Abstract
Amphotericin B (AMB) is the predominant antifungal drug, but the mechanism of resistance is not well understood. We compared the in vivo virulence of an AMB-resistant Aspergillus terreus (ATR) isolate with that of an AMB-susceptible A. terreus isolate (ATS) using a murine model for disseminated aspergillosis. Furthermore, we analyzed the molecular basis of intrinsic AMB resistance in vitro by comparing the ergosterol content, cell-associated AMB levels, AMB-induced intracellular efflux, and prooxidant effects between ATR and ATS. Infection of immunosuppressed mice with ATS or ATR showed that the ATS strain was more lethal than the ATR strain. However, AMB treatment improved the outcome in ATS-infected mice while having no positive effect on the animals infected with ATR. The in vitro data demonstrated that ergosterol content is not the molecular basis for AMB resistance. ATR absorbed less AMB, discharged more intracellular compounds, and had better protection against oxidative damage than the susceptible strain. Our experiments showed that ergosterol content plays a minor role in intrinsic AMB resistance and is not directly associated with intracellular cell-associated AMB content. AMB might exert its antifungal activity by oxidative injury rather than by an increase in membrane permeation.
INTRODUCTION
Invasive mold infections (IMI) are a significant determinant of morbidity and mortality in patients undergoing cancer chemotherapy, hematopoietic stem cell transplantation, or solid organ transplantation (1–3). These infections remain difficult to manage with therapeutic treatments because of a usually late diagnosis and complication of the treatment procedure by toxicity or interactions of drugs (4). The majority of IMI are caused by Aspergillus spp., and the most pathogenic species are Aspergillus fumigatus, Aspergillus terreus, and Aspergillus flavus (5). In particular, A. terreus, a widespread soil saprophyte and producer of several secondary metabolites, is a common cause of infection at the University Hospital of Innsbruck (UHI) in Austria (6–9). In vivo and in vitro data indicate that almost all A. terreus isolates are intrinsically resistant to amphotericin B (AMB), a fungicidal heptaene macrolide antimycotic, and a high mortality rate in patients is associated with this particular mold (10–12).
Previous work has shown that AMB binds to ergosterol, the principal sterol in the fungal cell membrane, and forms aqueous pores in the lipid bilayers. Subsequently, proteins and amino acids leak out, which in turn leads to disrupted membrane proton gradients (13–16). AMB resistance is rare, and it has been suggested that for A. flavus and Candida albicans, the ergosterol content (17), the composition of the fungal cell wall (18), and the ability to produce catalase might play a role in AMB resistance (19). Sokol-Anderson et al. speculated that AMB causes cell death in C. albicans by oxidative damage (19). Despite intensive research for over 50 years, the exact mechanism of action of AMB is still incompletely understood, and the principles of resistance need to be elucidated in more detail. Based on the conventional hypothesis of the AMB mode of action, we compared the responses of AMB-resistant A. terreus (ATR) and AMB-susceptible A. terreus (ATS) to AMB. The role of fungal ergosterol, cellular AMB uptake (cell-associated AMB content), efflux of intracellular compounds (e.g., potassium), and the presence of oxidative intracellular damages were analyzed in detail. A murine model for disseminated aspergillosis gives insight into the clinical relevance of in vitro AMB resistance and its correlation with fitness and virulence in vivo.
MATERIALS AND METHODS
Aspergillus terreus strains.
All A. terreus isolates used in this study were derived from clinical specimens, and the AMB MICs, tested according to EUCAST and CLSI guidelines (20, 21), were 0.5 μg/ml for ATS (n = 1) and 4 μg/ml for ATR (n = 4). Sublethal (0.1 μg/ml) and lethal (10 μg/ml) AMB deoxycholate (Bristol Meyer Squibb, Austria) concentrations were used for all experiments. AMB preparation was done according to the manufacturer's recommendations. Sublethal and lethal AMB concentrations were chosen according to the strain susceptibility patterns and MIC distribution. Strains with AMB MICs of >2 μg/ml were resistant (22). ATS and ATR were susceptible to other antifungal agents (i.e., echinocandins and azoles). To validate species identification, A. terreus strains (ATS and ATR) were analyzed by sequencing the internal transcribed spacer (the ITS3 to ITS4 region), calmodulin, β-tubulin, enolase, and cytochrome B (23–25).
Experimental animal model of ATS and ATR.
The pathogenicities of ATR (n = 1) and ATS (n = 1) with and without AMB (Bristol Meyer Squibb, Austria) treatment were compared in a murine model of invasive disseminated Aspergillus infection. Inbred BALB/c mice were used for our experiments to guarantee that the immunological backgrounds of the animals were the same, thus enabling optimal comparability. Twenty-four mice (average weight, 20 to 25 g; age, 14 to 16 weeks) were randomly divided in 4 groups and intravenously (i.v.) injected with 200 mg of cyclophosphamide/kg of body weight on day −3 and every 5th day to produce prolonged immunosuppression. All the mice were inoculated with ATR or ATS on day 0 by intravenous injection of 1 × 106 conidia (100 μl of a 107-conidia/ml stock solution in 0.9% NaCl) into the tail vein. To control the correct number and viability of the conidia, the final inoculum was checked by plating 100 μl onto Sabouraud glucose (SAB) agar plates for 48 h at 36 ± 1°C. Groups 1 and 3 received ATR, and groups 2 and 4 received ATS. The mice in groups 1 and 2 were intraperitoneally treated from days 1 to 21 with 5 mg/kg amphotericin B deoxycholate (26). Groups 3 and 4 served as untreated control groups and received daily 0.9% NaCl. The animals were observed daily for clinical symptoms (i.e., lack of food and water uptake, apathy, stiff movement, and forced ventilation). Clinical endpoints were set for moribund mice, which were sacrificed by cervical dislocation. The surviving mice were sacrificed on day 21 by cervical dislocation. Survival was the main focus; therefore, we did not perform CFU counts in this particular study. This animal study was performed in compliance with the Austrian Law for Experimental Animals and in accordance with the Austrian guidelines on care and use of animals for scientific purposes. The protocol was approved by the Austrian Ministry of Science and Research.
Cultivation of A. terreus strains.
ATR (n = 4) and ATS (n = 1) cultures (inoculum, 1 × 106 to 4 × 106 CFU/ml; the number and viability control were checked via plating on SAB agar plates and incubating for 48 h at 36 ± 1°C) were grown in RPMI 1640 medium (Sigma-Aldrich, Austria) containing 20 g/liter glucose at 37°C and gently shaken (200 rpm) for 24 h. Cell pellets were harvested, weighed to equivalent amounts, treated, and analyzed according to the following protocols.
Ergosterol quantification.
Ergosterol was extracted and saponified from a lyophilized biomass in hot methanol-ethanol-KOH (10 ml/2.5 ml/1 g) solution and extracted into hexane. After the evaporation of hexane, the residue was dissolved in methanol and centrifuged, and the ergosterol content was quantified by high-performance liquid chromatography (HPLC) on a reversed-phase C18 (5-cm) column with 95% (vol/vol) methanol (flow rate, 1.0 ml/min), detected at 282 nm. Ergosterol of the same structure (Sigma-Aldrich, Austria) was used as the standard (27).
Cell-associated AMB content.
The fungal biomass was weighed and treated in sterile ultrapure water with lethal and sublethal AMB (Bristol Meyer Squibb, Austria) concentrations, respectively, for 4 and 24 h. Hyphae were then filtered (2-μm filter), washed thrice with sterile ultrapure water, weighed, and finely ground in liquid nitrogen. After the addition of 10 ml methanol and 1 h of incubation at room temperature, AMB was quantified via HPLC as described previously (28).
Release of intracellular protein, amino acids, and potassium.
Cultures were filtered (2-μm filter), washed thrice with sterile ultrapure water, weighed, and resuspended in 50 ml ultrapure water. After treatment with lethal and sublethal AMB (Bristol Meyer Squibb, Austria) concentrations for 1 to 4 h, the biomass was separated by filtration, and the concentrations of the released intracellular compounds were analyzed. To highlight the AMB effect, the background efflux values were normalized to 0 for all strains. The measurement of each compound release was done as follows.
Protein was quantified by the Bradford method (29).
Amino acids were determined by the ninhydrin colorimetric method and expressed in terms of aspartic acid (Sigma-Aldrich) and glutamic acid (Sigma-Aldrich), which were used as standards. A ninhydrin (Sigma-Aldrich) solution (200 μl; stock, 0.35 g in 100 ml ethanol) was added to each sample (1 ml) and heated to 95°C for 4 min. After cooling to room temperature in an ice bath, the absorbance at 570 nm was recorded on a spectrophotometer (Beckman) (15).
Potassium release was detected with an ion chromatography (30) system (C4 100/4.0 column, IC conductivity detector; Metrohm). The eluent flow rate (1.7 mmol/liter nitric acid and 0.75 mmol/liter dipicolinic acid) was 0.9 ml/min.
Catalase activity.
The cultures were filtered (2-μm filter), washed thrice with sterile ultrapure water, weighed, and resuspended in 50 ml ultrapure water. H2O2 degradation by catalase was measured as described previously (31).
GAPDH activity.
From the hyphal pellets, 0.2 g each were weighed and incubated in 2 ml of AMB (Bristol Meyer Squibb, Austria) at lethal and sublethal concentrations for 10 to 30 min under continuous agitation at 37°C. Subsequently, fungi were pelleted by centrifugation at 1,800 × g for 5 min, and the supernatant was discarded. The pellet was homogenized for 5 min in a Mixer Mill homogenizer (Qiagen) after the addition of 1 ml cooled PBS and centrifuged at 18,000 × g. In the supernatant, conversion of NADH to NAD+ (glyceraldehyde-3-phosphate dehydrogenase [GAPDH] activity) was measured spectrophotometrically by the decrease of the peak at 340 nm, as described previously (32).
Lipid peroxidation.
Cultures were filtered (2-μm filter), washed thrice with sterile ultrapure water, weighed, and resuspended in 50 ml ultrapure water. After treatment with lethal and sublethal AMB concentrations for 4 and 24 h, hyphal lipid peroxidation was measured with some modifications (33). Mycelia were finely ground in fluid nitrogen, and 50 mM phosphate buffer (pH 7) was added. Sixty-seven microliters of this suspension was mixed with 933 μl 20% trichloroacetic acid (TCA) plus 0.5% thiobarbituric acid (TBA) and incubated at 95°C for 30 min. The tubes were cooled (to ∼5°C) in an ice bath and centrifuged for 10 min at 10,000 × g and 5°C. Following centrifugation, the specific and nonspecific absorbances were read at 532 and 600 nm, respectively. Twenty percent TCA plus 0.5% TBA was used as the reference standard. After subtraction of the nonspecific absorbance from the specific absorbance, the net absorbance at 532 nm was expressed in terms of the amount of protein in the solutions. The protein amount in the solution used for measurement was quantified by the Bradford method (29).
Statistics.
All in vitro assays were performed on three independent replicates. The results presented are the means ± standard errors. Statistical analyses were performed using Student's two-tailed t test or one-way analysis of variance (ANOVA) with the Bonferroni multiple-comparison test. Kaplan-Meier survival curves were analyzed with the log-rank test. P values of <0.05 were considered statistically significant.
RESULTS
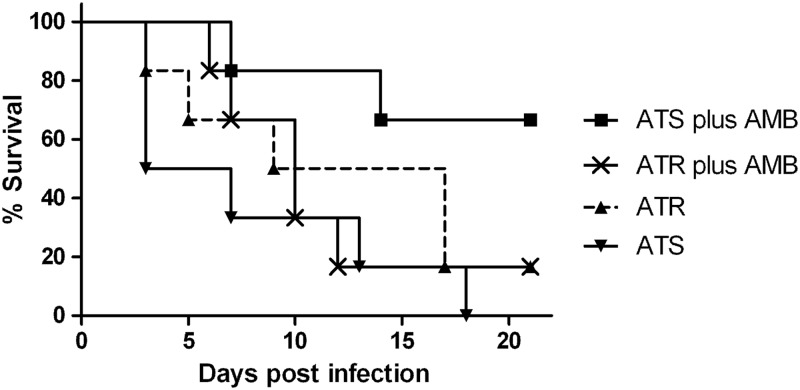
The survival rates of mice infected with ATS and those infected with ATR are shown in Fig. 1. ATS infection killed per se 50% of the mice by day 3 and was significantly more lethal than ATR (P < 0.05). At day 18, all mice infected with the ATS strain had died, whereas 17% of the animals that were infected with ATR and received no treatment survived. Treatment with AMB improved survival in ATS infection by up to 75%, while this was not the case with ATR infection (P < 0.05).
Fig 1.
Survival of amphotericin B (AMB)-treated and untreated Aspergillus terreus infection. Mice were infected with 1 × 106 conidia. Shown is the improved survival rate of AMB-susceptible A. terreus (ATS) plus AMB treatment compared to that of untreated ATS and AMB-resistant A. terreus (ATR) with and without treatment (P < 0.05, analyzed by the log-rank test).
Ergosterol and cell-associated AMB in ATS and ATR.
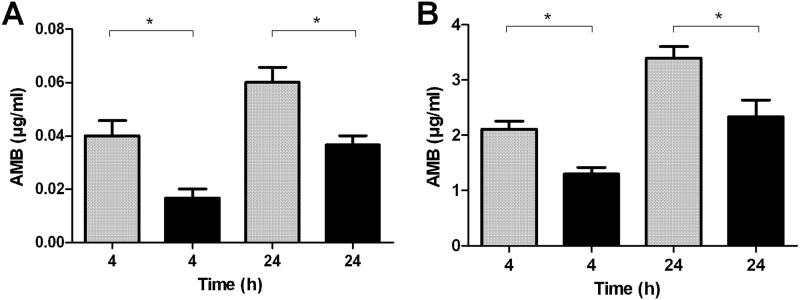
The ergosterol content of ATR and ATS showed no significant differences, as the mean ergosterol values were 5.41 ± 1.96 and 5.26 ± 1.34 μg/mg, respectively. ATR and ATS showed significant differences in cell-associated AMB content (Fig. 2). The accumulated AMB concentration was significantly higher in ATS than in ATR after 4 and 24 h of incubation with both concentrations (sublethal and lethal) tested (P < 0.05).
Fig 2.
Cell-associated amphotericin B (AMB) content of susceptible (gray columns) and resistant (black columns) Aspergillus terreus. Fungal hyphae were treated with different AMB concentrations for 4 and 24 h. (A) Cell-associated AMB content of resistant (ATR) and susceptible (ATS) A. terreus incubated with sublethal (0.1 μg/ml) AMB concentrations. (B) Cell-associated AMB content of ATR and ATS incubated with lethal (10 μg/ml) AMB concentrations. *, P < 0.05, analyzed with Student's two-tailed t test.
Differences in protein, amino acid, and potassium release of ATS versus ATR.
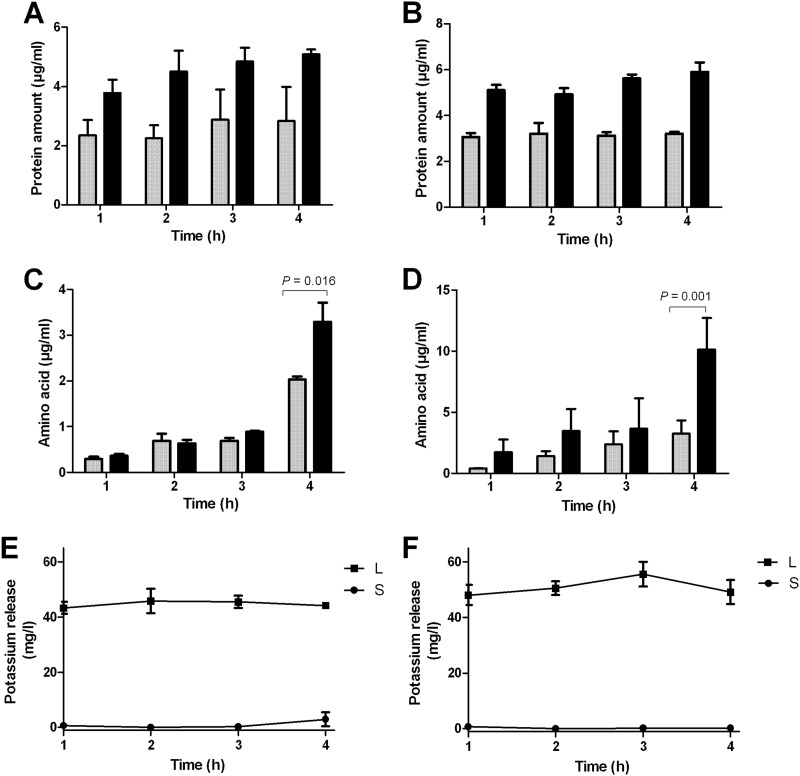
In response to AMB, intracellular protein, amino acid, and potassium release differed in ATR and ATS (Fig. 3). ATR showed higher protein (P < 0.05) and amino acid release than ATS did at both concentrations and time points tested. The highest amino acid peaks in ATR were detected after 4 h of AMB treatment with sublethal and lethal concentrations (3.29 ± 0.59 and 10.14 ± 1.9 μg/ml, respectively). Similar data were obtained for potassium efflux in ATR (54.41 ± 5.65 mg/liter). In ATS, the highest potassium release (46.81 ± 5.55 mg/liter) was measured after 2 h of incubation with the lethal concentration.
Fig 3.
Amphotericin B (AMB)-induced efflux of AMB-susceptible (ATS) (gray columns) and AMB-resistant (ATR) (black columns) Aspergillus terreus. (A) Protein efflux at sublethal (0.1 μg/ml) AMB concentrations. (B) Protein efflux at lethal (10 μg/ml) AMB concentrations. In all experiments, the level of protein release of ATR was significantly higher (P < 0.05). (C) Amino acid efflux at sublethal (0.1 μg/ml) AMB concentrations. (D) Amino acid efflux at lethal (10 μg/ml) AMB concentrations. (E) Potassium release in susceptible A. terreus treated with sublethal (S) and lethal (L) concentrations of AMB. (F) Potassium release in resistant A. terreus treated with sublethal and lethal concentrations of AMB. For statistical analyses, Student's two-tailed t test was used.
AMB-induced oxidative injury.
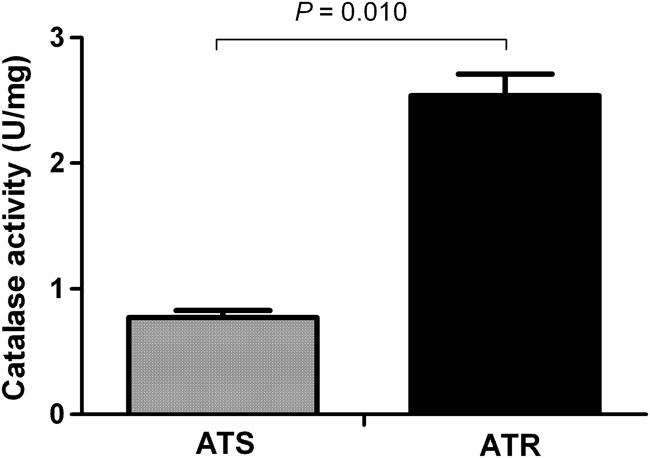
The basal catalase activity of ATR was significantly (P = 0.010) higher than that of ATS (Fig. 4). The mean catalase activity levels of ATR and ATS were 2.54 ± 0.24 and 0.77 ± 0.08 U/mg, respectively.
Fig 4.
Hyphal catalase activity of amphotericin B (AMB)-susceptible (ATS) and AMB-resistant (ATR) Aspergillus terreus species. Significant (P = 0.010, analyzed by Student's two-tailed t test) differences were seen between the hyphal catalase activity levels of ATR and ATS.
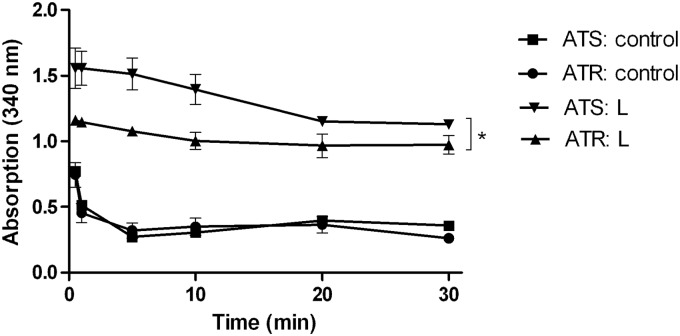
Conversion of NADH to NAD+ by the fungal GAPDH was demonstrated by the decrease of absorption at 340 nm (Fig. 5, controls) in both ATR and ATS. Oxidative GAPDH inactivation due to AMB is shown in Fig. 5 by the significantly lower decrease of NADH (P < 0.01) in both AMB-treated samples versus the controls. The absorbance values of ATS and ATR were also significantly different (P < 0.05).
Fig 5.
GAPDH activity in amphotericin B (AMB)-susceptible (ATS) and AMB-resistant (ATR) Aspergillus terreus with lethal (L) AMB treatment (10 μg/ml) and without (control) AMB treatment. *, P < 0.05, analyzed by one-way analysis of variance (ANOVA) with the Bonferroni multiple-comparison test.
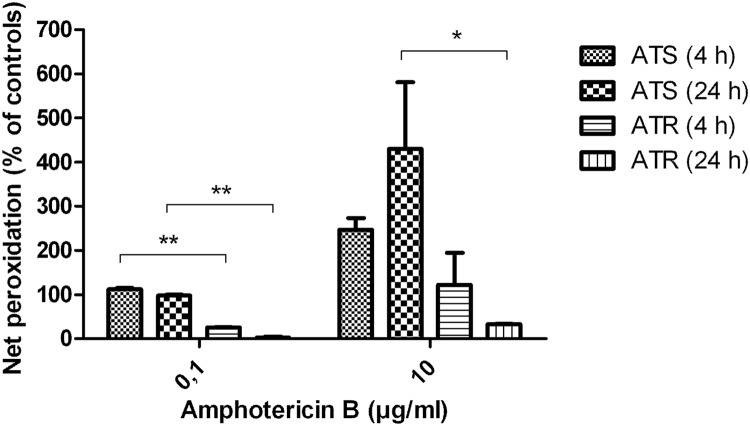
Lipid peroxidation was significantly higher in ATS than in ATR (P < 0.05), independent of AMB concentration and exposure time (Fig. 6). ATS treated with 0.1 μg/ml AMB showed nearly the same effect on peroxidation after 4 and 24 h of incubation. Ten micrograms per milliliter AMB increased lipid peroxidation dramatically, especially after 24 h of incubation. In general, ATR showed less peroxidation than ATS did, and after 24 h of AMB treatment, the lipid peroxidation level decreased.
Fig 6.
Lipid peroxidation of amphotericin B (AMB)-susceptible (ATS) and AMB-resistant (ATR) Aspergillus terreus normalized to that of untreated controls in response to AMB. *, P < 0.05, and **, P < 0.005, analyzed by Student's two-tailed t test. Incubation times are given in parentheses.
DISCUSSION
This study investigated the differences between the in vivo and in vitro responses of AMB-resistant and -susceptible A. terreus strains to AMB treatment. Our in vivo murine model of disseminated aspergillosis showed ATS (AMB MIC, 0.5 μg/ml) to be highly virulent, indicating that loss of fungal fitness is not associated with the appearance of AMB susceptibility. AMB therapy (5 mg/kg) significantly enhanced the in vivo outcome in mice infected with ATS, as 75% of them survived. The survival rates of ATR-infected mice with and without AMB treatment were similar in both groups. These data show the crucial clinical relevance of in vitro AMB MICs in A. terreus. Dannaoui and colleagues showed comparable survival rates of AMB-treated and untreated mice infected with AMB-resistant A. terreus (34).
In the second part of our study, we investigated the in vitro mechanisms related to AMB resistance in ATR and ATS. Membrane damage increased membrane permeability and intracellular oxidative damage, thought to be responsible for AMB action (14, 19). Reduced susceptibility to AMB has been associated with the upregulation of several ergosterol biosynthesis genes (ERG5, ERG6, and ERG25) and decreased ergosterol content in the fungal cell membrane (35–37). In contrast, our data suggest that the ergosterol content does not play a major role in AMB resistance in the A. terreus strains we tested. The mean ergosterol contents of ATR and ATS were equal. However, major differences were observed in cell-associated AMB content. ATS assimilated more AMB than ATR did. Therefore, we conclude that cell-associated AMB content is not directly related with membrane ergosterol. Reeves et al. showed AMB to induce amino acid efflux in A. fumigatus (15), and we also demonstrated an efflux of amino acids, intracellular proteins, and potassium in response to AMB, depending on the incubation time and concentration. In contrast to the expectation from these findings, ATR dispersed higher outflow levels of intracellular compounds in our study. The disruption of the cell membrane by AMB causes intracellular component efflux, but it obviously does not explain the fungicidal activity of AMB. Therefore, pore formation might play a minor role in AMB resistance in A. terreus.
Others have speculated that AMB leads to cell death by the formation of oxygen radicals, such as hydrogen peroxide (H2O2) (16, 19). Here, AMB induced intracellular oxidation, which in turn led to lipid peroxidation and, subsequently, to cell death. H2O2 permeates through biological membranes and causes peroxidation, but it might be neutralized via endogenous catalase (19). We showed significantly (P = 0.01) higher catalase activity in ATR than in ATS. Furthermore, ATR exhibited reduced lipid peroxidation at sublethal and lethal AMB concentrations, and ATR was able to reduce lipid peroxidation over time. These findings underline the fact that ATR better manages oxidative damage and possesses improved intracellular recovery systems.
Also, ATR seems to be best equipped with the intracellular enzyme activity responsible for survival strategies. This is supported by our data showing intracellular GAPDH activity, which can be used as an intracellular oxidation marker (38). Values of absorbance at 340 nm (λmax of NADH) were lower in ATS than in ATR in response to AMB. Although the shapes of both curves were similar, this might indicate a slightly lower GAPDH activity in AMB-treated ATS than in AMB-treated ATR. Thus, ATR might have an improved ability to prevent oxidative GAPDH inactivation, which might lead to improved survival.
In conclusion, AMB susceptibility in A. terreus is not necessarily associated with loss of in vivo virulence, but the outcome under therapy is improved in infections with AMB-susceptible A. terreus compared to those with resistant A. terreus. In contrast to current knowledge, our experiments show that ergosterol content plays a minor role in AMB resistance and is not directly associated with intracellular cell-associated AMB content. However, our results indicate that resistant A. terreus species absorb less AMB, discharge more intracellular compounds, and have better protection against oxidative damage than susceptible ones. AMB possibly exerts its antifungal activity by oxidative injury rather than by an increase of membrane permeation.
ACKNOWLEDGMENTS
This work was supported by TWF (2012-1-1069) and Gilead (PO 62051550).
We thank Anna Maria Tortorano (Faculty of Medicine and Surgery, University of Milan, Italy) for providing the AMB-susceptible A. terreus strain.
Footnotes
Published ahead of print 14 January 2013
REFERENCES
- 1. Fridkin S. 2005. The changing face of fungal infections in health care settings. Clin. Infect. Dis. 41:1455–1460 [DOI] [PubMed] [Google Scholar]
- 2. Martino R, Subira M. 2002. Invasive fungal infections in hematology: new trends. Ann. Hematol. 81:233–243 [DOI] [PubMed] [Google Scholar]
- 3. Singh N. 2001. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin. Infect. Dis. 33:1692–1696 [DOI] [PubMed] [Google Scholar]
- 4. Bruggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin. Infect. Dis. 48:1441–1458 [DOI] [PubMed] [Google Scholar]
- 5. Anaissie E. 1992. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin. Infect. Dis. 14:S43–S53 [DOI] [PubMed] [Google Scholar]
- 6. Blum G, Perkhofer S, Grif K, Mayr A, Kropshofer G, Nachbaur D, Kafka-Ritsch R, Dierich MP, Lass-Florl C. 2008. A 1-year Aspergillus terreus surveillance study at the University Hospital of Innsbruck: molecular typing of environmental and clinical isolates. Clin. Microbiol. Infect. 14:1146–1151 [DOI] [PubMed] [Google Scholar]
- 7. Lass-Flörl C, Grif K, Kontoyiannis DP. 2007. Molecular typing of Aspergillus terreus isolates in Houston, Texas, and Innsbruck, Austria: evidence of great genetic diversity. J. Clin. Microbiol. 45:2686–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lass-Flörl C, Rath PM, Niederwieser D, Kofler G, Würzner R, Kreczy A, Dierich MP. 2000. Aspergillus terreus infections in haematological malignancies: molecular epidemiology suggests association with in-hospital plants. J. Hosp. Infect. 46:31–35 [DOI] [PubMed] [Google Scholar]
- 9. Varga J, Toth B, Kocsube S, Farkas B, Szakacs G, Teren J, Kozakiewicz Z. 2005. Evolutionary relationships among Aspergillus terreus isolates and their relatives. Antonie van Leeuwenhoek 88:141–150 [DOI] [PubMed] [Google Scholar]
- 10. Abraham O, Manavathu E, Cutright JL, Chandrasekar PH. 1999. In vitro susceptibilities of Aspergillus species to voriconazole, itraconazole, and amphotericin B. Diagn. Microbiol. Infect. Dis. 33:7–11 [DOI] [PubMed] [Google Scholar]
- 11. Espinel-Ingroff A, Arthington-Skaggs BA, Igbal N, Ellis D, Pfaller M, Messer SA, Rinaldi M, Fothergill A, Gibbs D, Wang A. 2007. A multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin-B and caspofungin. J. Clin. Microbiol. 45:1811–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinbach W, Benjamin D, Kontoyiannis D, Perfect J, Lutsar I, Marr K, Lionakis M, Torres H, Jafri H, Walsh T. 2004. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 39:192–198 [DOI] [PubMed] [Google Scholar]
- 13. Abu-Salah KM. 1996. Amphotericin B: an update. Br. J. Biomed. Sci. 53:122–133 [PubMed] [Google Scholar]
- 14. Finkelstein A, Holz R. 1973. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes 2:377–408 [PubMed] [Google Scholar]
- 15. Reeves EP, Murphy T, Daly P, Kavanagh K. 2004. Amphotericin B enhances the synthesis and release of the immunosuppressive agent gliotoxin from the pulmonary pathogen Aspergillus fumigatus. J. Med. Microbiol. 53:719–725 [DOI] [PubMed] [Google Scholar]
- 16. Vanden Bossche H, Warnock DW, Dupont B, Kerridge D, Sen GS, Improvisi L, Marichal P, Odds FC, Provost F, Ronin O. 1994. Mechanisms and clinical impact of antifungal drug resistance. J. Med. Vet. Mycol. 32:189–202 [DOI] [PubMed] [Google Scholar]
- 17. Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U. S. A. 109:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seo K, Akiyoshi H, Ohnishi Y. 1999. Alteration of cell wall composition leads to amphotericin B resistance in Aspergillus flavus. Microbiol. Immunol. 43:1017–1025 [DOI] [PubMed] [Google Scholar]
- 19. Sokol-Anderson M, Brajtburg J, Medoff G. 1986. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 154:76–83 [DOI] [PubMed] [Google Scholar]
- 20. Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984 [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. CLSI Document M38-A2 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22. Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob. Agents Chemother. 55:5150–5154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balajee SA, Baddley JW, Peterson SW, Nickle D, Varga J, Boey A, Lass-Florl C, Frisvad JC, Samson RA. 2009. Aspergillus alabamensis, a new clinically relevant species in the section Terrei. Eukaryot. Cell 8:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang L, Yokoyama K, Miyaji M, Nishimura K. 2001. Identification, classification, and phylogeny of the pathogenic species Exophiala jeanselmei and related species by mitochondrial cytochrome b gene analysis. J. Clin. Microbiol. 39:4462–4467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yaguchi T, Horie Y, Tanaka R, Matsuzawa T, Ito J, Nishimura K. 2007. Molecular phylogenetics of multiple genes on Aspergillus section Fumigati isolated from clinical specimens in Japan. Nihon Ishinkin. Gakkai Zasshi. 48:37–46 [DOI] [PubMed] [Google Scholar]
- 26. Johnson EM, Oakley KL, Radford SA, Moore CB, Warn P, Warnock DW, Denning DW. 2000. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J. Antimicrob. Chemother. 45:85–93 [DOI] [PubMed] [Google Scholar]
- 27. Rössner H. 1993. Fungal biomass by ergosterol content, p 49–51 In Methods in soil biology. Springer, Berlin, Germany [Google Scholar]
- 28. Egger P, Bellmann R, Wiedermann CJ. 2001. Determination of amphotericin B, liposomal amphotericin B, and amphotericin B colloidal dispersion in plasma by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 760:307–313 [DOI] [PubMed] [Google Scholar]
- 29. Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 30. Thienpont LM, Van Nuwenborg JE, Stockl D. 1995. Ion chromatography as potential reference methodology for the determination of total sodium and potassium in human serum. J. Chromatogr. A 706:443–450 [DOI] [PubMed] [Google Scholar]
- 31. Aebi H. 1984. Catalase in vitro. Methods Enzymol. 105:121–126 [DOI] [PubMed] [Google Scholar]
- 32. Pullar JM, Winterbourn CC, Vissers MC. 1999. Loss of GSH and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am. J. Physiol. 277:H1505–H1512 [DOI] [PubMed] [Google Scholar]
- 33. Peever TL, Higgins VJ. 1989. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 90:867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dannaoui E, Borel E, Persat F, Piens MA, Picot S. 2000. Amphotericin B resistance of Aspergillus terreus in a murine model of disseminated aspergillosis. J. Med. Microbiol. 49:601–606 [DOI] [PubMed] [Google Scholar]
- 35. Barker KS, Crisp S, Wiederhold N, Lewis RE, Bareither B, Eckstein J, Barbuch R, Bard M, Rogers PD. 2004. Genome-wide expression profiling reveals genes associated with amphotericin B and fluconazole resistance in experimentally induced antifungal resistant isolates of Candida albicans. J. Antimicrob. Chemother. 54:376–385 [DOI] [PubMed] [Google Scholar]
- 36. Deak E, Wilson SD, White E, Carr JH, Balajee SA. 2009. Aspergillus terreus accessory conidia are unique in surface architecture, cell wall composition and germination kinetics. PLoS One 4:e7673 doi:10.1371/journal.pone.0007673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walsh T, Petraitis V, Petraitiene R, Field-Ridely A, Sutton DA, Ghannoum M, Sein T, Schaufele R, Peter J, Bacher J, Casler H, Armstrong D, Espinel-Ingroff A, Rinaldi M, Lyman CA. 2003. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J. Infect. Dis. 188:305–319 [DOI] [PubMed] [Google Scholar]
- 38. Vaidyanathan VV, Sastry PS, Ramasarma T. 1993. Regulation of the activity of glyceraldehyde 3-phosphate dehydrogenase by glutathione and H2O2. Mol. Cell. Biochem. 129:57–65 [DOI] [PubMed] [Google Scholar]