Abstract
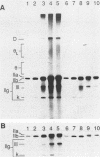
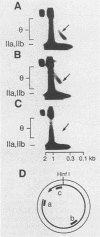
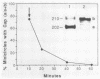
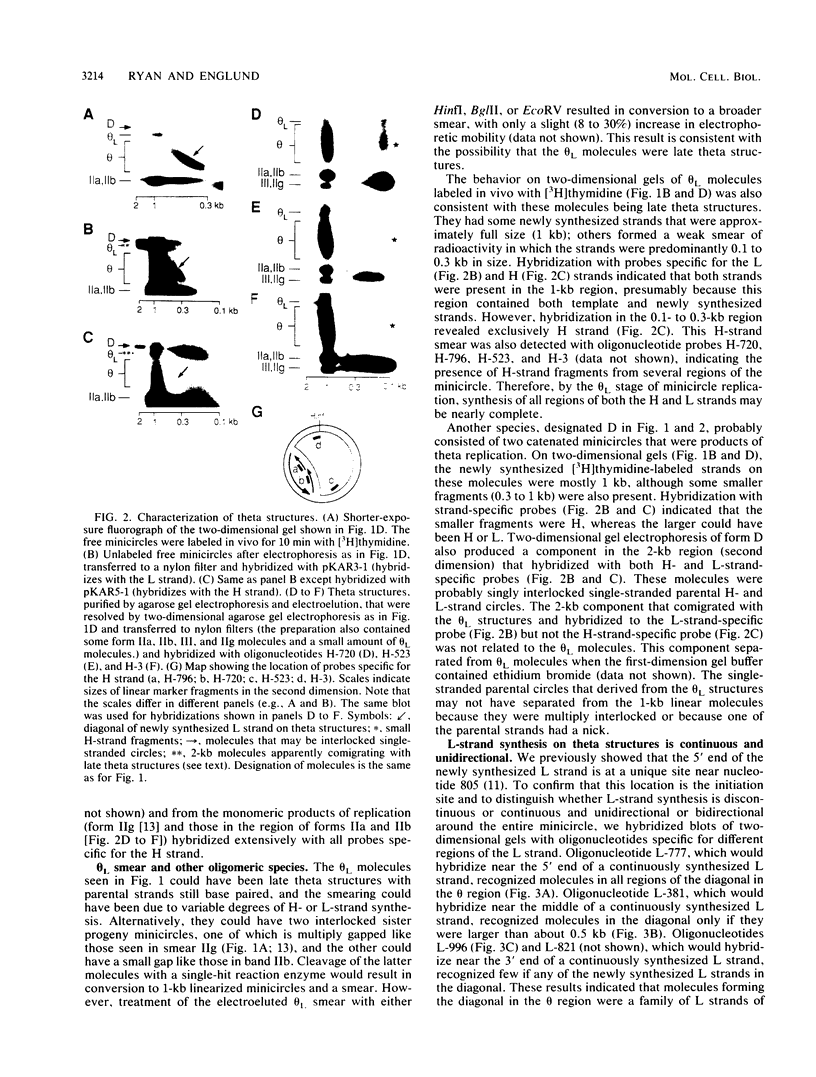
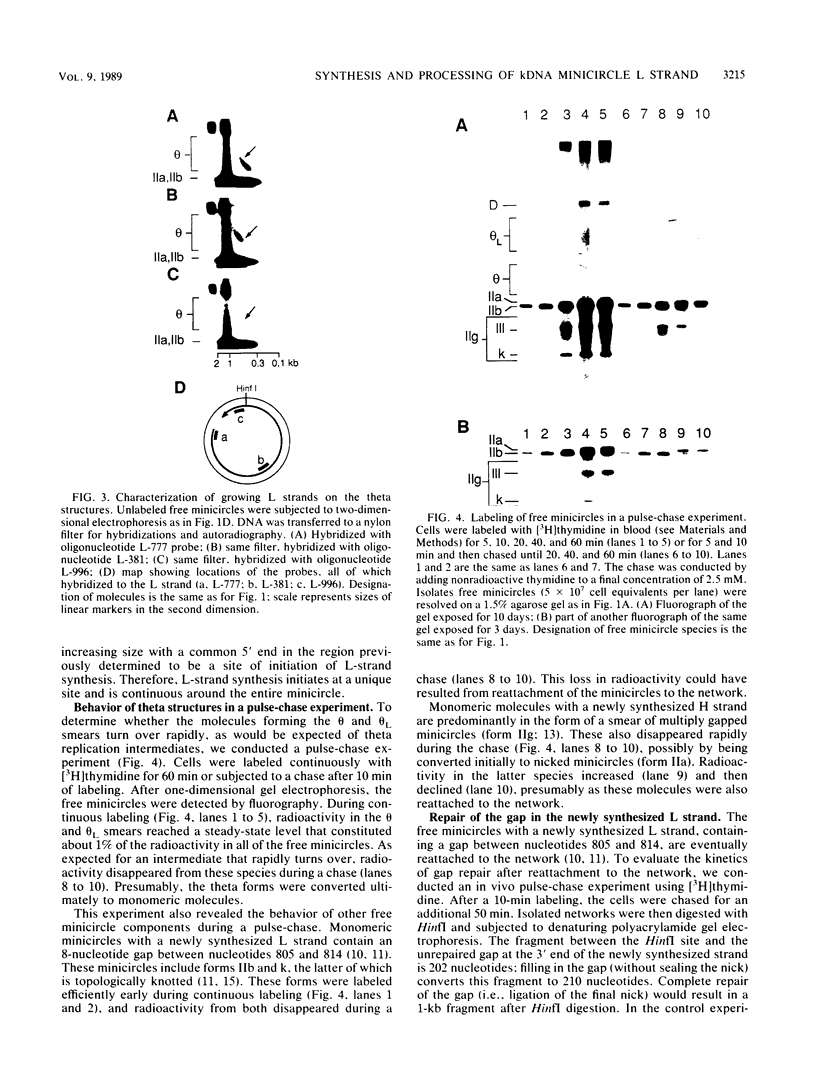
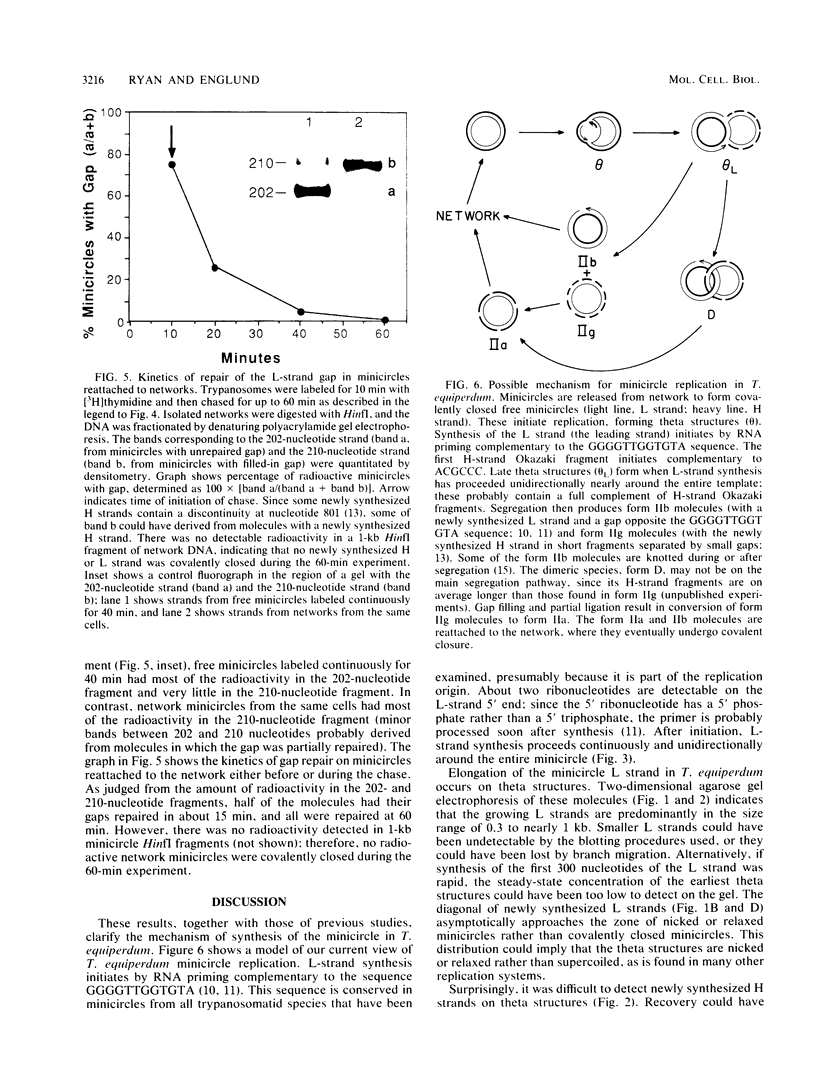
Kinetoplast DNA, the mitochondrial DNA in trypanosomes, is a giant network containing topologically interlocked minicircles. Replication occurs on free minicircles that have been detached from the network. In this paper, we report studies on the synthesis and processing of the minicircle L and H strands. Analysis of free minicircles from Trypanosoma equiperdum by two-dimensional agarose gel electrophoresis indicated that elongating L strands are present on theta structures. Hybridization studies indicated that L-strand elongation is continuous and unidirectional, starting near nucleotide 805 and proceeding around the entire minicircle. The theta structures segregate into monomeric progeny minicircles, and those with a newly synthesized L strand have a 8-nucleotide gap between nucleotides 805 and 814 (J. M. Ntambi, T. A. Shapiro, K. A. Ryan, and P. T. Englund, J. Biol. Chem. 261:11890-11895, 1986). These molecules are reattached to the network, where repair of the gap takes place. Of the molecules labeled during a 10-min pulse with [3H]thymidine, gap filling occurred on half within about 15 min and on virtually all by 60 min; however, there was no detectable covalent closure of the newly synthesized L strand by 60 min.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrois M., Riou G., Galibert F. Complete nucleotide sequence of minicircle kinetoplast DNA from Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3323–3327. doi: 10.1073/pnas.78.6.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeyer L., Ray D. S. Replication of kinetoplast DNA in isolated kinetoplasts from Crithidia fasciculata. Identification of minicircle DNA replication intermediates. J Biol Chem. 1986 Feb 15;261(5):2362–2368. [PubMed] [Google Scholar]
- Birkenmeyer L., Sugisaki H., Ray D. S. Structural characterization of site-specific discontinuities associated with replication origins of minicircle DNA from Crithidia fasciculata. J Biol Chem. 1987 Feb 15;262(5):2384–2392. [PubMed] [Google Scholar]
- Brack C., Delain E., Riou G. Replicating, convalently closed, circular DNA from kinetoplasts of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1642–1646. doi: 10.1073/pnas.69.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. Free minicircles of kinetoplast DNA in Crithidia fasciculata. J Biol Chem. 1979 Jun 10;254(11):4895–4900. [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Englund P. T. Intermediates in the replication of kinetoplast DNA minicircles. J Biol Chem. 1985 Mar 25;260(6):3844–3851. [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Fein B. I., Englund P. T. Gapped Minicircles. A novel replication intermediate of kinetoplast DNA. J Biol Chem. 1984 Dec 25;259(24):15532–15539. [PubMed] [Google Scholar]
- Ntambi J. M., Englund P. T. A gap at a unique location in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1985 May 10;260(9):5574–5579. [PubMed] [Google Scholar]
- Ntambi J. M., Shapiro T. A., Ryan K. A., Englund P. T. Ribonucleotides associated with a gap in newly replicated kinetoplast DNA minicircles from Trypanosoma equiperdum. J Biol Chem. 1986 Sep 5;261(25):11890–11895. [PubMed] [Google Scholar]
- Ray D. S. Kinetoplast DNA minicircles: high-copy-number mitochondrial plasmids. Plasmid. 1987 May;17(3):177–190. doi: 10.1016/0147-619x(87)90026-6. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Englund P. T. Replication of kinetoplast DNA in Trypanosoma equiperdum. Minicircle H strand fragments which map at specific locations. J Biol Chem. 1989 Jan 15;264(2):823–830. [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Englund P. T. Replication of kinetoplast DNA in trypanosomes. Annu Rev Microbiol. 1988;42:339–358. doi: 10.1146/annurev.mi.42.100188.002011. [DOI] [PubMed] [Google Scholar]
- Ryan K. A., Shapiro T. A., Rauch C. A., Griffith J. D., Englund P. T. A knotted free minicircle in kinetoplast DNA. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5844–5848. doi: 10.1073/pnas.85.16.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro T. A., Klein V. A., Englund P. T. Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J Biol Chem. 1989 Mar 5;264(7):4173–4178. [PubMed] [Google Scholar]
- Sheline C., Melendy T., Ray D. S. Replication of DNA minicircles in kinetoplasts isolated from Crithidia fasciculata: structure of nascent minicircles. Mol Cell Biol. 1989 Jan;9(1):169–176. doi: 10.1128/mcb.9.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. The mitochondrial genome of kinetoplastid protozoa: genomic organization, transcription, replication, and evolution. Annu Rev Microbiol. 1987;41:363–382. doi: 10.1146/annurev.mi.41.100187.002051. [DOI] [PubMed] [Google Scholar]
- Stuart K. Kinetoplast DNA, mitochondrial DNA with a difference. Mol Biochem Parasitol. 1983 Oct;9(2):93–104. doi: 10.1016/0166-6851(83)90103-2. [DOI] [PubMed] [Google Scholar]