Abstract
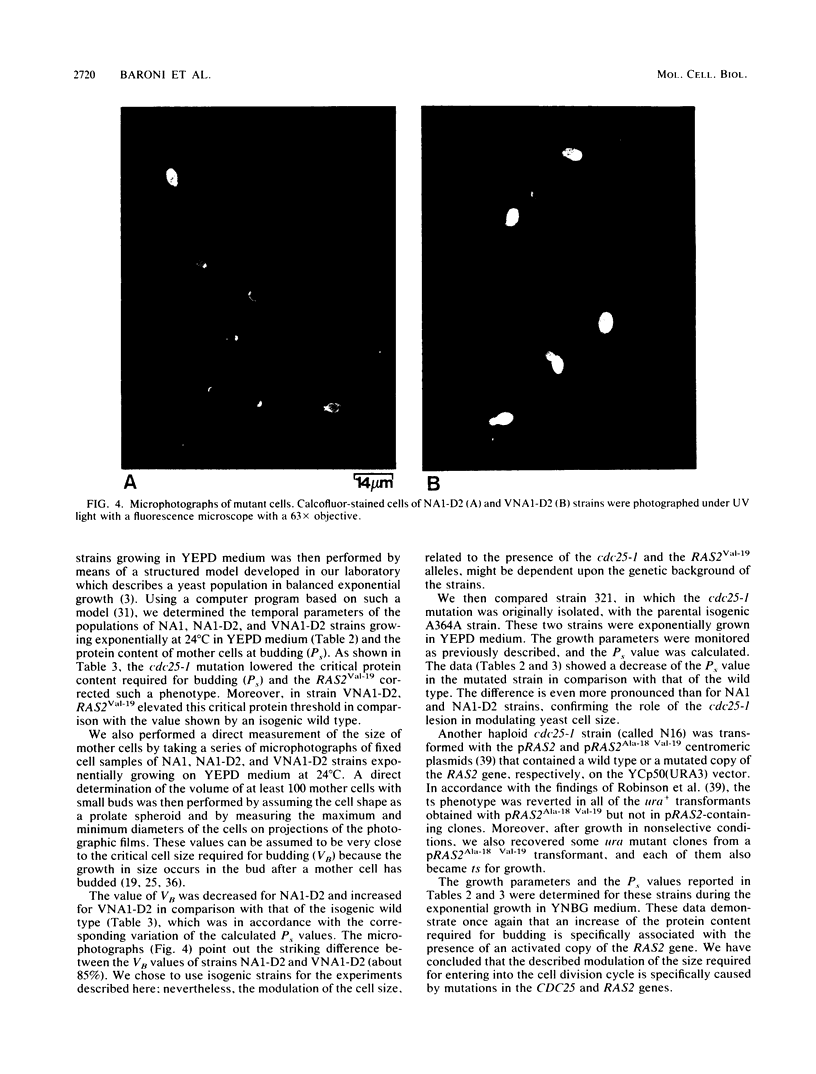
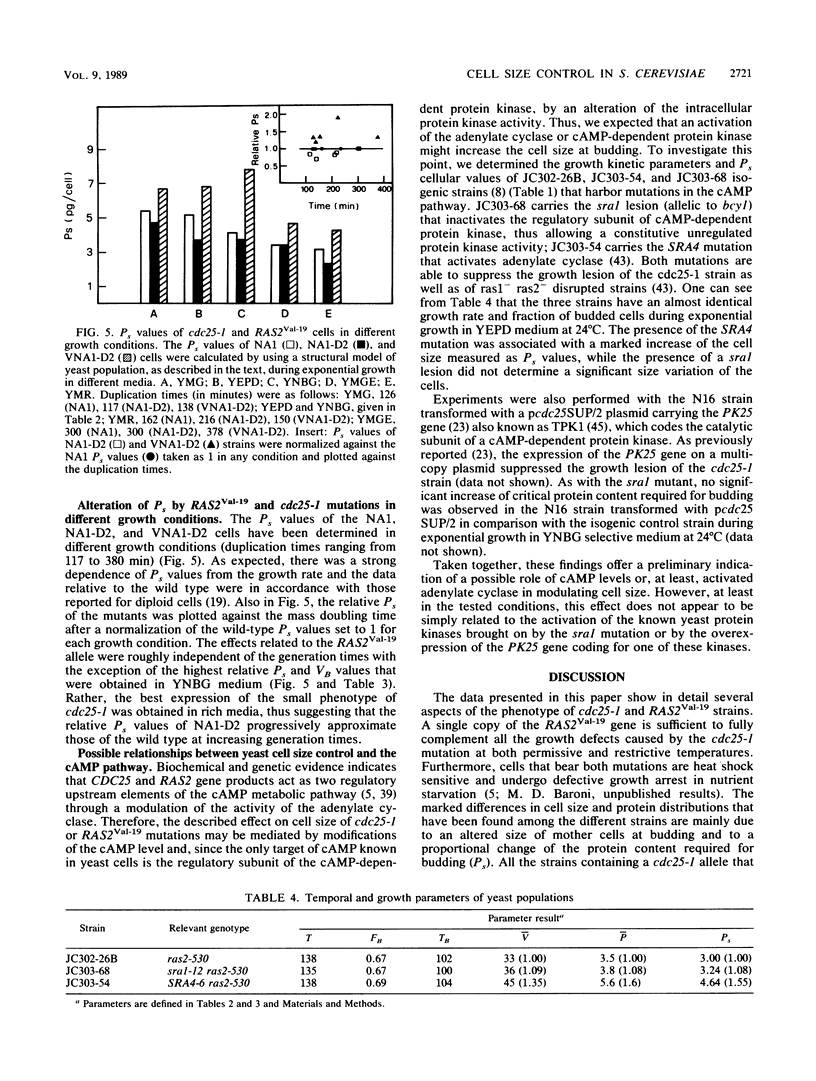
A detailed kinetic analysis of the cell cycle of cdc25-1, RAS2Val-19, or cdc25-1/RAS2Val-19 mutants during exponential growth is presented. At the permissive temperature (24 degrees C), cdc25-1 cells show a longer G1/unbudded phase of the cell cycle and have a smaller critical cell size required for budding without changing the growth rate in comparison to an isogenic wild type. The RAS2Val-19 mutation efficiently suppresses the ts growth defect of the cdc25-1 mutant at 36 degrees C and the increase of G1 phase at 24 degrees C. Moreover, it causes a marked increase of the critical cell mass required to enter into a new cell division cycle compared with that of the wild type. Since the critical cell mass is physiologically modulated by nutritional conditions, we have also studied the behavior of these mutants in different media. The increase in cell size caused by the RAS2Val-19 mutation is evident in all tested growth conditions, while the effect of cdc25-1 is apparently more pronounced in rich culture media. CDC25 and RAS2 gene products have been showed to control cell growth by regulating the cyclic AMP metabolic pathway. Experimental evidence reported herein suggests that the modulation of the critical cell size by CDC25 and RAS2 may involve adenylate cyclase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina L., Mariani L., Martegani E., Vanoni M. Analysis of protein distribution in budding yeast. Biotechnol Bioeng. 1983 May;25(5):1295–1310. doi: 10.1002/bit.260250510. [DOI] [PubMed] [Google Scholar]
- Boutelet F., Petitjean A., Hilger F. Yeast cdc35 mutants are defective in adenylate cyclase and are allelic with cyr1 mutants while CAS1, a new gene, is involved in the regulation of adenylate cyclase. EMBO J. 1985 Oct;4(10):2635–2641. doi: 10.1002/j.1460-2075.1985.tb03981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D., Toda T., Michaeli T., Levin L., Birchmeier C., Zoller M., Powers S., Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987 Mar 13;48(5):789–799. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Camonis J. H., Jacquet M. A new RAS mutation that suppresses the CDC25 gene requirement for growth of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2980–2983. doi: 10.1128/mcb.8.7.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camonis J. H., Kalékine M., Gondré B., Garreau H., Boy-Marcotte E., Jacquet M. Characterization, cloning and sequence analysis of the CDC25 gene which controls the cyclic AMP level of Saccharomyces cerevisiae. EMBO J. 1986 Feb;5(2):375–380. doi: 10.1002/j.1460-2075.1986.tb04222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. F., Gibbs J. B., Tatchell K. Suppressors of the ras2 mutation of Saccharomyces cerevisiae. Genetics. 1986 Jun;113(2):247–264. doi: 10.1093/genetics/113.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. L., Jagadish M. N. The relationship between cell size and cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1978 Mar 1;112(1):15–24. doi: 10.1016/0014-4827(78)90520-7. [DOI] [PubMed] [Google Scholar]
- Casperson G. F., Walker N., Bourne H. R. Isolation of the gene encoding adenylate cyclase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5060–5063. doi: 10.1073/pnas.82.15.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Messenguy F., Lacroute F., Glansdorff N. Cloning arg3, the gene for ornithine carbamoyltransferase from Saccharomyces cerevisiae: expression in Escherichia coli requires secondary mutations; production of plasmid beta-lactamase in yeast. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5026–5030. doi: 10.1073/pnas.78.8.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Becker J. M., Enari E., Levitzki A. The activation of adenylate cyclase by guanyl nucleotides in Saccharomyces cerevisiae is controlled by the CDC25 start gene product. Mol Cell Biol. 1987 Oct;7(10):3857–3861. doi: 10.1128/mcb.7.10.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vendittis E., Vitelli A., Zahn R., Fasano O. Suppression of defective RAS1 and RAS2 functions in yeast by an adenylate cyclase activated by a single amino acid change. EMBO J. 1986 Dec 20;5(13):3657–3663. doi: 10.1002/j.1460-2075.1986.tb04696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Marshall M. S., Scolnick E. M., Sigal I. S. Identification of guanine nucleotides bound to ras-encoded proteins in growing yeast cells. J Biol Chem. 1987 Aug 5;262(22):10426–10429. [PubMed] [Google Scholar]
- Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. Genetic Control of the Cell Division Cycle in Yeast: V. Genetic Analysis of cdc Mutants. Genetics. 1973 Jun;74(2):267–286. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Unger M. W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977 Nov;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Yahara I. Durable synthesis of high molecular weight heat shock proteins in G0 cells of the yeast and other eucaryotes. J Cell Biol. 1984 Jul;99(1 Pt 1):199–207. doi: 10.1083/jcb.99.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Ehrhardt C. W., Lorincz A., Carter B. L. Regulation of cell size in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):1–5. doi: 10.1128/jb.137.1.1-5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Lisziewicz J., Godany A., Förster H. H., Küntzel H. Isolation and nucleotide sequence of a Saccharomyces cerevisiae protein kinase gene suppressing the cell cycle start mutation cdc25. J Biol Chem. 1987 Feb 25;262(6):2549–2553. [PubMed] [Google Scholar]
- Lord P. G., Wheals A. E. Asymmetrical division of Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):808–818. doi: 10.1128/jb.142.3.808-818.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. S., Gibbs J. B., Scolnick E. M., Sigal I. S. Regulatory function of the Saccharomyces cerevisiae RAS C-terminus. Mol Cell Biol. 1987 Jul;7(7):2309–2315. doi: 10.1128/mcb.7.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martegani E., Baroni M., Wanoni M. Interaction of cAMP with the CDC25-mediated step in the cell cycle of budding yeast. Exp Cell Res. 1986 Feb;162(2):544–548. doi: 10.1016/0014-4827(86)90358-7. [DOI] [PubMed] [Google Scholar]
- Martegani E., Vanoni M., Baroni M. Macromolecular syntheses in the cell cycle mutant cdc25 of budding yeast. Eur J Biochem. 1984 Oct 15;144(2):205–210. doi: 10.1111/j.1432-1033.1984.tb08450.x. [DOI] [PubMed] [Google Scholar]
- Martegani E., Vanoni M., Delia D. A computer algorithm for the analysis of protein distribution in budding yeast. Cytometry. 1984 Jan;5(1):81–85. doi: 10.1002/cyto.990050112. [DOI] [PubMed] [Google Scholar]
- Martegani Enzo, Baroni Maurizio D., Frascotti Gianni, Alberghina Lilia. Molecular cloning and transcriptional analysis of the start gene CDC25 of Saccharomyces cerevisiae. EMBO J. 1986 Sep;5(9):2363–2369. doi: 10.1002/j.1460-2075.1986.tb04505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Control of cell division in Saccharomyces cerevisiae mutants defective in adenylate cyclase and cAMP-dependent protein kinase. Exp Cell Res. 1983 Jun;146(1):151–161. doi: 10.1016/0014-4827(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Ishikawa T. Genetic analysis of the role of cAMP in yeast. Yeast. 1985 Sep;1(1):15–24. doi: 10.1002/yea.320010103. [DOI] [PubMed] [Google Scholar]
- Mbonyi K., Beullens M., Detremerie K., Geerts L., Thevelein J. M. Requirement of one functional RAS gene and inability of an oncogenic ras variant to mediate the glucose-induced cyclic AMP signal in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1988 Aug;8(8):3051–3057. doi: 10.1128/mcb.8.8.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo F., Mazón M. J. The Saccharomyces cerevisiae start mutant carrying the cdc25 mutation is defective in activation of plasma membrane ATPase by glucose. J Bacteriol. 1986 Dec;168(3):1254–1257. doi: 10.1128/jb.168.3.1254-1257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzi B. M., Compagno C., Martegani E. Analysis of protein and cell volume distribution in glucose-limited continuous cultures of budding yeast. Biotechnol Bioeng. 1986 Feb;28(2):185–190. doi: 10.1002/bit.260280206. [DOI] [PubMed] [Google Scholar]
- Reed S. I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980 Jul;95(3):561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L. C., Gibbs J. B., Marshall M. S., Sigal I. S., Tatchell K. CDC25: a component of the RAS-adenylate cyclase pathway in Saccharomyces cerevisiae. Science. 1987 Mar 6;235(4793):1218–1221. doi: 10.1126/science.3547648. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Sharrow S. O., Gart J. J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3850–3854. doi: 10.1073/pnas.74.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P. E., Goodey A. R., Carter B. L. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature. 1980 Nov 27;288(5789):401–404. doi: 10.1038/288401a0. [DOI] [PubMed] [Google Scholar]
- Tatchell K., Robinson L. C., Breitenbach M. RAS2 of Saccharomyces cerevisiae is required for gluconeogenic growth and proper response to nutrient limitation. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3785–3789. doi: 10.1073/pnas.82.11.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Tyson C. B., Lord P. G., Wheals A. E. Dependency of size of Saccharomyces cerevisiae cells on growth rate. J Bacteriol. 1979 Apr;138(1):92–98. doi: 10.1128/jb.138.1.92-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoni M., Vai M., Popolo L., Alberghina L. Structural heterogeneity in populations of the budding yeast Saccharomyces cerevisiae. J Bacteriol. 1983 Dec;156(3):1282–1291. doi: 10.1128/jb.156.3.1282-1291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]