal RNA genes are attached to the nuclear matrix. J Mol Biol. 1986 Jan 5;187(1):15–21. doi: 10.1016/0022-2836(86)90402-x. [DOI] [PubMed] [Google Scholar]
- Käs E., Chasin L. A. Anchorage of the Chinese hamster dihydrofolate reductase gene to the nuclear scaffold occurs in an intragenic region. J Mol Biol. 1987 Dec 20;198(4):677–692. doi: 10.1016/0022-2836(87)90209-9. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Martini G., Toniolo D., Vulliamy T., Luzzatto L., Dono R., Viglietto G., Paonessa G., D'Urso M., Persico M. G. Structural analysis of the X-linked gene encoding human glucose 6-phosphate dehydrogenase. EMBO J. 1986 Aug;5(8):1849–1855. doi: 10.1002/j.1460-2075.1986.tb04436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R., Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987 Apr 2;326(6112):501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Godwin J., Mason D. W., Brazell I. A., Cook P. R. DNA is replicated at the nuclear cage. J Cell Sci. 1980 Dec;46:365–386. doi: 10.1242/jcs.46.1.365. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Blake C. C., Evans S. T., Orkin S. H. Structure of the human phosphoglycerate kinase gene and the intron-mediated evolution and dispersal of the nucleotide-binding domain. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6965–6969. doi: 10.1073/pnas.82.20.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Bruns G. A., Morton C. C., Orkin S. H. The human phosphoglycerate kinase multigene family. HLA-associated sequences and an X-linked locus containing a processed pseudogene and its functional counterpart. J Biol Chem. 1985 Jun 10;260(11):6982–6992. [PubMed] [Google Scholar]
- Migeon B. R., Axelman J., Stetten G. Clonal evolution in human lymphoblast cultures. Am J Hum Genet. 1988 May;42(5):742–747. [PMC free article] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Spierer P., Laemmli U. K. Genes and loops in 320,000 base-pairs of the Drosophila melanogaster chromosome. J Mol Biol. 1986 Jul 20;190(2):255–258. doi: 10.1016/0022-2836(86)90296-2. [DOI] [PubMed] [Google Scholar]
- Nguyen C., Pontarotti P., Birnbaum D., Chimini G., Rey J. A., Mattei J. F., Jordan B. R. Large scale physical mapping in the q27 region of the human X chromosome: the coagulation factor IX gene and the mcf.2 transforming sequence are separated by at most 270 kilobase pairs and are surrounded by several 'HTF islands'. EMBO J. 1987 Nov;6(11):3285–3289. doi: 10.1002/j.1460-2075.1987.tb02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico M. G., Viglietto G., Martini G., Toniolo D., Paonessa G., Moscatelli C., Dono R., Vulliamy T., Luzzatto L., D'Urso M. Isolation of human glucose-6-phosphate dehydrogenase (G6PD) cDNA clones: primary structure of the protein and unusual 5' non-coding region. Nucleic Acids Res. 1986 Mar 25;14(6):2511–2522. doi: 10.1093/nar/14.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Sander M., Hsieh T. S. Drosophila topoisomerase II double-strand DNA cleavage: analysis of DNA sequence homology at the cleavage site. Nucleic Acids Res. 1985 Feb 25;13(4):1057–1072. doi: 10.1093/nar/13.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Sam J., Simmer R. L., Keith D. H., Shively L., Teplitz M., Itakura K., Gartler S. M., Riggs A. D. Isolation of a cDNA clone for human X-linked 3-phosphoglycerate kinase by use of a mixture of synthetic oligodeoxyribonucleotides as a detection probe. Proc Natl Acad Sci U S A. 1983 Feb;80(3):802–806. doi: 10.1073/pnas.80.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. Nonrandom distribution of repeated DNA sequences with respect to supercoiled loops and the nuclear matrix. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5911–5915. doi: 10.1073/pnas.79.19.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. The association of transcribed genes with the nuclear matrix of Drosophila cells during heat shock. Nucleic Acids Res. 1985 Apr 11;13(7):2413–2431. doi: 10.1093/nar/13.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Vogelstein B. The anatomy of supercoiled loops in the Drosophila 7F locus. Nucleic Acids Res. 1985 Nov 11;13(21):7703–7713. doi: 10.1093/nar/13.21.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzner J. R., Muller M. T. A consensus sequence for cleavage by vertebrate DNA topoisomerase II. Nucleic Acids Res. 1988 Jun 24;16(12):5533–5556. doi: 10.1093/nar/16.12.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K., Singer-Sam J., Munns M., Yoshida A. Molecular cloning and structure of an autosomal processed gene for human phosphoglycerate kinase. Gene. 1985;35(1-2):11–18. doi: 10.1016/0378-1119(85)90152-0. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Martini G., Migeon B. R., Dono R. Expression of the G6PD locus on the human X chromosome is associated with demethylation of three CpG islands within 100 kb of DNA. EMBO J. 1988 Feb;7(2):401–406. doi: 10.1002/j.1460-2075.1988.tb02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Martin B. M., Kaslow D. C., Migeon B. R., Choudary P. V., Stubbleflied B. K., Mayor J. A., Murray G. J., Barranger J. A., Ginns E. I. Signal sequence and DNA-mediated expression of human lysosomal alpha-galactosidase A. Eur J Biochem. 1987 Jun 1;165(2):275–280. doi: 10.1111/j.1432-1033.1987.tb11438.x. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Dintzis S., Toniolo D., Persico G., Lunnen K. D., Axelman J., Migeon B. R. Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3' CpG clusters: implications for X chromosome dosage compensation. Nucleic Acids Res. 1984 Dec 21;12(24):9333–9348. doi: 10.1093/nar/12.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]