Abstract
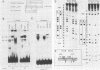
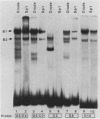
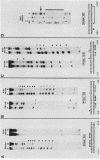
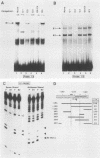
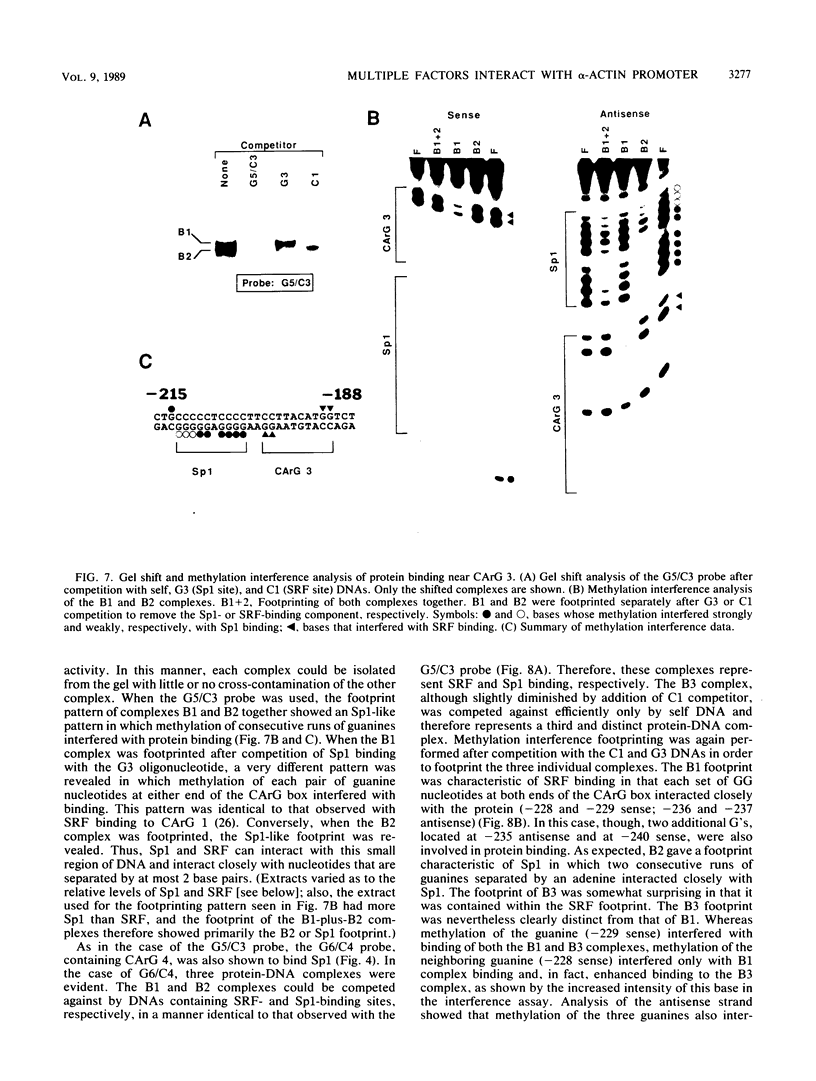
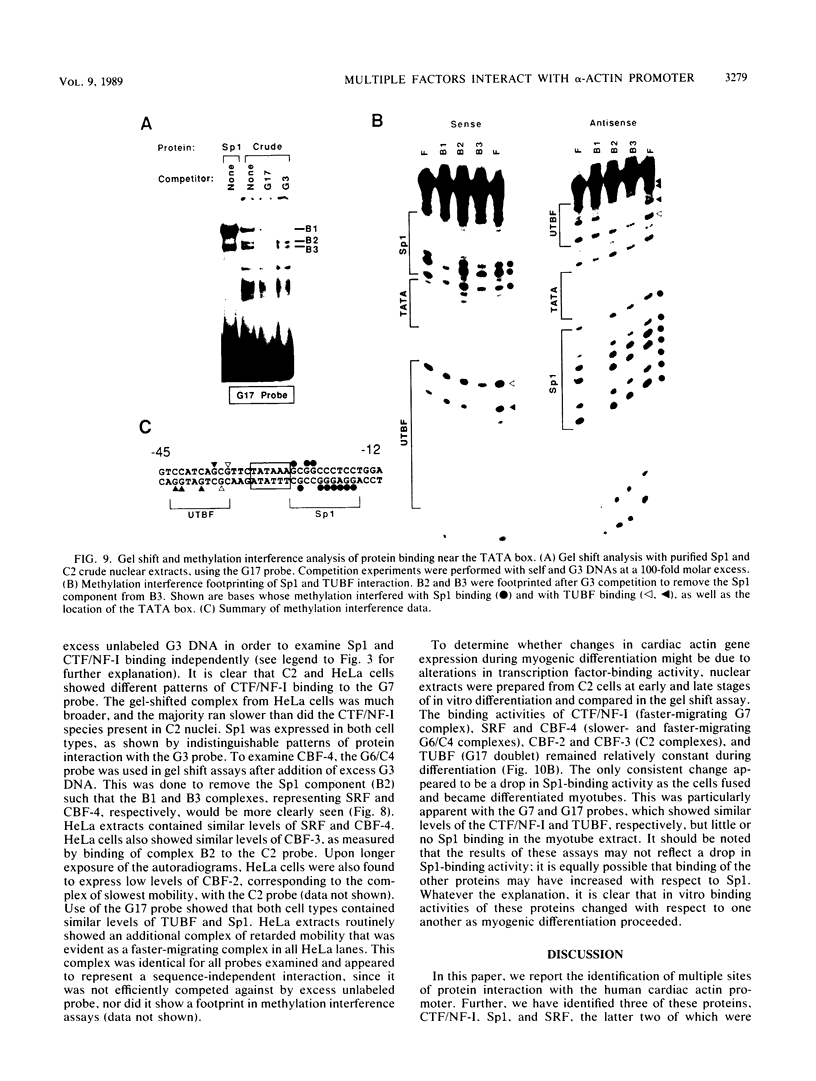
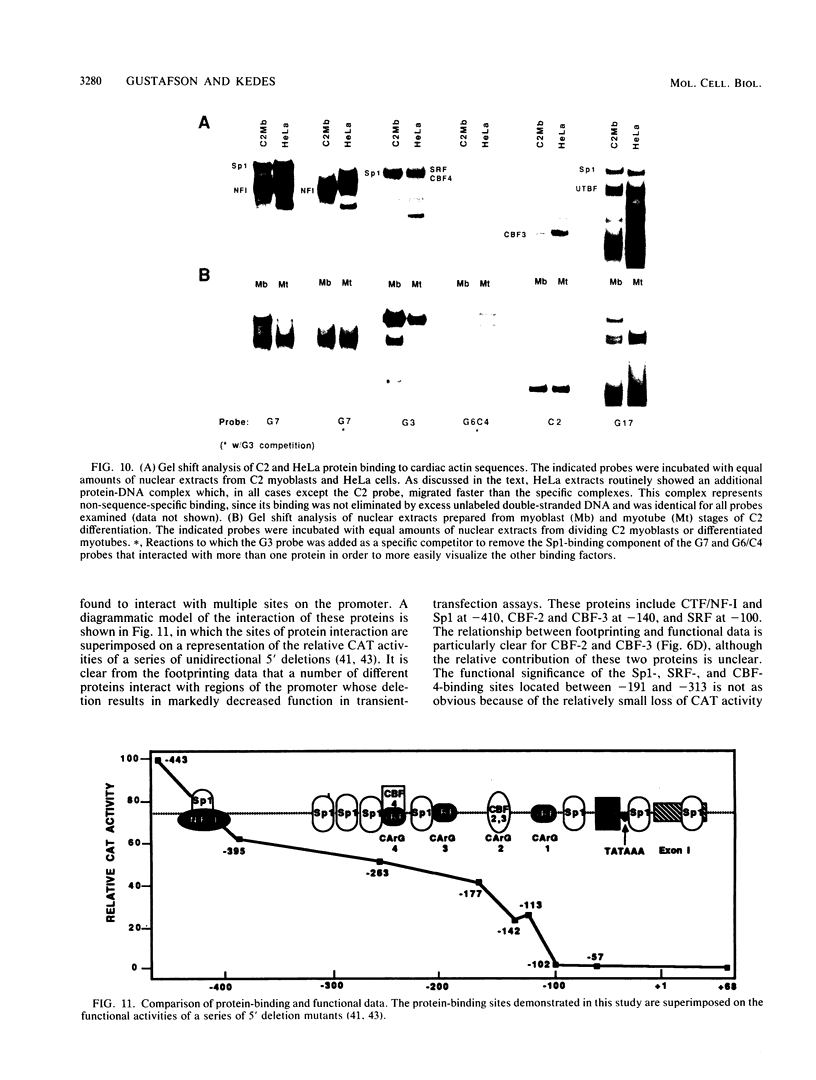
5' Sequences of the human cardiac alpha-actin gene are involved in the tissue-specific and developmental regulation of the gene. Deletion analyses combined with transient expression experiments in muscle cells have demonstrated three primary regions of functional importance (A. Minty and L. Kedes, Mol. Cell. Biol. 6:2125-2136, 1986; T. Miwa and L. Kedes, Mol. Cell. Biol. 7:2803-2813, 1987), and we have previously demonstrated binding of a protein indistinguishable from serum response factor (SRF) to the most proximal region (T.A. Gustafson, T. Miwa, L.M. Boxer, and L. Kedes, Mol. Cell. Biol. 8:4110-4119, 1988). In this report, we examine protein interaction with the remainder of the promoter. Gel shift and footprinting assays revealed that at least seven distinct nuclear proteins interacted with known and putative regulatory regions of the promoter. The transcription factor Sp1 bound to eight sites, as demonstrated by footprinting assays and gel shift analysis with purified Sp1. Purified CCAAT box-binding transcription factor CTF/NF-I and Sp1 were shown to interact with the far-upstream regulatory element at -410, and footprint analysis showed extensive overlap of these two sites. Two unidentified proteins with similar but distinct footprints interacted with the second region of functional importance at -140, which contains the second CArG motif [CC(A + T rich)6GG], and these proteins were shown to be distinct from SRF. SRF was found to bind to the remaining three CArG boxes, two of which were closely interdigitated with Sp1 sites. In addition, CArG box 4 was found to interact with SRF and another distinct protein whose footprint was contained within the SRF-binding site. Sequences surrounding the TATA box were also shown to bind proteins. Sp1 was shown to bind to a site immediately downstream from the TATA box and to a site within the first exon. Thus, each of the three functional upstream regions, as defined by transfection assays, was shown to interact with five factors: Sp1 and CTF/NF-I at the upstream site, two unidentified proteins at the central site, and SRF at the most proximal site. These results suggest that expression of the cardiac actin gene in muscle cells is controlled by complex interactions among multiple upstream and intragenic elements.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Kittler E. L., Emerson C. P., Jr Structure, evolution, and regulation of a fast skeletal muscle troponin I gene. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8080–8084. doi: 10.1073/pnas.82.23.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Barnhart K. M., Kim C. G., Banerji S. S., Sheffery M. Identification and characterization of multiple erythroid cell proteins that interact with the promoter of the murine alpha-globin gene. Mol Cell Biol. 1988 Aug;8(8):3215–3226. doi: 10.1128/mcb.8.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhueter S., Courtois G., Crabtree G. R. A variant nuclear protein in dedifferentiated hepatoma cells binds to the same functional sequences in the beta fibrinogen gene promoter as HNF-1. EMBO J. 1988 Aug;7(8):2485–2493. doi: 10.1002/j.1460-2075.1988.tb03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet P. F., Strehler E. E., White G. E., Strehler-Page M. A., Nadal-Ginard B., Mahdavi V. Multiple positive and negative 5' regulatory elements control the cell-type-specific expression of the embryonic skeletal myosin heavy-chain gene. Mol Cell Biol. 1987 Dec;7(12):4377–4389. doi: 10.1128/mcb.7.12.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Jaynes J. B., Hauschka S. D. Regulation of creatine kinase induction in differentiating mouse myoblasts. Mol Cell Biol. 1985 Mar;5(3):484–492. doi: 10.1128/mcb.5.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Hofmann S. L., van der Westhuyzen D. R., Südhof T. C., Brown M. S., Goldstein J. L. Sterol-dependent repression of low density lipoprotein receptor promoter mediated by 16-base pair sequence adjacent to binding site for transcription factor Sp1. J Biol Chem. 1988 Mar 5;263(7):3372–3379. [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., DeChiara T., Efstratiadis A. A promoter of the rat insulin-like growth factor II gene consists of minimal control elements. J Mol Biol. 1988 Jan 5;199(1):61–81. doi: 10.1016/0022-2836(88)90379-8. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner I., Minty A. J., Alonso S., Barton P. J., Buckingham M. E. A 5' duplication of the alpha-cardiac actin gene in BALB/c mice is associated with abnormal levels of alpha-cardiac and alpha-skeletal actin mRNAs in adult cardiac tissue. EMBO J. 1986 Oct;5(10):2559–2567. doi: 10.1002/j.1460-2075.1986.tb04535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Blau H., Kedes L. alpha-skeletal and alpha-cardiac actin genes are coexpressed in adult human skeletal muscle and heart. Mol Cell Biol. 1983 Nov;3(11):1985–1995. doi: 10.1128/mcb.3.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson T. A., Miwa T., Boxer L. M., Kedes L. Interaction of nuclear proteins with muscle-specific regulatory sequences of the human cardiac alpha-actin promoter. Mol Cell Biol. 1988 Oct;8(10):4110–4119. doi: 10.1128/mcb.8.10.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Allegretto E. A., Karin M., Green M. R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988 Oct;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
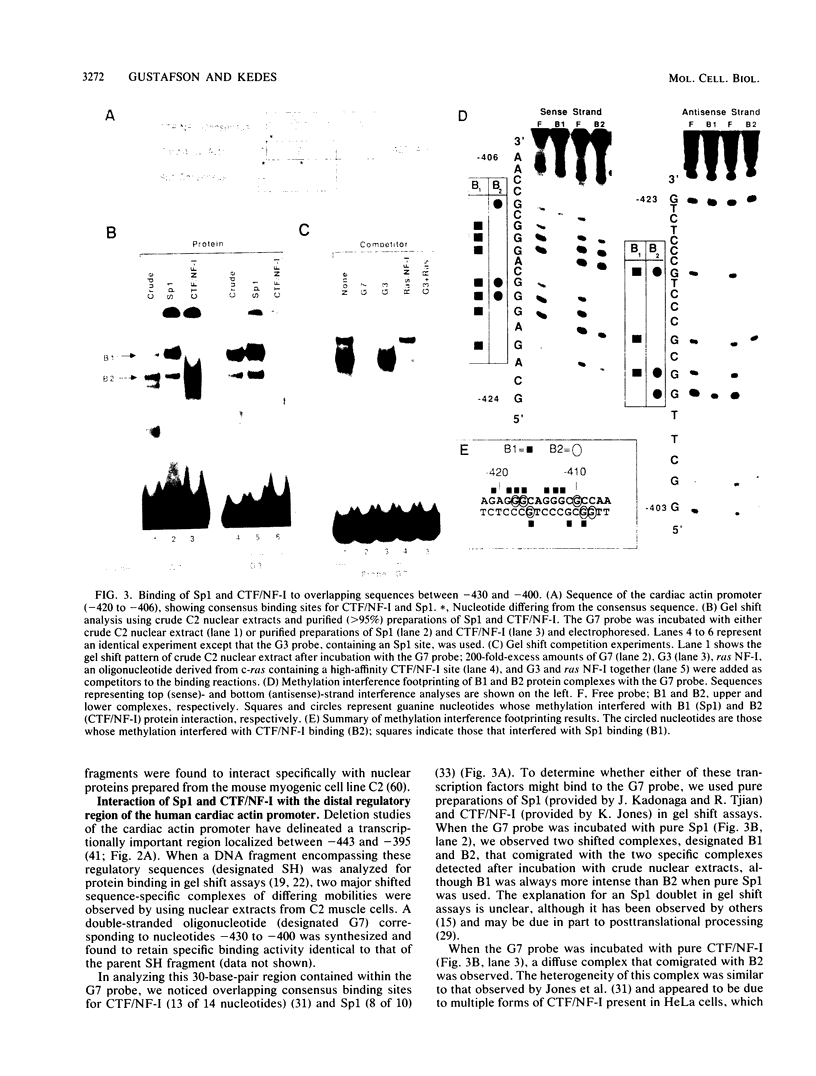
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kessler D. S., Levy D. E., Darnell J. E., Jr Two interferon-induced nuclear factors bind a single promoter element in interferon-stimulated genes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8521–8525. doi: 10.1073/pnas.85.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mayer Y., Czosnek H., Zeelon P. E., Yaffe D., Nudel U. Expression of the genes coding for the skeletal muscle and cardiac actions in the heart. Nucleic Acids Res. 1984 Jan 25;12(2):1087–1100. doi: 10.1093/nar/12.2.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Boxer L. M., Kedes L. CArG boxes in the human cardiac alpha-actin gene are core binding sites for positive trans-acting regulatory factors. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6702–6706. doi: 10.1073/pnas.84.19.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T., Kedes L. Duplicated CArG box domains have positive and mutually dependent regulatory roles in expression of the human alpha-cardiac actin gene. Mol Cell Biol. 1987 Aug;7(8):2803–2813. doi: 10.1128/mcb.7.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima-Sugano J., Roeder R. G. Cell-type-specific transcription of an immunoglobulin kappa light chain gene in vitro. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8511–8515. doi: 10.1073/pnas.83.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. G., Courtois G., Fourel G., Chodosh L. A., Campbell L., Evans E., Crabtree G. R. Sp1, a CAAT-binding factor, and the adenovirus major late promoter transcription factor interact with functional regions of the gamma-fibrinogen promoter. Mol Cell Biol. 1988 Jun;8(6):2628–2637. doi: 10.1128/mcb.8.6.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. D., Williams G. T., Morimoto R. I., Greene J., Kingston R. E., Tjian R. Two transcriptional activators, CCAAT-box-binding transcription factor and heat shock transcription factor, interact with a human hsp70 gene promoter. Mol Cell Biol. 1987 Mar;7(3):1129–1138. doi: 10.1128/mcb.7.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Gustafson T. A., Kedes L. A common factor regulates skeletal and cardiac alpha-actin gene transcription in muscle. Mol Cell Biol. 1988 Oct;8(10):4120–4133. doi: 10.1128/mcb.8.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan-Dinh-Tuy F., Tuil D., Schweighoffer F., Pinset C., Kahn A., Minty A. The 'CC.Ar.GG' box. A protein-binding site common to transcription-regulatory regions of the cardiac actin, c-fos and interleukin-2 receptor genes. Eur J Biochem. 1988 May 2;173(3):507–515. doi: 10.1111/j.1432-1033.1988.tb14027.x. [DOI] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga G., Barberis A., Schaffner G., Busslinger M. The -117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988 Oct;7(10):3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Bugaisky G., Buckingham M. Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem. 1986 Feb 5;261(4):1838–1843. [PubMed] [Google Scholar]
- Walsh K., Schimmel P. Two nuclear factors compete for the skeletal muscle actin promoter. J Biol Chem. 1987 Jul 15;262(20):9429–9432. [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Ferrandon D., Rosales R., Vigneron M., Macchi M., Ruffenach F., Chambon P. One cell-specific and three ubiquitous nuclear proteins bind in vitro to overlapping motifs in the domain B1 of the SV40 enhancer. EMBO J. 1987 Oct;6(10):3005–3013. doi: 10.1002/j.1460-2075.1987.tb02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]