Abstract
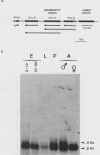
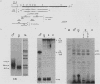
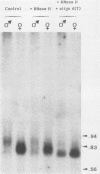
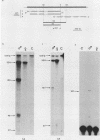
We investigated the structure and developmental pattern of expression of two genes clustered at the janus locus, located at 99D.3R. Data obtained from genomic and cDNA sequencing and from a combination of S1 mapping and primer extension experiments indicated a very unusual organization of this locus, which appeared to be composed of two partially overlapping genes, designated janA and janB. These two genes were found to be transcribed in the same direction. janA encoded one minor and two major transcripts. The 5' end of the janB mRNA mapped within the 3' untranslated region of the janA transcribed sequence. The overlapping region was 118 bases long. Similarities observed between these two genes with respect to both peptidic sequence and intron position strongly suggested that this locus originated from the duplication of an ancestral transcription unit. However, each of the resulting genes has acquired its own specificity of expression linked to sex determination. The janB transcript was detected only in males, and its expression at the adult stage was restricted to germ line cells. The janA gene displayed a much more complex expression; one of the major mRNAs was found in both sexes and at all stages, whereas the two other janA transcripts were expressed only in males.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S., Nagoshi R. N., Burtis K. C. Molecular genetic aspects of sex determination in Drosophila. Bioessays. 1987 Feb;6(2):66–70. doi: 10.1002/bies.950060206. [DOI] [PubMed] [Google Scholar]
- Belote J. M., Handler A. M., Wolfner M. F., Livak K. J., Baker B. S. Sex-specific regulation of yolk protein gene expression in Drosophila. Cell. 1985 Feb;40(2):339–348. doi: 10.1016/0092-8674(85)90148-5. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs R. T., Gregor P., Idriss S., Belote J. M., McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987 Aug 28;50(5):739–747. doi: 10.1016/0092-8674(87)90332-1. [DOI] [PubMed] [Google Scholar]
- Boswell R. E., Mahowald A. P. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985 Nov;43(1):97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Bownes M., Nöthiger R. Sex determining genes and vitellogenin synthesis in Drosophila melanogaster. Mol Gen Genet. 1981;182(2):222–228. doi: 10.1007/BF00269661. [DOI] [PubMed] [Google Scholar]
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Chapman K. B., Wolfner M. F. Determination of male-specific gene expression in Drosophila accessory glands. Dev Biol. 1988 Mar;126(1):195–202. doi: 10.1016/0012-1606(88)90253-9. [DOI] [PubMed] [Google Scholar]
- Chen P. S., Stumm-Zollinger E., Aigaki T., Balmer J., Bienz M., Böhlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988 Jul 29;54(3):291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Cline T. W. A female-specific lethal lesion in an X-linked positive regulator of the Drosophila sex determination gene, Sex-lethal. Genetics. 1986 Jul;113(3):641–663. doi: 10.1093/genetics/113.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W. Evidence that sisterless-a and sisterless-b are two of several discrete "numerator elements" of the X/A sex determination signal in Drosophila that switch Sxl between two alternative stable expression states. Genetics. 1988 Aug;119(4):829–862. doi: 10.1093/genetics/119.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C., Cline T. W. The Drosophila sex determination gene daughterless has different functions in the germ line versus the soma. Cell. 1987 Feb 13;48(3):479–487. doi: 10.1016/0092-8674(87)90198-x. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBenedetto A. J., Lakich D. M., Kruger W. D., Belote J. M., Baker B. S., Wolfner M. F. Sequences expressed sex-specifically in Drosophila melanogaster adults. Dev Biol. 1987 Jan;119(1):242–251. doi: 10.1016/0012-1606(87)90225-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Schäfer U., Schäfer M. Cis-acting regions sufficient for spermatocyte-specific transcriptional and spermatid-specific translational control of the Drosophila melanogaster gene mst(3)gl-9. EMBO J. 1988 Feb;7(2):447–454. doi: 10.1002/j.1460-2075.1988.tb02832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Valbuena O., Perry R. P. Isolation, purification, and properties of mouse heavy-chain immunoglobulin mRNAs. Biochemistry. 1978 May 2;17(9):1723–1733. doi: 10.1021/bi00602a022. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Wieschaus E. Is sex determination in germ line and soma controlled by separate genetic mechanisms? Nature. 1978 Mar 16;272(5650):249–251. doi: 10.1038/272249a0. [DOI] [PubMed] [Google Scholar]
- Monsma S. A., Wolfner M. F. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 1988 Sep;2(9):1063–1073. doi: 10.1101/gad.2.9.1063. [DOI] [PubMed] [Google Scholar]
- Palatnik C. M., Wilkins C., Jacobson A. Translational control during early Dictyostelium development: possible involvement of poly(A) sequences. Cell. 1984 Apr;36(4):1017–1025. doi: 10.1016/0092-8674(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Rao J. P., Zafar R. S., Sodja A. Transcriptional activity at the 3' end of the actin gene at 5C on the X chromosome of Drosophila melanogaster. Biochim Biophys Acta. 1988 May 6;950(1):30–44. doi: 10.1016/0167-4781(88)90070-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer U. The regulation of male-specific transcripts by sex determining genes in Drosophila melanogaster. EMBO J. 1986 Dec 20;5(13):3579–3582. doi: 10.1002/j.1460-2075.1986.tb04685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T. Autosomal mutations that interfere with sex determination in somatic cells of Drosophila have no direct effect on the germline. Dev Biol. 1982 Jan;89(1):117–127. doi: 10.1016/0012-1606(82)90300-1. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. Normal female germ cell differentiation requires the female X chromosome to autosome ratio and expression of sex-lethal in Drosophila melanogaster. Genetics. 1985 Mar;109(3):529–548. doi: 10.1093/genetics/109.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C. A., Gietz R. D., Hodgetts R. B. Overlapping transcription units in the dopa decarboxylase region of Drosophila. Nature. 1986 Jul 17;322(6076):279–281. doi: 10.1038/322279a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaslet C. A., O'Connell P., Izquierdo M., Rosbash M. Isolation and mapping of a cloned ribosomal protein gene of Drosophila melanogaster. Nature. 1980 Jun 26;285(5767):674–676. doi: 10.1038/285674a0. [DOI] [PubMed] [Google Scholar]
- Vincent A., Colot H. V., Rosbash M. Blastoderm-specific and read-through transcription of the sry alpha gene transformed into the Drosophila genome. Dev Biol. 1986 Dec;118(2):480–487. doi: 10.1016/0012-1606(86)90019-9. [DOI] [PubMed] [Google Scholar]
- Vincent A., Colot H. V., Rosbash M. Sequence and structure of the serendipity locus of Drosophila melanogaster. A densely transcribed region including a blastoderm-specific gene. J Mol Biol. 1985 Nov 5;186(1):149–166. doi: 10.1016/0022-2836(85)90265-7. [DOI] [PubMed] [Google Scholar]
- Vincent A., O'Connell P., Gray M. R., Rosbash M. Drosophila maternal and embryo mRNAs transcribed from a single transcription unit use alternate combinations of exons. EMBO J. 1984 May;3(5):1003–1013. doi: 10.1002/j.1460-2075.1984.tb01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]