Abstract
Studies in mice indicated that activation of the erythroid stress pathway requires the presence of both soluble KIT ligand (KITL) and the glucocorticoid receptor (GR). To clarify the relative role of KITL and GR in stress erythropoiesis in humans, the biological activities of soluble full length- (fl-, 26–190 aa), carboxy-terminus truncated (tr-, 26–162 aa) human (hKITL) and murine (mKITL) KITL in cultures of cord blood (CB) mononuclear cells (MNCs) and CD34pos cells that mimic either steady state (growth factors alone) or stress (growth factors plus dexamethasone [DXM]) erythropoeisis were investigated. In steady state cultures, the KITLs investigated were equally potent in sustaining growth of hematopoietic colonies and expansion of megakaryocytes (MK) and erythroid precursors (EBs). By contrast, under stress erythropoiesis conditions, fl-hKITL generated greater numbers of EBs (fold increase [FI]=140) than tr-hKITL or mKITL (FI=20–40). Flow cytometric analyses indicated that only EBs generated with fl-hKITL remained immature (>70% CD36pos/CD235aneg/low), and therefore capable to proliferate, until day 8–12 in response to DXM. Signaling studies indicated that all KITLs investigated induced EBs to phosphorylate signal transducer and activator of transcription 5 (STAT5) but that extracellular-signaling-regulated-kinases (ERK) activation was observed mainly in the presence of fl-hKITL. EBs exposed to fl-hKITL also expressed higher levels of GRα than those exposed to mKITL (and tr-hKITL) which were reduced upon exposure to the ERK inhibitor U0126. These data reveal a unique requirement for fl-hKITL in the upregulation of GRα and optimal EB expansion in cultures that mimic stress erythropoiesis.
Introduction
Red blood cells (RBCs) have a limited life-span and under steady state conditions their number is maintained constant by the continuous production of new RBCs from hematopoietic stem/progenitor cell compartments [1]. Erythropoiesis occurs mainly in the marrow and is regulated by the interplay between erythropoietin (EPO), a hormone produced by the kidney, and KIT ligand (KITL), a factor produced by stromal elements in the marrow. KITL is expressed as a membrane-bound protein which in concert with other components on the membrane of stromal cells regulates hematopoiesis in the stem/progenitor cell niche. Proteolytic cleavage of membrane KITL releases a soluble form of KITL into the microenvironment, which is also present in the circulation [2]. Soluble KITL is an effective inducer of erythroid maturation in vitro [3,4]. However, targeted mutant mice expressing exclusively the more stable membrane isoform of KITL, KITL2, lacking the major proteolytic cleavage site, have normal hematocrit values but recover poorly from radiation-induced anemia [5]. In wild-type mice, sublethal radiation induced a transient 4-fold increase of KITL in the serum (from <0.5 to >2 ng/mL) reaching a peak after 7 days. In contrast, the proteolytic cleavage mutant KITLKL2/KL2 mice did not release soluble KITL into the serum after sublethal radiation and survival was significantly diminished because of anemia [5]. These observations suggest that soluble KITL, although dispensable for steady state erythropoeisis, plays an important regulatory role under conditions of stress.
The pathway(s) that regulates erythropoiesis under conditions of acute or chronic anemia is starting to emerge [6]. Recent evidence suggests that, in addition to increasing EPO production by the kidney, this pathway activates microenvironmental cues that generate stress-specific hematopoietic compartments [7,8]. The importance of the glucocorticoid receptor (GR) in the control of stress erythropoiesis was established by studies in transgenic mice harboring a dimerization-defective GR (GRdim/dim mice) [9]. These mice have normal hematocrits under steady-state conditions but are unable to increase RBC production in response to hypoxia. Gene deletion studies established that GR facilitates stress erythropoiesis in mice by blocking maturation of erythroid precursors (EBs) and inducing them into a self-renewal state [10].
The identification of mechanisms that control erythropoiesis in humans relies on studies conducted using in vitro experimental systems and human forms of anemia resembling the phenotypes induced by genetic manipulations in mice. In culture, KITL drives mainly mast cell maturation when used alone and lineage-specific maturation when used in combination with lineage-specific growth factors [2]. For example, KITL, in combination with EPO, sustains unilineage erythroid differentiation of hematopoietic progenitor cells from various sources (human and mouse adult bone marrow, human adult blood [AB], cord blood [CB], and fetal liver) (reviewed in [11]). These cultures generate low numbers of EBs (fold increases [FI] in the order of 1–4 by days 10–15) and may be considered models of steady state erythropoiesis. Clinical observations in patients with Cushing's syndrome and those treated with glucocorticoids who develop polycythemia [12] have inspired the development of culture conditions employing stimulation with dexamethasone (DXM, the ligand for GR) in addition to growth factors (human erythroid massive amplification [HEMA] cultures) that generate great numbers of EBs (FI > 10 or >1000 when seeded with mononuclear cells [MNCs] or CD34pos cells, respectively) [13–16]. Similar to observations in mouse models of stress erythropoiesis, this great EB amplification occurs because DXM induces EBs into a self-renewal state [17–19], suggesting that HEMA culture may represent a model for stress erythropoiesis in humans. How soluble KITL and GR cooperate in eliciting the response of EBs to stress (in HEMA culture and/or in hypoxic mice) has not been investigated as yet.
The aim of this study was to assess whether the structure of KITL affects the levels of EB expansion observed in culture models of human steady state and stress erythropoiesis. The biological activity of soluble full-length human KITL (fl-hKITL, aa 26–190) and carboxy-terminus truncated KITL (tr-hKITL, aa 26–162) in hematopoietic colony assays and in liquid cultures tailored for megakaryocytic (MK) and EB expansion with or without DXM was compared. Given the reported similar activity of fl-hKITL and murine KITL (mKITL) in human mast cell cultures [20], parallel cultures were stimulated with mKITL as comparison. The data confirmed that fl-hKITL, tr-hKITL, and mKITL had similar activities in culture conditions mimicking steady state hematopoiesis (colony formation and MK/EB expansion in liquid cultures without DXM). However, under conditions mimicking stress erythropoiesis (HEMA cultures), fl-hKITL sustained greater EB expansion than either tr-hKITL or mKITL. In biochemical studies, fl-hKITL activated ERK phosphorylation and GRα expression while mKITL/tr-hKITL had poor/modest effects (absence of rapid/slightly increased sustained ERK activation and suppression of GRα expression). These data identify that KITL regulates GR expression in human EBs and establishes a link between the structural integrity of soluble hKITL and EB expansion under conditions of stress erythropoiesis.
Materials and Methods
Recombinant KITL
Recombinant fl-hKITL (26–190 aa) was purchased from R&D Systems (Minneapolis, MN). Recombinant tr-hKITL was produced in a baculovirus expression system as described previously as a C-terminal immunoglobulin fusion protein that after affinity purification was proteolytically processed to release the affinity tail [21]. Mass spectrometry analysis indicated that the protein included aa 26–162 of hKITL (kindly provided by Dmiitar Nikolov, Sloan Kettering Institute). mKITL was produced by baby hamster kidney (BHK) cells molecularly engineered with the murine gene (a gift of Patrice Mayeux). The concentration of the factor in the supernatant of the cell line was determined by standard dose/response curves in murine colony and UT7 proliferation assays as described by Smith and Zsebo [22].
Human subjects
CB was obtained as de-identified samples from the New York Blood Center, New York. Buffy coats from AB donations were provided by the transfusion center of “La Sapienza” University (Rome, Italy). CB and AB were provided according to guidelines established by the respective institutional review boards.
Cell separation
CB MNCs were separated by centrifugation over Ficoll-Hypaque (Amersham Pharmacia Biotec, Uppsala, Sweden). CD34pos cells were separated using the Human CB CD34 Selection Kit (STEMCELL Technologies, Inc., Vancouver, BC, Canada), as described by the manufacturer. CD34pos cells were >90% pure upon re-analyses and stored cryopreserved in standard fetal bovine serum (FBS) containing 10% (v/v) dimethyl sulfoxide.
Colony forming assay
CB MNCs (2×105 cells/mL) and CD34pos cells (500 cells/mL) were cultured in semisolid methylcellulose cultures (MethoCult; STEMCELL Technologies, Inc.) supplemented with 30% (v/v) FBS and 10% (w/v) bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) and stimulated with interleukin (IL)-3 (1 ng/mL) (Biosource, San Jose, CA), granulocyte-macrophage colony-stimulating factor (GM-CSF, 10 ng/mL) (Leukine, Genzyme, Cambridge, MA), granulocyte colony-stimulating factor (G-CSF, 100 ng/mL) (Neupogen, Amgen, Thousand Oaks, CA) plus increasing concentration of tr-hKITL or concentrations of fl-hKITL and mKITL previously shown to sustain optimal growth of hematopoietic colonies (10 ng/mL) [3]. In selected cultures, EPO (5 U/mL) (Epogen, Amgen, Thousand Oaks, CA) was added to stimulate the growth of burst forming unit-erythroid (BFU-E)-derived colonies. The cultures were incubated at 37°C in a fully humidified 5% pCO2 atmosphere and scored after 14 days for the growth of hematopoietic colonies, which were recognized according to standard morphological criteria.
Ex vivo expansion of human MKs and EBs in the absence of DXM
Purified CB CD34pos cells (3×105/mL) were cultured up to 12 days in serum-free X-Vivo medium (Lonza Walkersville, Inc., Walkersville, MD) supplemented with human IL-3 (3 ng/mL), thrombopoietin (TPO) (3% of a supernatant of BHK cells molecularly engineered to express the human TPO gene, generously provided by K. Kaushansky), and either the fl-hKITL, tr-hKITL, or mKITL (all at 50 ng/mL), as described by Gobbi et al. [23]. The cultures were replenished with fresh cytokines and medium every 3 days.
Ex vivo expansion of human EBs
CD34pos cells and MNCs (5×104 and 106 cells/mL, respectively) were cultured in Iscove's modified Dulbecco's medium (Lonza Walkersville, Inc.) supplemented with FBS (PAA Laboratories, Dartmouth, MA) (20% v/v), human BSA (15% w/v), EPO (3 U/mL), human IL-3 (10 ng/mL), l-glutamine (200 mM; Lonza Walkersville, Inc.), antibiotics (penicillin [10,000 units/mL], streptomycin sulfate [10,000 μg/mL], and amphotericin B [25 μg/mL]; Lonza Walkersville, Inc.), β-mercaptoethanol (10−6 M; Sigma Aldrich), and either fl-hKITL (R&D Systems, Minneapolis, MN), tr-hKITL, or mKITL (50 ng/mL in all cases), as described by Migliaccio et al. [16]. The cultures were performed with (HEMA culture) and without DXM and estradiol (10−6 M for both) (Sigma-Aldrich) and kept for up to 10–16 days at 37°C in a fully humidified 5% pCO2 incubator.
Cell viability and phenotypic analysis
Cell numbers and viability were assessed by microscopic evaluation after trypan blue staining (Boston Bioproducts, Ashland, MA). Erythroid and MK maturations were assessed according to standard morphological criteria by visual examination of cytocentrifuged cell preparations (Cytospin 3; Shandon, Astmoor, England) stained with May-Grünwald-Giemsa (Fisher Scientific, Pittsburg, PA) with the Axioscope light microscope equipped with a Coolsnap video camera (Zeiss, Oberkochen, Germany). Alternatively, cells were suspended in Ca2+/Mg2+-free phosphate-buffered saline, supplemented with 1% BSA, stained with either phycoerythrin (PE)–conjugated CD36 (antithrombospondin receptor) [24] and allophycocyanin (APC)–conjugated CD235a (anti-glycophorin A), or PE-coniugated CD41a (which recognizes the GPIIb/IIIa complex) and APC-CD42 (which recognizes GPIb) or appropriate isotype controls (all from Becton Dickinson Biosciences, Franklin Lakes, NJ), and analyzed with the fluorescence activated cell sorting (FACS) Canto (Becton Dickinson Biosciences) equipped with 3 air-cooled solid state lasers (488, 633, and 407 nm). In selected experiments, the levels of cKIT expressed by the cells were determined on the basis of the cyanine 5.5-CD117 signaling (BioLegend, San Diego, CA). Dead cells were excluded by propidium iodide (PI, 5 μg/mL; Sigma) staining.
Quantitative real-time polymerase chain reaction determination of GRα expression
Total RNA was isolated from 106 cells using RNeasy Mini Kit (Quiagen Sciences, Germantown, MD), following the manufacturer's instructions. One microgram of RNA quantified by NanoDrop Technology (Thermo Scientific, Wilmington, DE) was reverse transcribed using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. cDNA was diluted with the Universal PCR Master Mix (KAPA Biosystem, Boston, MA), and subjected to real-time PCR analysis (ABI7000; Applied Biosystem) following standard protocols using primers as described by Pedersen and Vedeckis [25]. Expression levels were calculated with the algorithm ΔCt=CtX – CtGAPDH, where Ct is the average threshold cycle and X is the GRα cDNA and presented as relative expression units with respect to controls.
Western blot analysis
Whole cell extracts were prepared from day 11 EBs collected from HEMA culture and from those incubated in media deprived of growth factors (GFD) for 4 h and then stimulated with either fl-hKITL, tr-hKITL, or mKITL (50 ng/mL in all cases) for increasing lengths of time (15 min, 1 h, 2 h, and 4 h) as described by Varricchio et al. [17]. In selected experiments, EBs were exposed to 100 μM of the mitogen ERK kinase 1/2 (MEK 1/2) inhibitor U0126 (cat No. 9903; Cell Signalling, Lake Placid, NY) before stimulation with fl-hKITL. Proteins (30 μg) were separated on sodium dodecylsulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes that were first incubated with anti-GRα (H300, sc-8992; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-ERK (cat No. 9102) or anti-pERK (cat No. 9101 and No. 4060), anti-AKT (cat No. 9272) or anti-phospho AKT(p-AKT) (cat No. 9271) (all from Cell Signalling), and anti-STAT5 (cat No. sc835; Santa Cruz Biotechnology, Inc.) or anti-STAT5pTyr (cat No. 9351S; Cell Signaling) antibodies, and then with appropriate horseradish peroxidase-coupled secondary antibodies (Calbiochem, San Diego, CA). Lysates from the breast epithelial cell line MCF10A were used as positive control of pAKT [26]. All the antibodies were used at dilutions of 1:1000. Immune complexes were detected with an enhanced chemiluminescence kit (Amersham, Buckingamshire, UK).
Statistical analysis
Statistical analysis was performed by analysis of variance test with the computer software Origin 5.0 for Windows (Microcal Software, Inc., Northampton, MA).
Results
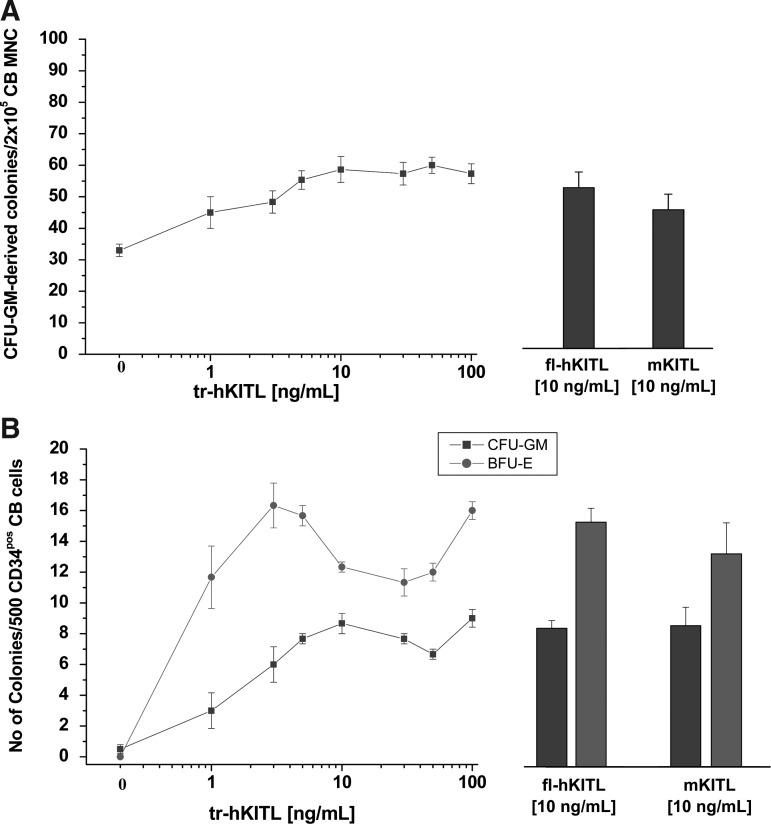
Similar numbers of CFU-GM- and BFU-E-derived colonies are observed in semisolid cultures of human cells stimulated with fl-hKITL, tr-hKITL, and mKITL
The tr-hKITL concentration/response curves for hematopoietic colonies generated by human CB MNCs and CD34pos cells are presented in Fig. 1. Results are compared with those observed with optimal concentrations of fl-hKITL and mKITL. In both cases, the number of GM-derived colonies reached a plateau at a concentration of tr-hKITL of 10 ng/mL while that of BFU-E-derived colonies reached a plateau at a concentration of 3 ng/mL. The maximal numbers of colonies observed in cultures stimulated with tr-hKITL were similar to those observed in cultures stimulated with fl-hKITL and mKITL (10 ng/mL). The concentrations of tr-hKITL that induced maximal colony growth are similar to those reported in a previous study [3].
FIG. 1.
tr-hKITL is as potent as fl-hKITL and mKITL in sustaining the growth of CFU-GM- and BFU-E-derived colonies from CB MNCs and CD34pos cells. (A) tr-hKITL concentration/response curve for CFU-GM-derived colonies from CB MNCs. Results are compared with those obtained in parallel cultures stimulated with optimal concentrations of fl-hKITL and mKITL (10 ng/mL for both, bar graphs on the right). (B) tr-KITL concentration/response curves for CFU-GM- (squares) and BFU-E-derived colonies (circles) from CB CD34pos cells. The number of erythroid bursts (light gray bars) and granulo-monocytic colonies (dark gray bars) generated by CD34pos cells in the presence of either fl-hKITL or mKITL (10 ng/mL for both) are presented on the right, for comparison. Results are presented as mean (±SD) of 3 independent experiments. hKITL, human KIT ligand; tr, carboxy-terminus truncated; fl, full length; mKITL, murine KIT ligand; CFU, colony-stimulating factor; GM, granulocyte-macrophage; CB, cord blood; MNC, mononuclear; BFU-E, burst forming unit-erythroid; SD, standard deviation.
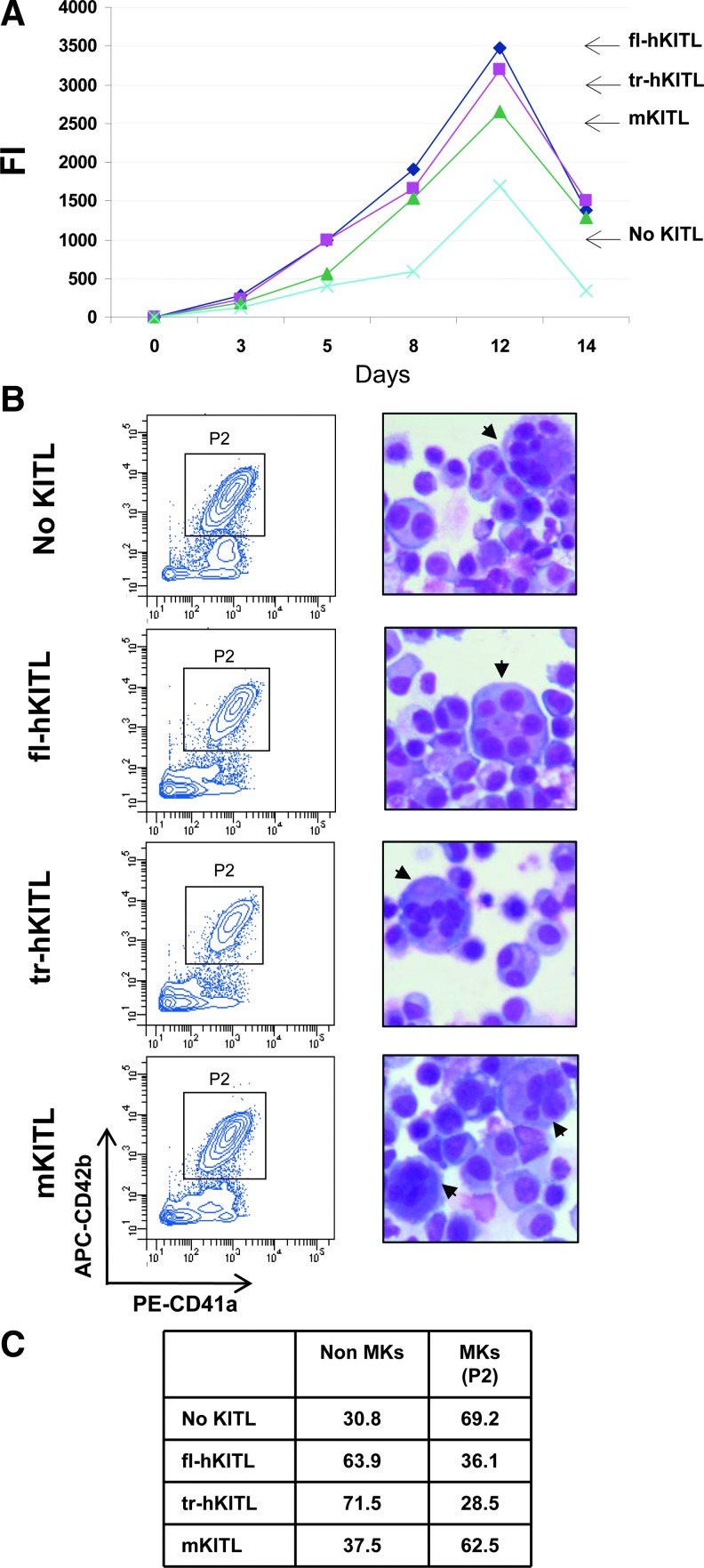
fl-hKITL, tr-hKITL, and mKITL are equally potent in sustaining MK and EB expansion from CB CD34pos cells in cultures that do not contain DXM
The effects of the 3 forms of KITL on the number of MK and EB generated by CB CD34pos cells in the absence of DXM are described in Fig. 2. In all cases, maximal numbers of cells were detected by day 12 of culture. In the absence of KITL, expansion was modest (FI < 1000) and most (>60%) of the cells observed were MK (>60% CD41aposCD42bpos). By contrast, great levels of expansion (FI=2500–3500-fold) were observed by day 12 in cultures stimulated with all the 3 forms of KITL investigated. Flow cytometrical and morphological analyses indicated that in cultures stimulated with mKITL the majority of cells generated were represented by MK (>60%), but in those stimulated with fl-hKITL and tr-hKITL the majority of the cells were represented by EBs (identified by morphology) with MK (identified by FACS) representing <40% of the cells.
FIG. 2.
fl-hKITL, tr-hKITL, and mKITL are equally potent in sustaining MK and EB expansion from CB CD34pos cells in cultures not supplemented with DXM. (A) Growth curves of CB CD34pos cells cultured for up to 14 days in the presence of IL-3 and TPO alone (no KITL, blue) or with either fl-hKITL (black), tr-hKITL (red), or mKITL (green) (50 ng/mL in all cases), as indicated. Data are expressed as FI with respect to the numbers of CD34pos cells seeded at day 0. (B) FACS profile for CD41a and CD42b expression (on the left) and morphology (by May-Grunwald staining, on the right) of cells obtained after 12 days in cultures. Arrowheads indicate representative MKs. Magnification 40×. (C) Frequency of MK and non-MK populations (mostly EBs) in the flow cytometric analysis shown in (B). MK, megakaryocytic; EBs, erythroid precursors; DXM, dexamethasone; IL, interleukin; FI, fold increase.
Thus, the 3 forms of KITL investigated had similar effects on the numbers of cells generated by CB CD34pos cells in the absence of DXM although the presence of hKITL (both fl-hKITL and tr-hKITL) favored EB expansion.
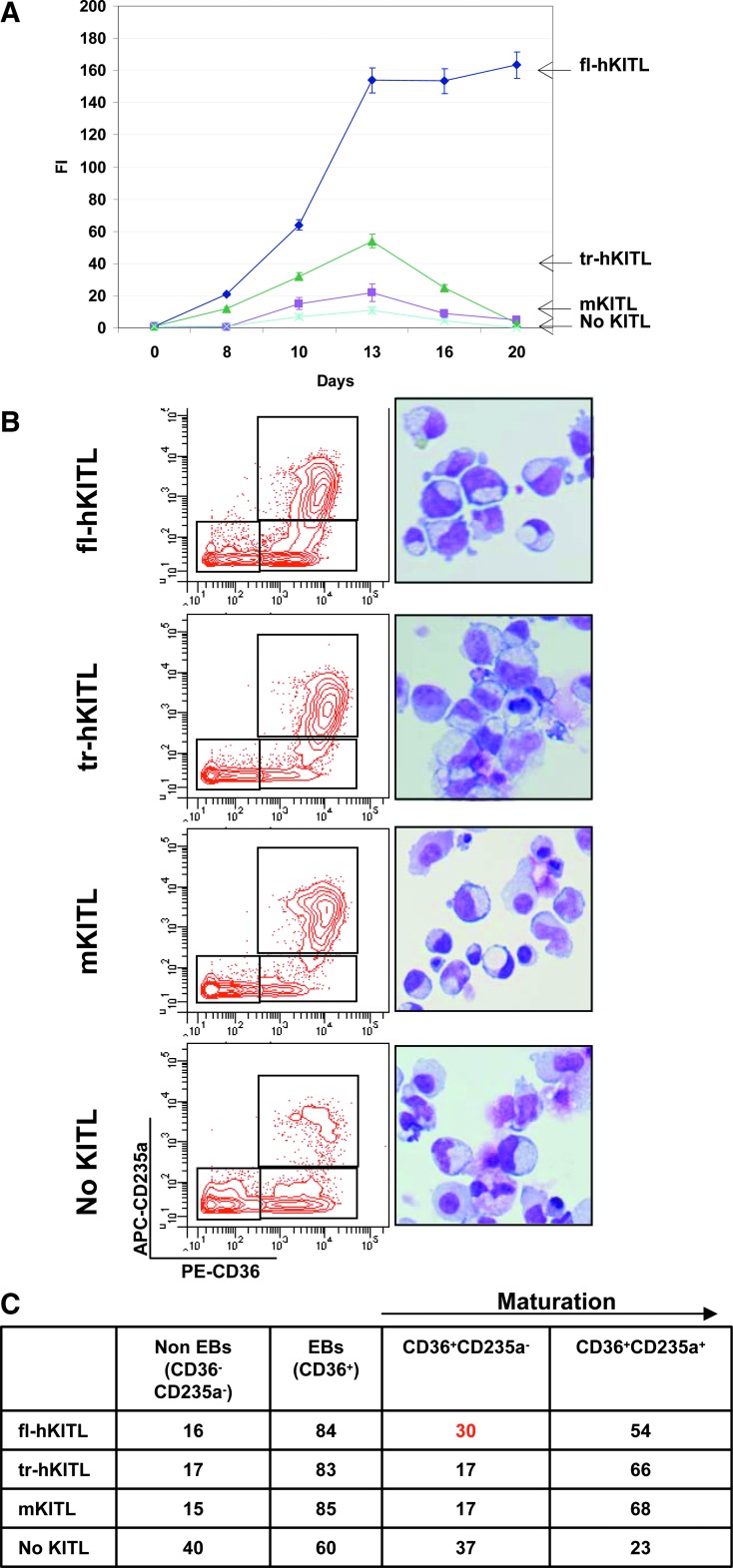
fl-hKITL is superior to both tr-hKITL and mKITL in sustaining ex vivo amplification of EBs from CB MNCs and CD34pos cells in cultures containing DXM
The effect of the 3 forms of KITL on the levels of EB amplification achieved from human CB MNCs in cultures containing DXM is compared in Fig. 3. In the absence of KITL, modest levels of amplification were observed that reached a peak (FI < 5) by day 13. FACS and morphology analyses indicated that >40% of the cells present at day 13 in cultures without KITL were not erythroid. The addition of either mKITL (FI=20) or tr-hKITL (FI=50) modestly increased the numbers of cells generated in these cultures that reached a peak by day 13 (FI=20–50). However, these cultures contained mostly erythroid cells (>80% CD36pos) demonstrating advanced maturation (>65% CD235apos). The addition of fl-hKITL induced great levels of cell amplification that reached a maximum by day 13 (FI > 150) and was sustained up to day 20. Most of the cells observed at day 13 in these cultures were EBs (>80% CD36pos). A great proportion of the day 13 EBs observed in these cultures was immature (30% CD235aneg/low). Similar results were observed in 1 experiment performed with MNCs from adult blood (results not shown).
FIG. 3.
fl-hKITL is superior to both tr-hKITL and mKITL in sustaining ex vivo amplification of EBs from CB MNCs in cultures containing DXM (HEMA conditions). (A) Growth curves of EBs in cultures stimulated with DXM, estradiol, IL-3, and EPO but without KITL (blue) or with fl-hKITL (black), tr-hKITL (purple), or mKITL (green), as indicated. Data are expressed as FI with respect to the number of cells seeded at day 0. Results are shown as mean (±SD) of 3 separate experiments. (B) FACS profile for CD36 and CD235a expression (on the left) and morphology (by May-Grunwald staining, on the right) of cells obtained after 13 days in culture. Magnification 40×. (C) Frequency of non-EBs (CD36negCD235aneg/low cells), total EBs (CD36pos cells), and immature (CD36posCD235aneg/low) and mature (CD36posCD235apos) EBs in the flow cytometry analysis is presented in (B). Results from a representative experiment are shown (see also Fig. 4). HEMA, human erythroid massive amplification; EPO, erythropoietin.
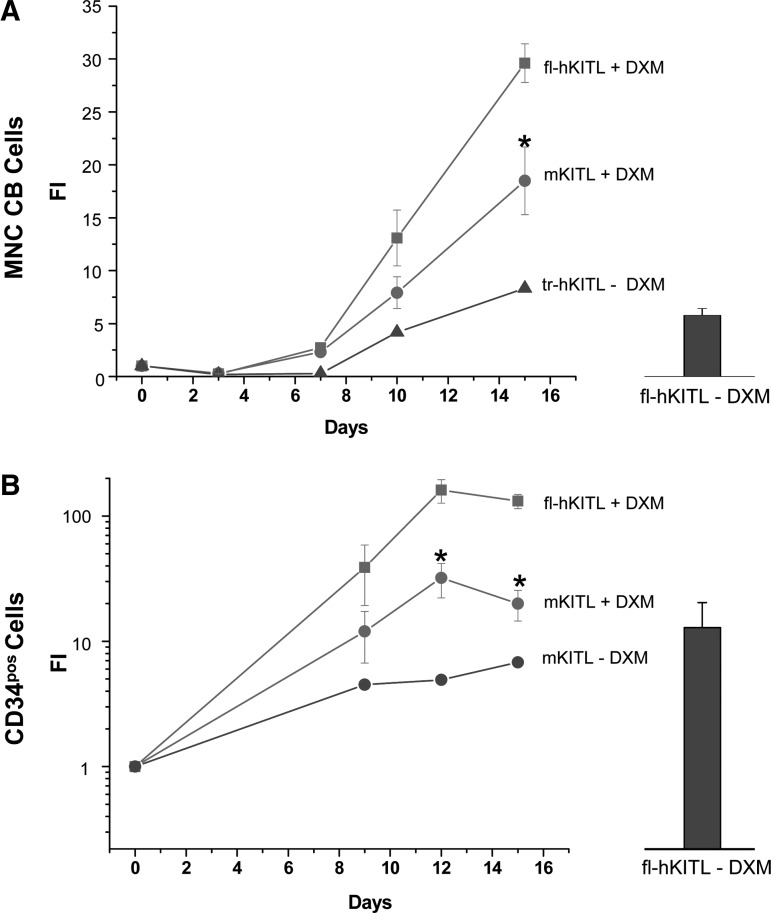
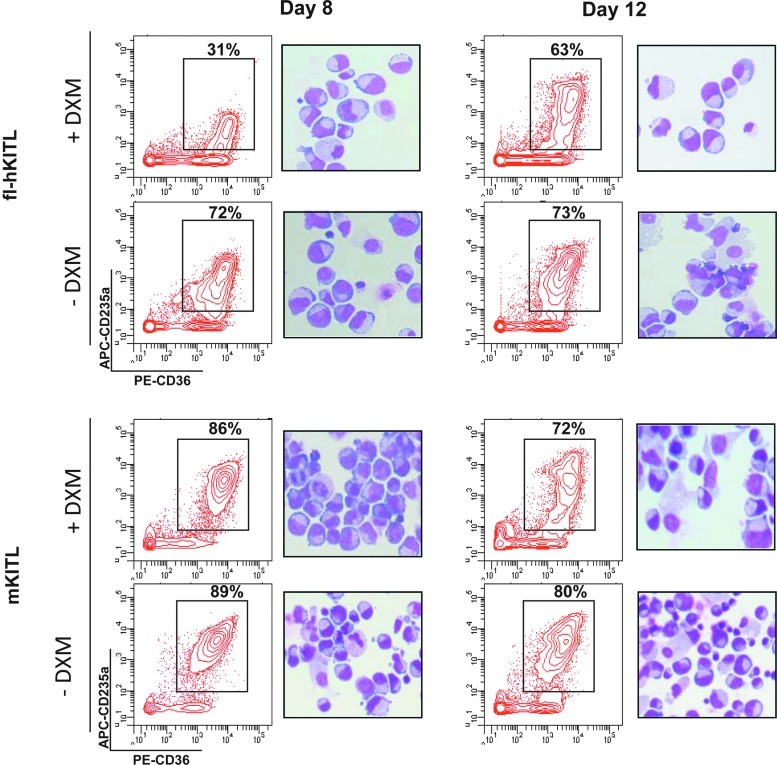
The effect of fl-hKITL, tr-hKITL, and mKITL on EB amplification in cultures without and with steroid is compared in Figs. 4 and 5. Similar levels of amplification were observed in cultures of fl-hKITL and mKITL that did not contain DXM (Fig. 4). Also the maturation state of the EBs generated with the 2 forms of KITL was similar (>70% CD36posCD235apos by day 8) (Fig. 5). By contrast, in cultures containing DXM, fl-hKITL generated significantly (P<0.01) greater number of EBs than mKITL, both in cultures of CB MNCs (day 15 FI=30 vs. 15, respectively) and CB CD34pos (day 12 FI=200 vs. 20, respectively). In this case, a great difference was observed in the maturation levels of EBs generated in the presence of the 2 KITL (Fig. 5). A great proportion (69% at day 8 and 37% at day 12) of the EBs generated with fl-hKITL were immature (CD235aneg/low) while the frequency of immature EBs generated in cultures stimulated with mKITL was similar to that observed in cultures not containing DXM (>86%–70% CD235apos). These results were confirmed by morphological analysis (Fig. 5).
FIG. 4.
fl-hKITL is superior to mKITL in sustaining EB expansion from both CB MNCs and CD34pos cells in cultures containing DXM (HEMA cultures). Growth curves of EBs in HEMA cultures of CB MNCs (top panel) and CD34pos cells (bottom panel) stimulated with either fl-hKITL (squares) or mKITL (circles). Growth curves observed in cultures stimulated with fl-hKITL (top panel) or mKITL (bottom panel) but without DXM are presented as controls. Results are expressed as FI and are presented as mean (±SD) of 3 separate experiments with 3 separate donors and are compared with average FI obtained in cultures stimulated with fl-hKITL, IL-3, and EPO but not DXM (columns on the right) as previously published [3,4]. FI that is statistically different (P<0.01) between DXM-stimulated cultures stimulated with fl-hKITL or mKITL is indicated by asterisks.
FIG. 5.
Addition of DXM retards EB maturation in HEMA cultures stimulated with fl-hKITL but not in those stimulated with mKITL. Representative flow cytometry (for CD36/CD235a expression) and morphology (by May-Grunwald staining) of EBs generated by CB CD34pos cells at days 8 and 12 in cultures stimulated with either fl-hKITL or mKITL without or with DXM. Similar results were observed in 2 additional experiments (see also Fig. 4). Magnification 40×. APC, allophycocyanin; PE, phycoerythrin.
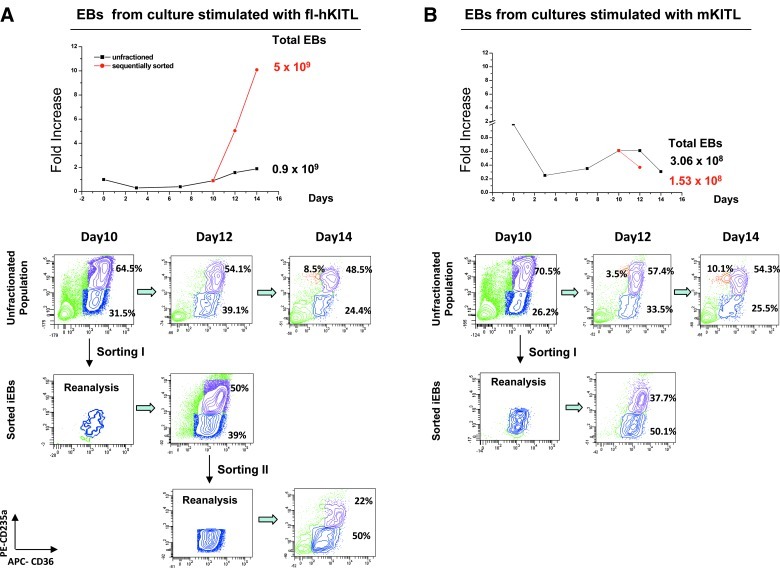
The proliferation potential of immature EBs generated at day 10 in HEMA cultures stimulated with either fl-hKITL or mKITL was compared by sequencial purification/reculture experiments (Fig. 6). As previously described [18], immature day 10 EBs generated in the presence of fl-hKITL could be sequencially sorted and recultured 2 times generating a total number of 5×109 erythroid cells (vs. only 9×108 cells generated in the parallel cultures on unfractionated EBs; Fig. 6A). By contrast, immature EBs isolated from day 10 HEMA cultures stimulated with mKITL matured but did not increase in cell number upon re-culture (Fig. 6B).
FIG. 6.
Comparison of the proliferation potential of immature EBs generated by day 10 in HEMA cultures stimulated with either fl-hKITL (A) or mKITL (B). Top panels: Growth curves of adult MNCs in HEMA culture (squares) stimulated with either fl-hKITL (A) or mKITL (B) and of the corresponding immature day 10 EBs sequentially sorted (every 2 days) and expanded in culture again (circles). The theoretical number of EBs generated from the MNCs from an entire blood donation cultured under the various conditions is presented on the right (total EBs). Bottom panels: Maturation profile (CD36/CD235a staining) of the day 10–14 progeny of adult MNCs and of the sorted populations. Immature EBs generated in the presence of fl-hKITL could be sorted and re-cultured twice generating great numbers of erythroid cells while those generated with mKITL could be purified and cultured only once due to lack of growth.
In conclusion, in the presence of DXM, the presence of fl-hKITL allowed for the generation of greater numbers of EBs from both CB MNCs and CD34pos cells than either tr-hKITL or mKITL and immature EBs were present with greater frequencies and retained proliferation potential upon re-culture.
Human KITL (both full-length and deleted) but not mKITL induces the rapid and sustained ERK phosphorylation
To clarify the mechanism that retains immature EBs in cultures stimulated with fl-hKITL, the levels of cKIT expressed by EBs generated at day 12 in HEMA cultures stimulated with fl-hKITL and the signaling activated in these cells by treatment with fl-hKITL, tr-hKITL, and mKITL were determined (Fig. 7). EBs were identified as CD36pos cells and divided into immature and mature on the basis of CD235a expression (CD235aneg/low and CD235apos, respectively).
FIG. 7.
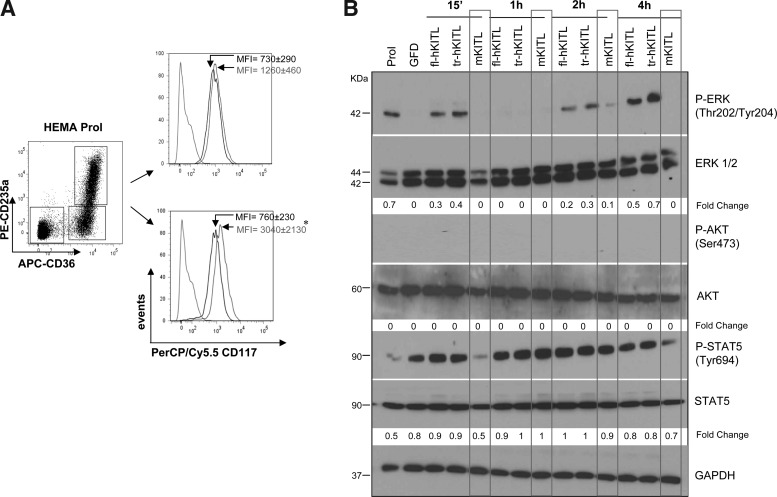
Day 10 EBs express high levels of cKIT and respond to hKITL (both fl-hKITL and tr-hKITL) but not mKITL by activating both the rapid and the sustained ERK pathway. (A) Representative flow cytometric analysis for the expression of cKIT (CD117) of immature (bottom panel) and mature (upper panel) EBs. EBs were obtained at day 10 of HEMA cultures stimulated with fl-hKITL and divided into immature and mature populations on the basis of the CD36CD235a gates presented on the left. Cells were analyzed either as obtained from the culture (black histogram) or after 2 h of GFD (gray histogram). The dotted histogram indicates the isotype control. The MFI (±SD) CD117 expression observed in 5 separate experiments is indicated within the quadrants (*P<0.05). (B) Levels of ERK, STAT5, and AKT phosphorylation in CB EBs obtained at day 10 of fl-hKITL-stimulated HEMA cultures (Prol) and then GFD for 4 h and treated with fl-hKITL, tr-hKITL, or mKITL for 15 min, 1, 2, or 4 h, as indicated. Levels of total STAT5, AKT, ERK, and GAPDH expression were analyzed as quantitative control. The intensity of the bands corresponding to the phosphorylated and total form of each protein was quantified by densitometry and expressed as fold change below each line, for comparison. GFD, growth factor deprived; MFI, mean fluorescent intensity; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Day 12 EBs, both immature and mature, express KIT (mean fluorescence intensity [MFI]=760±230 and 730±290, respectively). The receptor is likely to be more active in the immature cells because 2 h of GFD increased by 4-fold (P<0.05) the levels of KIT expressed by these cells while had modest (not statistically significant) effects on the levels of KIT expressed by mature EBs (Fig. 7A).
Day 12 EBs expressed robust levels of key mediators of the major signaling pathways (ERK, AKT, and STAT5) (Fig. 7B). GFD and KITL stimulation had no effect on the AKT and STAT5 content of these cells. By contrast, GFD increased ERK content which in the case of mKITL stimulation returned to baseline levels by 15 min. EBs expressed detectable levels of endogenous ERK phosphorylation that were greatly reduced by 4 h of GFD. Exposure to hKITL (both fl-KITL and tr-hKITL) induced both transient (15 min) and sustained (2–4 h) ERK phosphorylation. By contrast, exposure to mKITL induced low levels of ERK phosphorylation only at the 2 h time point.
As previously described [17,19], day 12 EBs did not express significant levels of STAT5 phosphorylation that was instead induced in these cells by the GFD treatment (Fig. 7B). Exposure of the cells to hKITL (both fl-hKITL and tr-hKITL) rapidly (15 min) increased the levels of STAT5 phosphorylation that remained high up to 4 h. By contrast, exposure of EBs to mKITL induced a rapid decrease (15 min) in the levels of STAT5 phosphorylation that returned to high levels after the 1 h time point. None of the KITLs analyzed activated AKT in human EBs (Fig. 7B) although a strong p-AKT signal was observed with MCF10A cells [26] analyzed in parallel as positive control (data not shown).
These data indicate that the phenotype of human EBs capable of sustained proliferation that are generated under HEMA conditions (KITposCD36posCD235aneg/low) is similar to that of stress-specific BFU-E recently described to be generated in mice under conditions of stress erythropoiesis {KITpos, CD71pos (an antigen that recognizes the same EB populations recognized by CD36pos [18,27]), and TER119pos (an antibody that recognizes glycophorin A [28])} [6]. The human EBs capable of extensive proliferation generated under HEMA culture have a pro-erythroblast morphology similar to that of the expandable pro-erythroblast pool that has been described to be generated in mice during EPO treatment or anemia challenge [29]. The fact that the phenotype of these murine pro-erythroblasts is CD71highTER119neg but KITneg may be due to the levels of sensitivity of KIT detection by flow of the murine and human antibodies. In addition, tr-hKITL and mKITL behave differently in these biochemical determinations.
EBs exposed to human KITL (both fl-hKITL and tr-hKITL) express greater levels of GRα than EBs exposed to mKITL
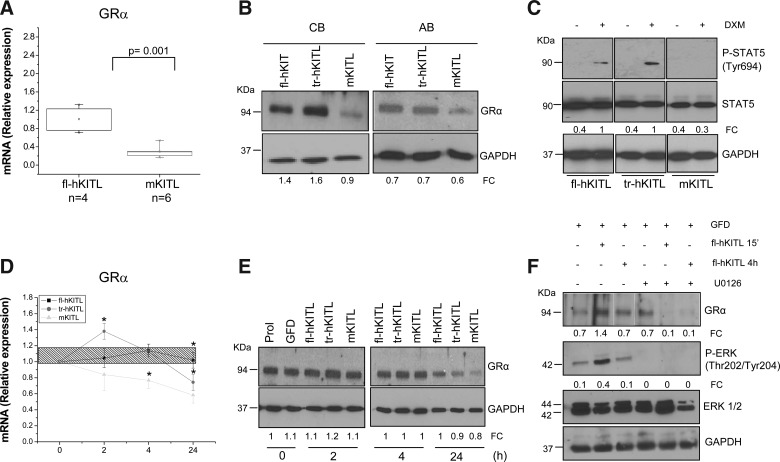
To further investigate the molecular mechanism underlying the greater EB expansion activity in HEMA cultures of fl-hKITL, the levels of GRα mRNA and protein expressed by EBs generated in the presence of hKITL and mKITL were first compared (Fig. 8A, B). fl-hKITL-generated EBs expressed 4–6 times more (P<0.001) GRα mRNA and protein than that of mKITL-derived EBs. On the other hand, tr-hKITL-derived EBs contained levels of GRα protein similar to those contained by fl-hKITL-derived EBs (Fig. 8B).
FIG. 8.
fl-hKITL regulates the expression of GRα in human EBs. (A) Quantitative RT-PCR of the levels of GRα mRNA expressed by day 10 EBs generated in HEMA cultures stimulated with either hKITL or mKITL, as indicated. The number of independent experiments included in the analyses (each one with a different donor) is indicated by n. mRNA levels are presented in relative units calculated using the average 2−ΔCt observed with EBs generated with fl-hKITL as 1. (B) WB analysis for GRα protein content of day 10 EBs generated in the presence of fl-hKITL, tr-hKITL, and mKITL, as indicated. Two representative experiments, 1 with CB-derived and 1 with AB-derived EBs are presented. GAPDH was analyzed as loading control. For further details see legend of Fig. 7. (C) Levels of STAT5 phosphorylation expressed by day 10 EBs obtained in HEMA cultures stimulated with either fl-hKITL, tr-hKITL, or mKITL and then exposed or not to DXM for 15 min. (D) Quantitative RT-PCR and (E) western blot analysis of the levels of GRα mRNA and protein expressed by day 10 EBs generated in HEMA cultures stimulated with fl-hKITL [shaded area in (C) and Prol in (D)], GFD for 4 h (time 0), and then exposed to either fl-hKITL, tr-hKITL, or mKITL (50 ng/mL in all cases) for up to 4 h as indicated. mRNA and protein levels were analyzed on the same cells. mRNA levels are expressed in relative units using 2−ΔCt obtained with GFD EBs as 1 and presented as mean (±SD) of 3 separate determinations. Asterisk (*) indicates <0.01 with respect to the corresponding values observed with fl-hKITL. (F) Western blot analysis of the levels of GRα, phospho-extracellular signal regulated kinases (P-ERK), and total ERK 1/2 and GAPDH (as loading control) in day 10 EBs generated in the presence of fl-hKITL, and exposed to GFD for 4 h. In some of the samples, the last hour of GFD occurred in the presence of U0126 (100 μM). GFD with or without U0126 EBs was then exposed to fl-hKITL for 15 min and 1 h, as indicated. The FC of the various bands with respect to the levels of GAPDH is also indicated. Similar results were observed in a separate experiment. GR, glucocorticoid receptor; RT-PCR, real-time polymerase chain reaction; AB, adult blood.
The levels of GRα content in the EBs generated with the different KITL were correlated with those of GRα signaling by measuring the levels of STAT-5 phosphorylation induced by DXM in the different EB populations (Fig. 8C). DXM rapidly, within 15 min, induced STAT-5 phosphorylation in EBs generated in the presence of hKITL (both fl and tr) but was ineffective in EBs generated with mKITL.
To determine whether the effect of fl-hKITL on GRα expression was mediated through ERK activation, the effects of fl-hKITL (15 min and 24 h) on the GRα content expressed by fl-hKITL-generated EBs with or without pre-exposure to the U0126 ERK inhibitor were evaluated (Fig. 8F). Exposure to the U0126 alone did not affect GRα content of day 10 EBs. Exposure to fl-hKITL preserved the GRα content in EBs that had not been pre-treated with the ERK inhibitor for up to 24 h but GRα was barely detectable in EBs pre-treated with the inhibitor upon fl-hKITL exposure. These results suggest that in erythroid cells fl-hKITL regulates the GRα content through the mitogen activated protein (MAP) kinase pathway.
These results reveal that KITL exerts a structure-specific regulation on the level of GRα expressed by human EBs and that exposure to fl-hKITL uniquely sustains GRα expression, possibly through the ERK pathway.
Discussion
Here, data are presented indicating that fl-hKITL induces 2–3 times greater EB expansion than tr-hKITL and mKITL (Figs. 3 and 4) in cultures containing DXM in spite of the similar biological activity observed in cultures without DXM (Figs. 1, 2 and 4). This greater expansion was associated with (1) EB retention in the CD117posCD36posCD235aneg/low state, an immature phenotype with proliferation potential similar to that of erythroid cells generated in mice under condition of stress erythropoiesis (Figs. 3, 5 and 6) [6,29]; (2) greater levels of ERK and/or STAT5 activation (Fig. 7); and (3) greater levels of GRα and ability of the cells to elicit a biochemical response to DXM (Fig. 8). The observation that exposure of human EBs to an inhibitor of ERK signaling reduces GRα expression (Fig. 8) suggests that fl-KITL may regulate GRα expression through the ERK pathway. These biochemical differences suggest that the superiority of fl-hKITL to expand EBs in cultures containing DXM may be due both to its greater ability to trigger a proliferation signal and to prime EBs to respond to DXM by activating GR expression.
Several studies have investigated the relationship between KITL signaling and cell fate. The biological activity of fl-hKITL on hematopoietic progenitor cells is concentration dependent. Progressively greater concentrations are required for optimal survival (1 ng/mL), maturation (10 ng/mL), and expansion (50–100 ng/mL) [2]. In human and murine erythroid progenitors, KITL induces rapid—within 15 min—ERK activation that lasts only 1 h [30,31]. In human erythroleukemic K562 and myeloid MO7e cells, rapid KITL-dependent ERK activation is associated with proliferation while a late (by 2 h) KITL-dependent sustained ERK activation is instead responsible for differentiation [32,33]. STAT5 activation exerts a level-dependent control on hematopoietic progenitor cell fate with low levels favoring maturation and high levels proliferation [34,35]. Whether KITL activates the STAT5 pathway in erythroid cells is controversial. On one hand, KITL was found to be unable to activate STAT5 in prospectively isolated human erythroid progenitor cells [30]. On the other hand, more recent data analyzing single cells by FACS indicate that KITL activates STAT5 in bipotent erythroid/MK but not in myelomonocytic progenitor cells [36]. Finally, KITL has been described to activate the PI-3K/AKT pathway in murine erythroid progenitors [31] and in human MO7e cells [33]. In this study, KITL was shown to be able to activate ERK and STAT5 but not PI-3K in human EBs although differences were observed in the signaling activated by the 3 forms of KITL investigated. fl-hKITL and tr-hKITL induced both the rapid (similar levels) and sustained (more efficiently by tr-hKITL) ERK activation while mKITL induced low levels of sustained ERK activation only. Although all the forms of KITL investigated activated STAT5, early (15 min) STAT5 phosphorylation was inhibited by mKITL. The greater differences in signaling activity observed at the level of ERK are consistent with the prediction that minor differences in ligand structure, such as those existing among fl-hKITL, tr-hKITL, and mKITL [2], may promote kinase activation and/or influence receptor turnover to differing degrees and thus affect ERK activation in a quantitative fashion [2,37].
The signaling properties of the 3 forms of KITL investigated, and their predicted effects on cell fate, explain only in part the differences in EB expansion sustained by each of them in HEMA culture. In the case of mKITL, low levels of EB amplification associated with maturation may be explained by absence of rapid ERK activation, which inhibits proliferation [30,31], and retention of sustained ERK and reduced STAT5 activation, both of which sustain maturation [30,31,34,35]. However, in the case of tr-hKITL these biological effects were observed in association with modest reductions in the levels of ERK or STAT-5 activation. In addition, if differences in EB amplification were only due to the signaling properties of the 3 KITLs investigated, they should be observed in cultures with and without DXM. The fact that they were observed only in cultures with DXM suggested to us that fl-hKITL sustains the generation of EBs with a superior ability to respond to glucocorticoids.
GR has a complex genomic organization with multiple transcription start sites and splice sites conserved in evolution that allow fine tuning of tissue-specific activation during cell maturation and in response to a variety of stimuli [38]. Environmental stimuli, such as glucocorticoids themselves and the pro-inflammatory protein tumor necrosis factor alpha (TNFα), are known to exert a tissue-specific transcriptional, translational, and post-translational regulation on GR expression. In human respiratory epithelial cells and myofibroblasts, glucocorticoids exert a cycloeximide-independent repression on GRα expression [39,40]. Suppression of GRα expression by TNFα was first described in mice that are protected from TNFα-induced toxic shock only if DXM is administered before TNFα challenge [41]. TNFα inhibits glucocorticoid signaling by suppressing GRα expression also in human lymphoid CEMC7 and epithelial cells [42,43]. Since this suppression is mediated by the MAP kinase/ERK pathway [44], a pathway also activated by DXM [45], we hypothesized that also KITL may regulate GR expression through the MAP kinase/ERK pathway and that KITLs with different ERK activities may elicit different GR expression. In agreement with this hypothesis, EBs obtained or exposed to fl-hKITL, which was the most efficient ERK activator (Fig. 7), expressed greater levels of GRα than EBs exposed to tr-hKITL or mKITL and the levels of GRα expressed by EBs exposed to fl-hKITL were reduced by pre-treating the cells with the ERK inhibitor U0126 (Fig. 8). The regulation of GR expression is complex and may involve transcriptional, translational, and/or post-translational mechanisms. The fact that U0126-treated EBs lost GRα content within 15 min of fl-hKITL stimulation suggests that the MAP kinase signaling prevents the degradation of GRα protein. However, the reduction of GRα mRNA observed in EBs stimulated for 24 h with tr-hKITL and mKITL suggests that fl-hKITL regulates also the mRNA levels of this protein. A separate study will be dedicated to the identification of the full spectrum of the effects of fl-hKITL on GR regulation.
Ex vivo–expanded EBs in the presence of glucocorticoids are currently under consideration for use as alternative transfusion products and many laboratories have devoted time and resources to biochemical profile of these cells (reviewed in [45]). However, for reason unknown, results obtained in different laboratories are not always consistent. Our data clarify that at least one of the reasons for this inconsistency may be represented by the source of KITL used for the study. In fact, one of the barriers to produce erythroid cells for transfusion is that large quantities of growth factors (including fl-hKITL) are required for their production. Cost considerations have therefore inspired many investigators to explore the possibility to produce these cells using less expensive sources of KITL. Based on the similar activity of fl-hKITL and mKITL in human mast cell cultures [20], several investigators expanded human EBs in the presence of a supernatant of BHK cells expressing high titers of mKITL developed by Normal Iscove (Marieke von Lindern, Personal communication). We explored the use of tr-hKITL generated in large quantities by Dr. Nikolov (SKI) for structural studies in the baculovirus system. However, the expansion and biological properties of cells generated with fl-hKITL and those generated with tr-hKITL or mKITL differed significantly, and therefore neither tr-hKITL nor mKITL is suitable for the expansion of human EBs in the presence of DXM.
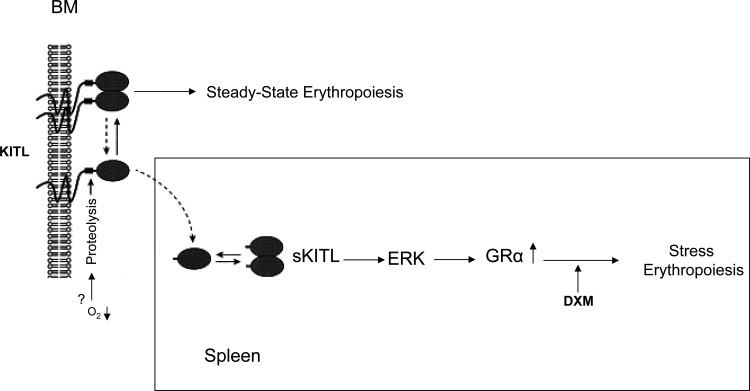
In conclusion, our data indicate a KITL-structure-dependent regulation of the EB response to DXM through regulation of GR expression, and suggest a model for the activation of stress erythropoiesis summarized in Fig. 9, which could also explain why KITLKL2/KL2 [5] and GRdim/dim [9] mice have a similar erythroid stress unresponsive phenotypes. According to this model, under steady state conditions, erythropoiesis is regulated by membrane-bound hKITL and is GR independent. The establishment of a proteolytic environment as a consequence of a stress signal cleaves hKITL releasing fl-hKITL that is uniquely capable, possibly through ERK signaling, of activating GRα expression allowing EBs to respond to glucocorticoids.
FIG. 9.
A model for the regulation of stress erythropoiesis based on the exquisite role of fl-hKITL as regulator of GR expression in human EBs. Under steady state conditions, erythropoiesis is regulated by membrane-bound hKITL and is GR independent. Microenvironmental cues activated by a stress signal activate the proteolytic cleavage of hKITL and fl-hKITL that are released in the plasma. Soluble fl-hKITL, although less efficient than the membrane-bound growth factor to activate the signal cascade [46], is uniquely capable, possibly through the ERK pathway, of activating GRα expression, making EBs responsive to glucocorticoids.
Acknowledgments
This study was supported by a grant from the NY-STAR foundation (C-06066), by a grant by the Italian “Centro Nazionale Sangue,” and by institutional funds from the Mount Sinai School of Medicine and Istituto Superiore Sanità. The authors gratefully acknowledge Dmiitar Nikolov (Sloan Kettering Institute) for the gift of tr-KITL and of Hedive Erdjument-Bromage of the Micro-Chemistry and Proteomics Facilities at Sloan Kettering Institute for the mass spectrometric analysis of tr-hKITL. Further, we would like to thank Valentina Tirelli for assistance with flow cytometry KIT determinations.
Author Disclosure Statement
The authors have no commercial affiliation and conflict of interest in connection with this article to disclose.
References
- 1.Papayannopoulou T. Migliaccio AR. Abkowitz JL. D'Andrea AD. Biology of erythropoiesis, erythroid differentiation, and maturation. In: Hoffman R, editor; Benz EJ, editor; Shattil S, editor. Hematology: Basic Principles and Practise. 5. Churchill Livingstone; New York: 2009. pp. 276–294. [Google Scholar]
- 2.Besmer P. Kit-ligand-stem cell factor. In: Garland JM, editor; Quesenberry PJ, editor; Hilton DJ, editor. Colony-Stimulating Factors: Molecular and Cellular Biology. Marcel Dekker; New York: 1997. pp. 369–404. [Google Scholar]
- 3.Migliaccio G. Migliaccio AR. Druzin ML. Giardina PJ. Zsebo KM. Adamson JW. Effects of recombinant human stem cell factor (SCF) on the growth of human progenitor cells in vitro. J Cell Physiol. 1991;148:503–509. doi: 10.1002/jcp.1041480324. [DOI] [PubMed] [Google Scholar]
- 4.Migliaccio G. Migliaccio AR. Druzin ML. Giardina PJ. Zsebo KM. Adamson JW. Long-term generation of colony-forming cells in liquid culture of CD34+ cord blood cells in the presence of recombinant human stem cell factor. Blood. 1992;79:2620–2627. [PubMed] [Google Scholar]
- 5.Tajima Y. Moore MA. Soares V. Ono M. Kissel H. Besmer P. Consequences of exclusive expression in vivo of Kit-ligand lacking the major proteolytic cleavage site. Proc Natl Acad Sci U S A. 1998;95:11903–11908. doi: 10.1073/pnas.95.20.11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson RF. Shi L. Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18:139–145. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry JM. Harandi OF. Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109:4494–4502. doi: 10.1182/blood-2006-04-016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harandi OF. Hedge S. Wu DC. McKeone D. Paulson RF. Murine erythroid short-term radioprotection requires a BMP4-dependent, self-renewing population of stress erythroid progenitors. J Clin Invest. 2010;120:4507–4519. doi: 10.1172/JCI41291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer A. Tronche F. Wessely O. Kellendonk C. Reichardt HM. Steinlein P. Schutz G. Beug H. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolznig H. Grebien F. Deiner EM. Stangl K. Kolbus A. Habermann B. Kerenyi MA. Kieslinger M. Moriggl R. Beug H. Mullner EW. Erythroid progenitor renewal versus differentiation: genetic evidence for cell autonomous, essential functions of EpoR, Stat5 and the GR. Oncogene. 2006;25:2890–2900. doi: 10.1038/sj.onc.1209308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliaccio AR. Vannucchi AM. Migliaccio G. Molecular control of erythroid differentiation. Int J Hematol. 1996;64:1–29. doi: 10.1016/0925-5710(96)00465-3. [DOI] [PubMed] [Google Scholar]
- 12.Gursoy A. Dogruk Unal A. Ayturk S. Karakus S. Nur Izol A. Bascil Tutuncu N. Guvener Demirag N. Polycythemia as the first manifestation of Cushing's disease. J Endocrinol Invest. 2006;29:742–744. doi: 10.1007/BF03344186. [DOI] [PubMed] [Google Scholar]
- 13.Fibach E. Rachmilewitz EA. The two-step liquid culture: a novel procedure for studying maturation of human normal and pathological erythroid precursors. Stem Cells. 1993;1:36–41. doi: 10.1002/stem.5530110608. [DOI] [PubMed] [Google Scholar]
- 14.von von Lindern M. Zauner W. Mellitzer G. Steinlein P. Fritsch G. Huber K. Lowenberg B. Beug H. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94:550–559. [PubMed] [Google Scholar]
- 15.Panzenbock B. Bartunek P. Mapara MY. Zenke M. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92:3658–3668. [PubMed] [Google Scholar]
- 16.Migliaccio G. Di Pietro R. di Giacomo V. Di Baldassarre A. Migliaccio AR. Maccioni L. Galanello R. Papayannopoulou T. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol Dis. 2002;28:169–180. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- 17.Varricchio L. Masselli E. Alfani E. Battistini A. Migliaccio G. Vannucchi AM. Zhang W. Rondelli D. Godbold J, et al. The dominant negative beta isoform of the glucocorticoid receptor is uniquely expressed in erythroid cells expanded from polycythemia vera patients. Blood. 2011;118:425–436. doi: 10.1182/blood-2010-07-296921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migliaccio G. Masiello F. Tirelli V. Sanchez M. Varricchio L. Whitsett C. Migliaccio AR. Under HEMA conditions, self-replication of human erythroblasts is limited by autophagic death. Blood Cells Mol Dis. 2011;47:182–197. doi: 10.1016/j.bcmd.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Stellacci E. Di Noia A. Di Baldassarre A. Migliaccio G. Battistini A. Migliaccio AR. Interaction between the glucocorticoid and erythropoietin receptors in human erythroid cells. Exp Hematol. 2009;37:559–572. doi: 10.1016/j.exphem.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsui H. Furitsu T. Dvorak AM. Irani AM. Schwartz LB. Inagaki N. Takei M. Ishizaka K. Zsebo KM. Gillis S. Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-kit ligand. Proc Natl Acad Sci U S A. 1993;90:735–739. doi: 10.1073/pnas.90.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semavina M. Saha N. Kolev MV. Goldgur Y. Giger RJ. Himanen JP. Nikolov DB. Crystal structure of the Nogo-receptor-2. Protein Sci. 2011;20:684–689. doi: 10.1002/pro.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith K. Zsebo KM. Measurement of human and murin stem cell factor (c-kit ligand) In: Shevach EM, editor. Current Protocol in Immunology. John Wiley & Sons; New York: 1992. pp. 6.17.11–16.17.11. [DOI] [PubMed] [Google Scholar]
- 23.Gobbi G. Mirandola P. Sponzilli I. Micheloni C. Malinverno C. Cocco L. Vitale M. Timing and expression level of protein kinase C epsilon regulate the megakaryocytic differentiation of human CD34 cells. Stem Cells. 2007;25:2322–2329. doi: 10.1634/stemcells.2006-0839. [DOI] [PubMed] [Google Scholar]
- 24.Kieffer N. Bettaieb A. Legrand C. Coulombel L. Vainchenker W. Edelman L. Breton-Gorius J. Developmentally regulated expression of a 78 kDa erythroblast membrane glycoprotein immunologically related to the platelet thrombospondin receptor. Biochem J. 1989;262:835–842. doi: 10.1042/bj2620835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen KB. Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42:10978–10990. doi: 10.1021/bi034651u. [DOI] [PubMed] [Google Scholar]
- 26.Menard RE. Jovanovski AP. Mattingly RR. Active p21-activated kinase 1 rescues MCF10A breast epithelial cells from undergoing anoikis. Neoplasia. 2005;7:638–645. doi: 10.1593/neo.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L. Gao Z. Zhu J. Rodgers GP. Identification of CD13+CD36+ cells as a common progenitor for erythroid and myeloid lineages in human bone marrow. Exp Hematol. 2007;35:1047–1055. doi: 10.1016/j.exphem.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auffray I. Marfatia S. de Jong K. Lee G. Huang CH. Paszty C. Tanner MJ. Mohandas N. Chasis JA. Glycophorin A dimerization and band 3 interaction during erythroid membrane biogenesis: in vivo studies in human glycophorin A transgenic mice. Blood. 2001;97:2872–2878. doi: 10.1182/blood.v97.9.2872. [DOI] [PubMed] [Google Scholar]
- 29.Dev A. Fang J. Sathyanarayana P. Pradeep A. Emerson C. Wojchowski DM. During EPO or anemia challenge, erythroid progenitor cells transit through a selectively expandable proerythroblast pool. Blood. 2010;116:5334–5346. doi: 10.1182/blood-2009-12-258947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sui X. Krantz SB. You M. Zhao Z. Synergistic activation of MAP kinase (ERK1/2) by erythropoietin and stem cell factor is essential for expanded erythropoiesis. Blood. 1998;92:1142–1149. [PubMed] [Google Scholar]
- 31.Agosti V. Karur V. Sathyanarayana P. Besmer P. Wojchowski DM. A KIT juxtamembrane PY567-directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis. Exp Hematol. 2009;37:159–171. doi: 10.1016/j.exphem.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racke FK. Lewandowska K. Goueli S. Goldfarb AN. Sustained activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway is required for megakaryocytic differentiation of K562 cells. J Biol Chem. 1997;272:23366–23370. doi: 10.1074/jbc.272.37.23366. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ. Yoon JH. Song KS. Chrysin inhibited stem cell factor (SCF)/c-Kit complex-induced cell proliferation in human myeloid leukemia cells. Biochem Pharmacol. 2007;74:215–225. doi: 10.1016/j.bcp.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Schuringa JJ. Chung KY. Morrone G. Moore MA. Constitutive activation of STAT5A promotes human hematopoietic stem cell self-renewal and erythroid differentiation. J Exp Med. 2004;200:623–635. doi: 10.1084/jem.20041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierenga AT. Vellenga E. Schuringa JJ. Maximal STAT5-induced proliferation and self-renewal at intermediate STAT5 activity levels. Mol Cell Biol. 2008;28:6668–6680. doi: 10.1128/MCB.01025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L. Wierenga AT. Rozenveld-Geugien M. van de Lande K. Vellenga E. Schuringa JJ. Single-cell STAT5 signal transduction profiling in normal and leukemic stem and progenitor cell populations reveals highly distinct cytokine responses. PLoS One. 2009;4:e7989. doi: 10.1371/journal.pone.0007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lev S. Blechman JM. Givol D. Yarden Y. Steel factor and c-kit protooncogene: genetic lessons in signal transduction. Crit Rev Oncog. 1994;5:141–168. doi: 10.1615/critrevoncog.v5.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J. Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Pujols L. Mullol J. Perez M. Roca-Ferrer J. Juan M. Xaubet A. Cidlowski JA. Picado C. Expression of the human glucocorticoid receptor alpha and beta isoforms in human respiratory epithelial cells and their regulation by dexamethasone. Am J Respir Cell Mol Biol. 2001;24:49–57. doi: 10.1165/ajrcmb.24.1.4024. [DOI] [PubMed] [Google Scholar]
- 40.Whorwood CB. Donovan SJ. Wood PJ. Phillips DI. Regulation of glucocorticoid receptor alpha and beta isoforms and type I 11beta-hydroxysteroid dehydrogenase expression in human skeletal muscle cells: a key role in the pathogenesis of insulin resistance? J Clin Endocrinol Metab. 2001;86:2296–2308. doi: 10.1210/jcem.86.5.7503. [DOI] [PubMed] [Google Scholar]
- 41.Van Bogaert T. Vandevyver S. Dejager L. Van Hauwermeiren F. Pinheiro I. Petta I. Engblom D. Kleyman A. Schutz G. Tuckermann J. Libert C. Tumor necrosis factor inhibits glucocorticoid receptor function in mice: a strong signal toward lethal shock. J Biol Chem. 2011;286:26555–26567. doi: 10.1074/jbc.M110.212365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster JC. Oakitley RH. Jewell CM. Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onda K. Nagashima M. Kawakubo Y. Inoue S. Hirano T. Oka K. Mitogen-activated protein kinase kinase 1/extracellular signal-regulated kinase (MEK-1/ERK) inhibitors sensitize reduced glucocorticoid response mediated by TNFalpha in human epidermal keratinocytes (HaCaT) Biochem Biophys Res Commun. 2006;351:266–272. doi: 10.1016/j.bbrc.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Van Bogaert T. De Bosscher K. Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev. 2010;21:275–286. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Migliaccio AR. Masselli E. Varricchio L. Whitsett C. Ex-vivo expansion of red blood cells: how real for transfusion in humans? Blood Rev. 2011;26:81–95. doi: 10.1016/j.blre.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyazawa K. Williams DA. Gotoh A. Nishimaki J. Broxmeyer HE. Toyama K. Membrane-bound steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]