Abstract
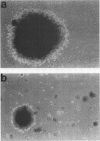
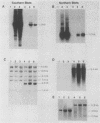
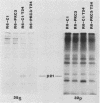
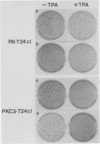
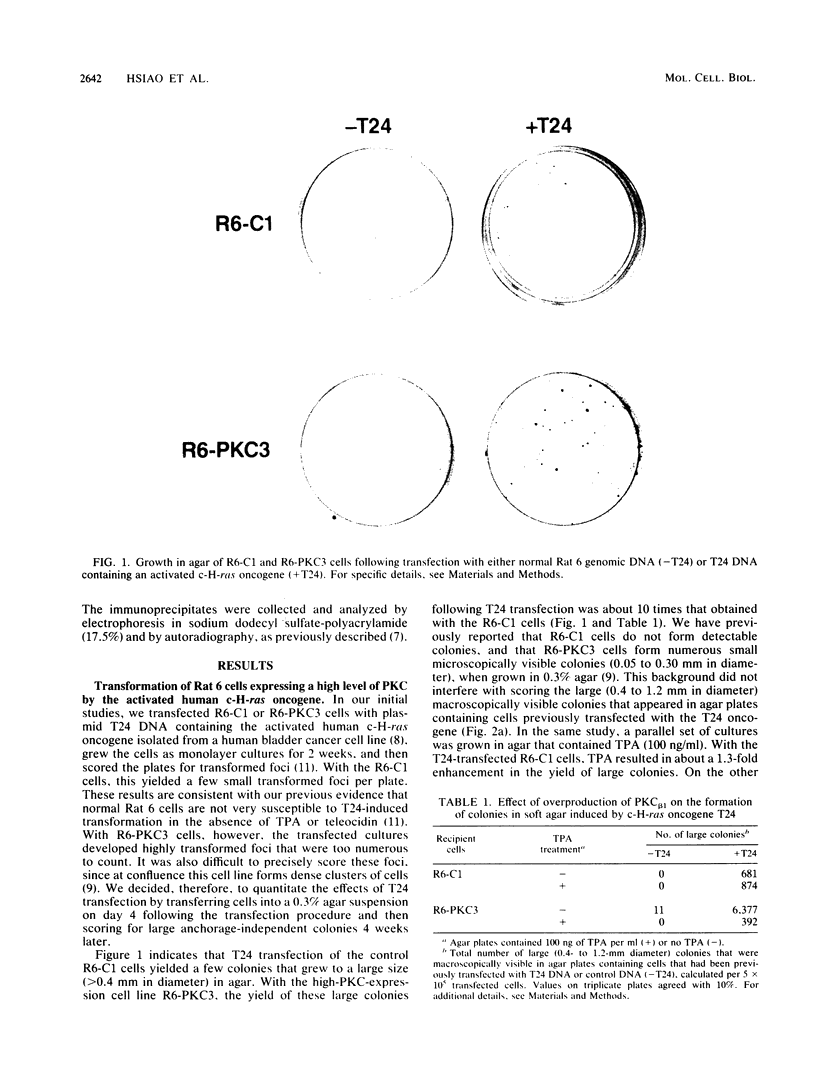
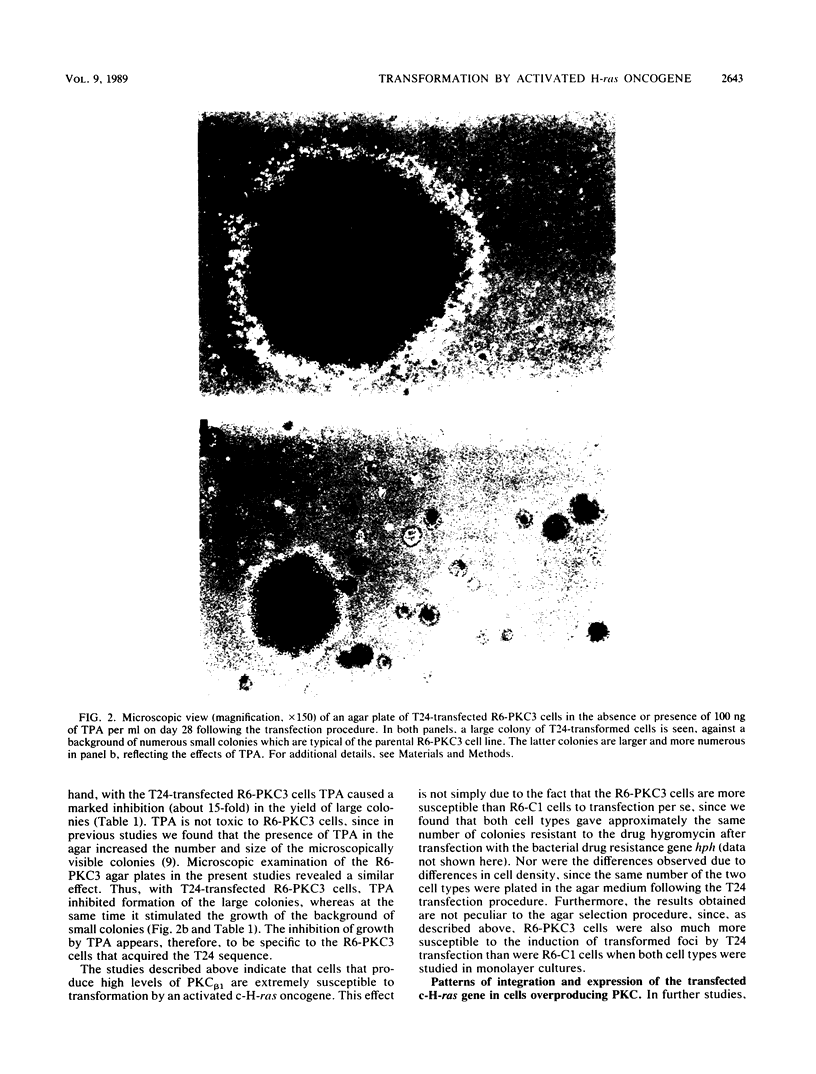
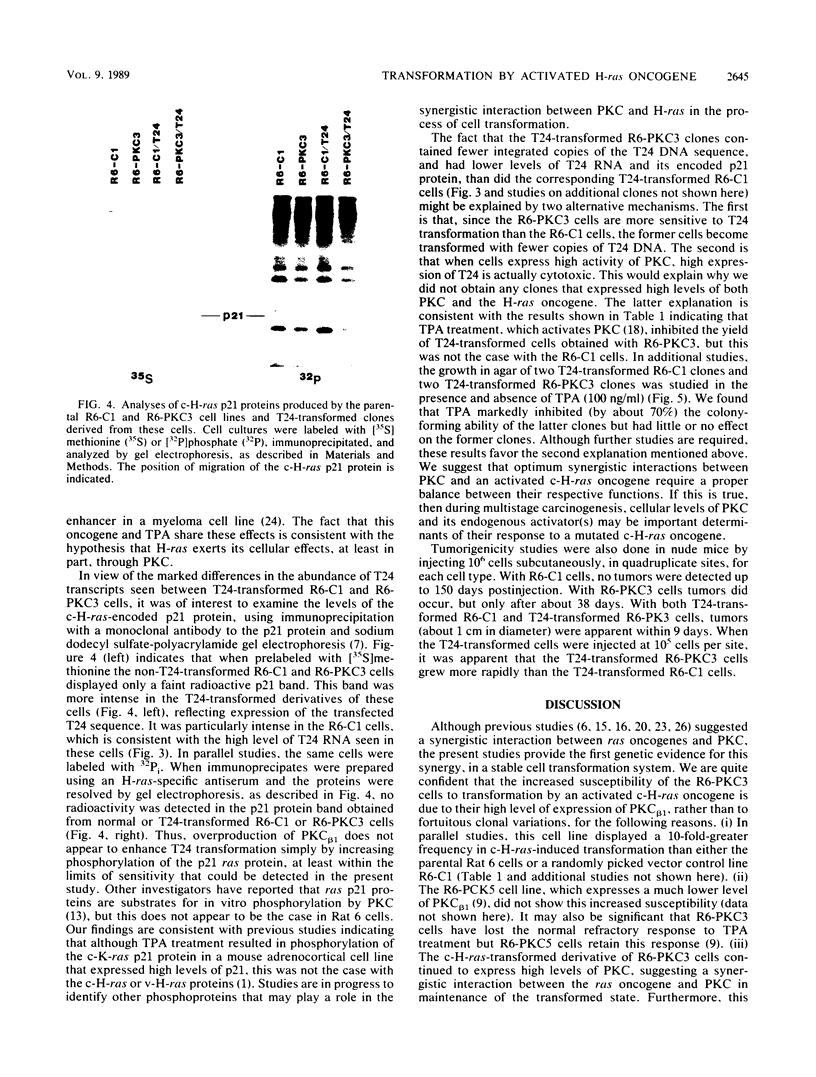
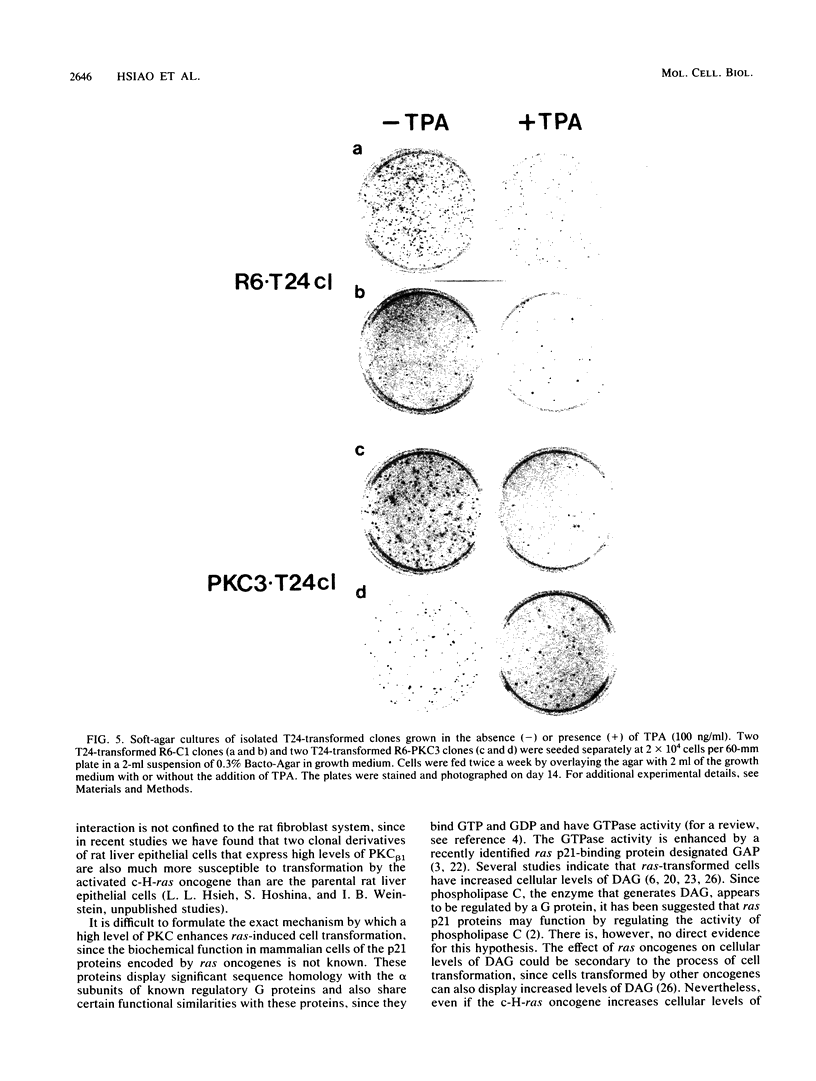
We recently developed rat fibroblast cell lines that stably overproduce high levels of the beta 1 form of protein kinase C (PKC). These cells display several disorders in growth control and form small microscopic colonies in agar. In the present study we demonstrate that one of these cell lines, R6-PKC3, is extremely susceptible to transformation by an activated human bladder cancer c-H-ras oncogene (T24). Compared with control cell line R6-C1, T24-transfected R6-PKC3 cells yielded a 10-fold increase in the formation of large colonies in agar. Cell lines established from these colonies displayed a highly transformed morphology, expressed the T24-encoded p21 ras protein, continued to express high levels of PKC, and were highly tumorigenic in nude mice. These results provide genetic evidence that PKC mediates some of the effects of the c-H-ras oncogene on cell transformation. Data are also presented suggesting that optimum synergistic effects between c-H-ras and PKC require critical levels of their respective activities. These findings may be relevant to the process of multistage carcinogenesis in tissues containing cells with an activated c-H-ras oncogene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballester R., Furth M. E., Rosen O. M. Phorbol ester- and protein kinase C-mediated phosphorylation of the cellular Kirsten ras gene product. J Biol Chem. 1987 Feb 25;262(6):2688–2695. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Shimizu K., Perucho M., Wigler M. Isolation and preliminary characterization of a human transforming gene from T24 bladder carcinoma cells. Nature. 1982 Apr 1;296(5856):404–409. doi: 10.1038/296404a0. [DOI] [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Oncogene-induced transformation of C3H 10T1/2 cells is enhanced by tumor promoters. Science. 1984 Nov 2;226(4674):552–555. doi: 10.1126/science.6436974. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Wu T., Weinstein I. B. Oncogene-induced transformation of a rat embryo fibroblast cell line is enhanced by tumor promoters. Mol Cell Biol. 1986 Jun;6(6):1943–1950. doi: 10.1128/mcb.6.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. L., Yoshida Y., Nakabayashi H., Knopf J. L., Young W. S., 3rd, Huang K. P. Immunochemical identification of protein kinase C isozymes as products of discrete genes. Biochem Biophys Res Commun. 1987 Dec 31;149(3):946–952. doi: 10.1016/0006-291x(87)90500-6. [DOI] [PubMed] [Google Scholar]
- Jeng A. Y., Srivastava S. K., Lacal J. C., Blumberg P. M. Phosphorylation of ras oncogene product by protein kinase C. Biochem Biophys Res Commun. 1987 Jun 15;145(2):782–788. doi: 10.1016/0006-291x(87)91033-3. [DOI] [PubMed] [Google Scholar]
- Kamata T., Sullivan N. F., Wooten M. W. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987 Mar;1(1):37–46. [PubMed] [Google Scholar]
- Lacal J. C., Fleming T. P., Warren B. S., Blumberg P. M., Aaronson S. A. Involvement of functional protein kinase C in the mitogenic response to the H-ras oncogene product. Mol Cell Biol. 1987 Nov;7(11):4146–4149. doi: 10.1128/mcb.7.11.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., McNally M. A., Okarma T. B., Lerch L. B. Inducible gene expression from multiple promoters by the tumor-promoting agent, PMA. Nucleic Acids Res. 1987 Nov 11;15(21):9043–9055. doi: 10.1093/nar/15.21.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. Identification of three additional members of rat protein kinase C family: delta-, epsilon- and zeta-subspecies. FEBS Lett. 1987 Dec 21;226(1):125–128. doi: 10.1016/0014-5793(87)80564-1. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Schaeffer W. I., Friend K. Efficient detection of soft agar grown colonies using a tetrazolium salt. Cancer Lett. 1976 May;1(5):259–262. doi: 10.1016/s0304-3835(75)97506-0. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Perez-Mutul J., Wasylyk B. The c-Ha-ras oncogene and a tumor promoter activate the polyoma virus enhancer. Cell. 1987 Feb 13;48(3):525–534. doi: 10.1016/0092-8674(87)90203-0. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Tong L., Milburn M. V., Matias P. M., Jancarik J., Noguchi S., Nishimura S., Miura K., Ohtsuka E., Kim S. H. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988 Feb 19;239(4842):888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]