Abstract
Prostate cancer (PCa) is the most commonly diagnosed male malignancy and the second biggest cause of cancer death in men of the Western world. Higher incidences of PCa occur in men from North America, Oceania and Western countries, whereas men from Asia and North Africa have a much lower PCa incidence rate. Investigations into this population disparity of PCa incidence, in order to identify potential preventive factors or targets for the therapeutic intervention of PCa, have found differences in both environmental and genetic variations between these populations. Environmental variations include both diet and lifestyle, which vary widely between populations. Evidence that diet comes into play has been shown by men who immigrate from Eastern to Western countries. PCa incidence in these men is higher than men in their native countries. However the number of immigrants developing PCa still doesn’t match native black/white men, therefore genetic factors also contribute to PCa risk, which are supported by familial studies. There are a number of genetic polymorphisms that are differentially presented between Western and Eastern men, which are potentially associated with PCa incidence. Androgen and its receptor (AR) play a major role in PCa development and progression. In this study, we focus on genes involved in androgen biosynthesis and metabolism, as well as those associated with AR pathway, whose polymorphisms affect androgen level and biological or physiological functions of androgen. While many of the genetic polymorphisms in this androgen/AR system showed different frequencies between populations, contradictory evidences exist for most of these genes investigated individually as to the true contribution to PCa risk. More accurate measurements of androgen activity within the prostate are required and further studies need to include more African and Asian subjects. As many of these genetic polymorphisms may contribute to different steps in the same biological/physiological function of androgen and AR pathway, an integrated analysis considering the combined effect of all the genetic polymorphisms may be necessary to assess their contribution to PCa initiation and progression.
Keywords: Prostate cancer, ethnical disparity, risk factors, genetic polymorphism, androgen, androgen receptor
Introduction
Prostate cancer (PCa) is the most common male malignancy and the second leading cause of cancer mortality among men in Western countries [1]. However, there is significant disparity between the incidence and mortality of the disease among different countries and races. People in North America, Oceania, North and Western Europe have a much higher disease incidence than Asian and North African populations [1]. There are evidences to show that PCa development is due to multiple factors, such as environmental exposure, diet and genetic variation; and these factors, differentially present in different populations, may be associated with prostate carcinogenesis through the induction of certain somatic genomic alterations, which are detected at different frequencies between populations [2-4]. Important evidence that diet and environmental factors contribute to PCa include studies on Asian immigrants in North America and Europe, who have a significantly higher incidence of PCa than residents in Asia [5,6]. Fat consumption is higher in the Western population than Asian, and is associated with around 2-fold increased PCa risk [7]. In contrast to the elevated PCa risk by saturated fat intake, soy products and green tea, which are more popular in Asian men, were shown to associate with decreasing PCa risk [8-12]. However, although Asian immigrants in North America and Europe have a higher incidence of PCa than residents in Asia, it is still lower than white and black men in those regions [5,13,14]. Immigrant studies in the US also showed that, even under the same environmental conditions and medical care system, there were significant differences in mobility and mortality of PCa between white and African American men [15]. Asian American men presented with lower clinical stage PCa but a more adverse biopsy grade than Caucasian and African American men [16,17]. These data suggest that genetic factors also play an important role in the racial and regional difference in PCa incidence and mortality. In addition, evidence of the importance of the genetic contribution to PCa is supplied by the study of familial disease, which accounts for approximately 10-15% of PCa cases. A meta-analysis study found that first degree family members of PCa patients are at a 2.53-fold lifetime risk of developing the disease [18]. Another review also showed that the risk of developing PCa is about 15% if a first line relative has suffered, this risk increases to 20% if a father or brother under the age of 60 have suffered from the disease [19]. While it can be argued that family members have a similar life style and environmental exposure, twin studies have provided more convincing evidence of the genetic effect of PCa. Monozygotic twins, who are genetically identical, were found to have a higher risk of developing PCa than dizygotic twins, who only share 50% of their genes [20-22].
We will review the ethnical disparities of genetic polymorphism and its association with differences in PCa incidence or progression. As androgen and androgen receptor (AR) play a critical role in both normal prostate and PCa growth [23,24], in this article we will focus on genes involved in androgen biosynthesis and metabolism, as well as those associated with AR pathways, whose polymorphisms affect androgen level and androgen biological or physiological functions.
Androgen in prostate cancer development and ethnic differences in androgen levels
The growth of normal prostate epithelial cells or PCa cells depends on androgen. PCa is extremely rare in men castrated before puberty [25] and androgen deprivation is currently still the standard therapy for advanced PCa. Androgens have also been implicated in the occurrence of the TMPRRS: ERG fusion gene. This fusion gene has been found at different frequencies between populations, occurring in 50% of PCa samples from Western men, in comparison to around 10% in Chinese men [2]. This fusion gene can be induced in the PCa cell line LNCaP following treatment for 24h with DHT and in non-cancer cell line PNT1A and PNT2 following long term exposure to DHT [26-29]. Therefore, androgen levels may be an important factor in PCa risk.
Some studies have reported variation in the serum levels of androgen between different ethnic backgrounds, consistent with variation in PCa incidence between different ethnicities. They reported black men had higher serum levels of testosterone, free testosterone and dihydrotestosterone (DHT) than white men [30,31], and DHT to testosterone ratios were highest in African-American, intermediate in white, and lowest in Asian-American men [31,32]. This indicates that a high androgen level is a risk for PCa. However, studies stratified by age found that the difference in serum testosterone only exists in young men. Ellis et al [33] compared 525 African American men and 3654 non-Hispanic white men in different age categories. In the 31 to 34 year-old group, African-American men had a 6.6% higher mean serum testosterone level than white men, yet in the 40 to 50 year-old group, the difference was only 0.5%. Kubricht et al [34] reported that African American and white men aged over 40 had comparable serum testosterone levels. Moreover, some studies found no racial differences in circulating testosterone and DHT [35,36].
Regarding the association between androgen level and PCa, most case-control studies did not support that serum androgen levels contribute to PCa development [37-53], only two studies reported positive association between PCa and circulating testosterone level [54,55]. A large pooled analysis including 18 prospective studies also reported that PCa risk is not associated with serum levels of testosterone, free testosterone, or DHT [56]. Although the pooled analysis showed a negative result, Hsing et al [57] indicated circulating levels of testosterone might not reflect androgen action in the prostate. In the serum, the concentration of DHT, which has a higher affinity for AR was far less than the concentration of testosterone, whereas the concentration of DHT in prostatic tissue was several times higher than that of testosterone [57,58]. Due to technical limitation, it is difficult to directly measure the androgen level of prostate tissue, particularly in the healthy population. There is only one study reporting androgen level analysis in prostate tissue. The study found that black men had a higher tissue concentration of sex hormone-binding globulin (SHGB) and androstenedione than white men, but the testosterone and DHT levels in prostate tissue are similar in black and white men [59]. Recent studies of androgen induced gene proximity and fusion genes [26-29] indicate that high androgen levels at a certain physiological developmental stage, is a risk factor for inducing genomic alterations in prostate cells, and consequently increases the risk of PCa. The conflicting results either support the association between androgen level and PCa risk, or oppose it in different studies using different research approaches. This may be due to the complexity in measuring the real effective androgen or associated protein levels. The ideal way to measure the impact of androgens on PCa should be the DHT levels within the prostate tissue from the period of puberty, when there is a boost of androgen levels, to age 50 or 60, when PCa occurs. However, currently it is technically difficulty to do this. Novel techniques to repeatedly measure tissue androgen levels in an individual through a long period are urgently required. For now we have to estimate the action of androgen in the prostate by other means.
Genes involved in androgen synthesis and metabolism
While it is difficult to identify the form of androgen that potentially contributes to prostate carcinogenesis and difficult to quantitatively measure the active form of androgen in the prostate, many studies have focused on genes involved in androgen synthesis and metabolism.
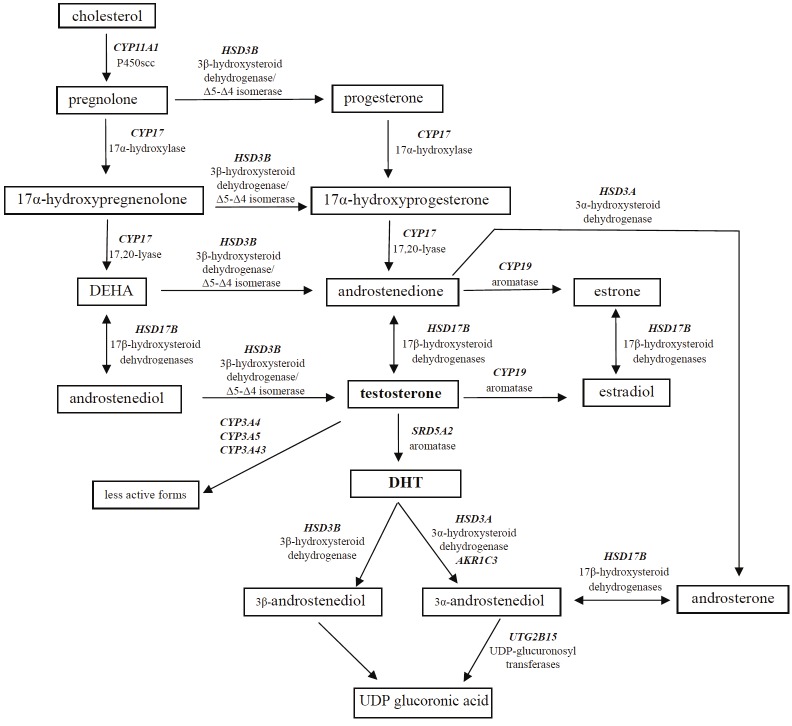
Many of these genes have been found to harbour genetic polymorphisms. These polymorphisms can potentially change androgen levels in prostate tissue, and therefore, may give a better idea of the action androgen is playing in prostate tissue than measuring levels of circulating androgen. Here we summarize the reported genetic polymorphisms in these pathways (Figure 1), which have been reported at differential racial frequencies and implicated in variations of PCa risk between different populations (Table 1).
Figure 1.
Androgen pathway and genes involved in androgen biosynthesis and metabolism. Abbreviations, DHEA: dehydroepiandrosterone; DHT: dihydrotestosterone.
Table 1.
Genetic polymorphisms in genes associated with androgen biosynthesis/metabolism and AR which show differential racial frequencies and potential association with prostate cancer
| Gene | Variant | Racial frequency difference | Association with prostate cancer risk | ||
|---|---|---|---|---|---|
|
| |||||
| Asian | White | Black | |||
| CYP11A1 | (TAAAA)n repeat | More 6-repeats in Japanese, 4-repeats in European and African | IC | IC | IC |
| CYP17 | T>C (rs743572) | Highest in Asians, intermediate in Caucasians, lowest in Blacks | No | No | Yes |
| SRD5A2 | A49T | Sparse in whites and blacks, not detected in Asians | IC | IC | IC |
| V89L | Higher in Asians than Caucasians and Blacks | IC | IC | IC | |
| (TA)n repeat | Longer repeat higher in whites than Asians | Yes | Yes | NA | |
| HSD3B1 | N367T (rs1047303) | Higher frequency in Caucasians, middle in African Americans and lower in Asians | IC | IC | IC |
| HSD3B2 | (TG)n(TA)n (CA)n repeat | Most common alleles occurred at different frequencies | NA | Yes | No |
| HSD3B2 | rs1819698 and rs1538989 | Higher in African-Americans than Caucasians | NA | No | Yes |
| CYP19A1 | rs2470164 | Higher in Caucasians than African Americans | NA | Yes | No |
| Arg264Cys (rs700519) | Higher frequency in Indians than African Americans and Caucasians | Yes | IC | No | |
| (TTTA) n | Short repeat (A1) at high frequency in Asians | IC | IC | IC | |
| CYP3A4 | A>G (rs2740574) | Higher in African descents than Caucasians and Asians | IC | IC | IC |
| CYP3A5 | CYP3A5*3C (rs10249369) | More in Caucasians than Africans | NA | Yes | No |
| CYP3A43 | CYP3A43*3 (rs680055) | More in African Americans than Caucasians | NA | No | Yes |
| HSD17B1 | Haplotype CAGC | More in whites and blacks than Asians | Yes | No | No |
| AKR1C3 | A>G (rs3763676) | More in Caucasians than Asians | NA | Yes | NA |
| SHBG | D356N | More in whites than blacks | NA | IC | IC |
| AR | CAG repeat | Longest in Asians, intermediate in whites, shortest in blacks | IC | IC | IC |
| GGN repeat | Longest in Asians, medium in whites, shortest in blacks | IC | IC | IC | |
| UGT2B15 | D85Y | More 85D allele in Asians than Caucasians | IC | IC | IC |
NA: no report in the population; IC: Inconclusive.
CYP11A1
CYP11A1 gene on 15q23–q24 encodes the P450scc enzyme, which is the first and also rate-limiting step of biosynthesis for both testosterone and estrogen, it catalyzes cholesterol to pregnenolone. There is a pentanucleotide (TAAAA)n repeat located in the 5’UTR of the gene, ranging from 4 to 10-repeat sequences [60]. Although the association between (TAAAA)n repeat and androgen level is unclear, population studies have found higher prevalence of a 6-repeat allele in Japanese populations compared with the higher prevalence of a 4-repeat allele in European and African populations [60,61]. Japanese PCa patients without the 4-repeat allele had an increased risk of metastatic PCa compared to those with the 4-repeat allele [61]. However, a positive association with PCa risk was not identified from a few studies of European populations [62-66].
CYP17
CYP17 gene is located on chromosome 10 and encodes the cytochrome P450 17 enzyme (17a-hydroxylase/17, 20-lyase). This enzyme catalyzes two reactions in the biosynthesis of testosterone in the gonad and adrenal glands. The first step is conversion of pregnenolone to 17-hydroxypregnenolone (hydroxylase activity), and the second step is the subsequent conversion to dehydroepiandrosterone (lyase activity) [67,68]. The 5′-untranslated promoter region of the CYP17 gene contains a single nucleotide polymorphism, a T to C substitution, that gives rise to A1 (T) and A2 (C) alleles (rs743572). This T to C transition creates a potential Sp1 binding site (CCACC box) or promoter region, which was suspected to increase the transcription of the CYP17 gene [69]. However contradictory results were reported from a later study [70].
Frequency of the A2 allele was highest in Asian, intermediate in Caucasian and lowest in Black men [71,72]. However, case-control studies for association between the A2 allele and PCa risk were inconsistent. More than half of the studies indicated that the A2 allele may be associated with an increasing PCa risk [73-80]. However, a number of studies suggested no association [81-84] and a few studies even showed a possible increased risk of PCa from the A1 allele [85-87]. It is a paradox that the Asian population, with a higher frequency of the A2 allele, have a lower incidence of PCa than Black and white populations. Results from two meta-analysis studies may partially explain this contradiction. They found that a significant association between A2 polymorphism and PCa risk only existed in the black population, but not in Caucasian or Asian populations [71,88]. Therefore, the A2 type of CYP17 may cooperate with other genetic or environmental factors existing in the black population to contribute to the risk of PCa.
SRD5A2
Steroid 5α-reductase irreversibly converts testosterone into DHT. Two forms of steroid 5α-reductase exist, steroid 5α-reductase type 1 (SRD5A1) and steroid 5α-reductase type 2 (SRD5A2). SRD5A1 is expressed more abundantly in extra-prostatic tissues, such as the skin and SRD5A2 is exclusively expressed in the prostate [89]. 5α-reductase activity was lower in Asian than white and black men [90,91].
The SRD5A2 enzyme is encoded by the SRD5A2 gene, which is located on chromosome 2p23. A substitution polymorphism A49T (rs9282858) results in replacement of an alanine (A) residue at codon 49 with threonine (T), which has been reported to increase the activity of 5α-reductase 5-fold, both in vitro and in vivo [92]. The prevalence of the T allele was 2-2.8% and 1% in control subjects of European and African descent. It is absent in men of Asian descent [93-95]. The association between A49T and PCa risk has been extensively investigated, but the results are controversial. Three meta-analyses for this polymorphism have been published. In a study by Ntais et al [95] (7 studies with a total of 1594 cases and 2137 controls), the T allele has shown a modest effect on PCa susceptibility. However, the meta-analysis results from Li et al [94] (24 studies with a total of 4,998 cases and 5,451 controls) indicated A49T was probably not associated with PCa risk. A recent meta-analysis including 31 association studies with 14,726 PCa cases and 15,802 controls also found that the T allele had no significant effect on the overall PCa risk, but the T allele significantly elevated the risk of high stage (Stages III-IV) disease [93]. The prevalence of the T allele is sparse in the general population, with a frequency of T/T homozygosity of only 0.5% in healthy Caucasians, who have a relatively higher frequency of the T allele than other populations [93]. This rarity of T allele cases may also contribute partly to the inconclusive results for the association between A49T and PCa risk.
The V89L (rs523349) polymorphism results in a valine (V) to leucine (L) substitution at condon 89, which decreases 5α-reductase activity [96]. Men with the LL genotype had almost a 30% reduction of activity of 5α-reductase than men with VL or VV genotypes [96]. The L allele was more commonly found in Asian (46.9-50%) than Caucasian (28.1-37.5%) and African men (25-33.5%) [93-95]. The V89L variant was also common in men in Greenland who had a low risk of PCa [97]. These studies suggested that the V89L substitution may be a protective factor for PCa, but some recent case-control studies did not find significant association between the V89L polymorphism and PCa risk [87,98,99]. All meta-analysis studies also excluded such an association in all ethnic groups [93-95], except one, which found a small increase in PCa risk in Europeans with the L allele [100].
The TA dinucleotide repeat polymorphism is present in the 3′ untranslated region of SRD5A2. It has three main polymorphsims with different numbers of TA-dinucleotide repeats, (TA)0, (TA)9 and (TA)18 [101]. The frequencies of (TA)9 and (TA)18 alleles were 14% and 9% in healthy subjects of European and Asian descent, respectively [95], but 32% in African American men [102]. (TA)18, in particular, is present in a much higher frequency in African American than Caucasian and Asian American populations, where this allele is rare [95,102]. Although it is expected that the longer TA alleles, presenting more frequently in the high PCa risk African American group, may increase cancer risk. Interestingly, meta-analysis studies (4 studies, 1109 cases and 1378 controls) presented the opposite result, the longer alleles were associated with a modest PCa risk reduction in Caucasian men [95]. Case-control studies in Chinese and Indian men (191 and 157 cases respectively) also reported that homozygous (TA)0 leads to higher PCa risk than longer alleles [99,103]. It is not clear whether the longer repeat alleles also have a protective role in the Black population, due to lack of case-control studies in men of African descent.
HSD3B family
The HSD3B1 and HSD3B2 genes, located on 1p13.1, encode 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase 1 and 2 isoenzymes (3β-HSD types 1 and 2). The proteins are bifunctional enzymes that catalyze androstendione production in steroidogenic tissues and convert the active DHT into inactive metabolites in steroid target tissues [104].
A N367T (AAC>ACC, rs1047303) polymorphism in HSD3B1 has been reported to present at a high frequency in Caucasian (31%), inter-medium frequency in African American (11.7%) and low frequency in Asian men (8.5%), although the variant has a similar activity to the wild type [105]. Chang et al [106] reported that the N type variant increased PCa risk moderately in Caucasian men, but this was not supported by further studies [64,107].
A complex (TG)n(TA)n(CA)n dinucleotide repeat polymorphism was found in intron 3 of the HSD3B2 gene [108]. The common alleles occurred at very variable frequencies in different racial populations, with the longer alleles more commonly found in Asian men [109,110]. The longer the allele length, the more stable the hairpin structures they formed and subsequently, the faster the degradation rate of DHT. Short alleles have been found to be associated with an increased PCa risk in Caucasian but not in African American men [109].
Beuten et al [111] found that two SNPs in HSD3B2, rs1819698 and rs1538989, were more common in African American than Caucasian men and increased PCa risk in African American but not Caucasian men.
The interaction between HDS3B1 and HSD3B2 polymorphisms has also been investigated. Although the N367T polymorphism in HSD3B1 is only weakly associated with PCa risk, the combination with HSD3B2 rs1819698 greatly enhanced the association [106].
CYP19A1
The CYP19A1 gene, located on chromosome 15q21.1, encodes the enzyme aromatase, which catalyzes the irreversible conversion of C19 androgens, androstenedione and testosterone, to the C18 estrogens, estrone and estradiol respectively. More than 30 SNPs have been detected in different populations. Several SNPs (rs2470152, rs749292, rs727479) were confirmed to be associated with serum estradiol of men [112,113].
Beuten et al [111] explored polymorphisms of CYP19 by genotyping 2,452 samples from Caucasian and African American men, some of these polymorphisms (rs2470152, rs12439137, rs3751592, rs2470164) were associated with PCa risk and had different racial distributions. Particularly rs2470164, which was reported to increase PCa risk in Caucasian men, had a dramatically different frequency among healthy Caucasian (50%) and African American men (5.6%).
The tetranucleotide repeat (TTTA)n is located in intron 4 of CYP19A1, TTTA repeat numbers range from 7 to 13 and are designated as A1 to A7 according to the repeat number. Most studies for this polymorphism were among Asian men. A1 was found to occur more frequently (about 50% of the population) than all other alleles in Asian men [114,115]. Suzuki et al [116] reported that the shorter repeat (A1 and A2 alleles) significantly increased familial PCa risk in Japanese men. Huang et al [115] found the homozygous A1 genotype had a significantly greater risk of developing PCa in Taiwanese. However, in conflict with their results, another study in Japanese men found a longer repeat was significantly associated with increased PCa risk [114]. Latil et al [82] reported that some specific repeat lengths were associated with PCa risk in men of White French ethnogeographic origin, but studies among US men reported no association with PCa [64].
The polymorphism Arg264Cys substitution (rs700519) was found at a higher frequency in Indian men (27%) [117] in comparison to African American (16.8%) and Caucasian men (4-8.1%) [107,111,118]. Studies among Caucasian and Indian men showed a tendency for this polymorphism to increase risk [117,118], but large case studies failed to confirm the results in Caucasian men [107,111,113].
CYP3A family
Cytochrome P450 3A (CYP3A) enzymes hydroxylate testosterone and dehydroepiandrosterone to less active metabolites. The CYP3A locus consists of four genes in humans, CYP3A4, CYP3A5, CYP3A7 and CYP3A43, all of which reside in a 231 kb region of chromosome 7q21-22.1 [119].
CYP3A4 is involved in the oxidative deactivation of testosterone, an A to G mutation (CYP3A4*1B, rs2740574) was reported to decrease CYP3A4 protein activity, thus increase the availability of testosterone [120]. CYP3A4*1B has a higher frequency in men from African descent than Caucasian and is absent in Asian men [121-125], but case-control studies didn’t find an association between CYP3A4*1B and PCa risk in men of African descent who had a high frequency of the variant [78,111,124,126]. In addition, reports in Caucasian men were contradictory [111,124,127]. Studies for the association between CYP3A4*1B and the progression of PCa were also inconclusive, some studies reported CYP3A4*1B is associated with aggressive PCa in Caucasian and African American men [122,123,125,127,128], however others studies disagreed [129-131].
CYP3A5 catalyzes 6β-hydroxylation of testosterone, it has been suggested that CYP3A5 is expressed at high levels in the non-tumoral prostate tissue, specifically in the basolateral cells, and that this expression does not occur in the tumor. An A to G transition (A6986G) within intron 3 leads to a variant in the CYP3A5 mRNA expression in human prostatic tissue [132]. The allele CYP3A5*1 (A allele) produces a correctly spliced transcript leading to high levels of full-length CYP3A5 mRNA and protein [125,133]. The allele CYP3A5*3 (rs776746, G allele) creates a cryptic splice site leading to the inclusion of a novel exon, and ultimately a premature stop codon [133]. CYP3A5*3/*3 decreases CYP3A5 mRNA content 13-fold compared to CYP3A5*1/*3 [132]. CYP3A5*1 has a higher frequency in African American individuals than Caucasian or Asian men [133]. CYP3A5*1 was suggested to show obvious linkage disequilibrium with CYP3A4*1B in Caucasian and African men [127,133,134], and the CYP3A4*1B/CYP3A5*1 haplotype was inversely associated with risk among Caucasian men with less aggressive disease [125]. Studies in Japanese men whose CYP3A4*1B was absent may help confirm that the CYP3A5*1 allele is associated with PCa risk, however cannot exclude that CYP3A4*1B may also be a risk factor. They reported CYP3A5*1/*1 men had lower risk of developing a low-grade localized PCa than CYP3A5*3/*3 men [135]. On the other hand, although CYP3A5*3 was reported not to associate with PCa in either white or African men [126], the CYP3A4*1B/CYP3A5*3 haplotype is significantly associated with increasing PCa risk in European American but not in African American men [125,136]. Moreover, CYP3A5 is also reported to interact with SRD5A2 or KLK3 which could influence development of PCa [137].
CYP3A43 is predominantly expressed in the prostate [138]. The CYP3A43*3 allele (rs680055) frequency was significantly higher in African American than Caucasian men [127,139]. There was a 2.6-fold increase in PCa risk among individuals with the CYP3A43*3 homozygous genotype compared with those with the CYP3A43*1 homozygous genotype in African American, but not in Caucasian men [127,139].
There are very few studies on CYP3A7, Simense et al [140] found the CYP3A7*1C (rs11568825) G allele decreased levels of estrone sulphate, dehydroepiandrosterone sulfate, androstenedione and estrone, however no significant association was observed for CYP3A7 genotypes with PCa risk.
HSD17B family
The 17β-hydroxysteroid dehydrogenases (17β-HSDs) are involved in regulation of estrogens and androgens by catalyzing the reduction of 17-ketosteroids or the oxidation of 17β-hydroxysteroids.
17β-HSD1, encoded by HSD17B1 on 17q21 plays a role in estrogen and testosterone biosynthesis. Cunningham et al [64] reported a polymorphism of HSD17B1 (Ser313Gly, rs605059), detected in 40% of patients, mainly Caucasian men, had a possible association with either familial or sporadic cases of PCa. However, large numbers of multi-ethnic studies (The Breast and Prostate Cancer Cohort Consortium, BPC3) have since found no association [141]. BPC3 detected four common SNPs (rs676387, rs605059, rs598126, rs2010750). Although none were found to be associated with PCa risk, they reported some haplotypes that consisted of the four SNPs had varying frequencies between different races. The haplotype CAAC was only common in African American men, CAGC was more prevalent in white and black than Asian men, and CAGC was inversely associated with PCa risk in Latino and Japanese American but not in African American, Native Hawaiian, or white men [141].
17β-HSD2 encoded by HSD17B2 on 16q24 is involved in the conversion of active androgens into their less active forms. SNPs in HSD17B2 (rs1424151) were found to have significant associations between plasma testosterone level in Caucasian men [142], but no association with PCa was detected [64,142].
17β-HSD3 encoded by HSD17B3 on 9q22 catalyzes androstenedione to testosterone. The frequency of the G289S polymorphism (rs2066479) of HSD17B3, was 4.3-7.3% in Caucasian men and was reported to significantly increase PCa risk in Italian men [143], but studies in Finnish and Swedish men found no positive associations [107,144].
The HSD17B4 gene on 5q21 encodes androgen/estrogen inactivating enzyme 17β-HSD4. It was reported to be associated with the outcome of PCa patients [145,146].
Human 17β-HSD5 belongs to the aldo-keto reductase (AKR) superfamily and is formally known as AKR1C3 encoded by the AKR1C3 gene on 10p14-p15, it catalyzes the conversion of androstenedione to testosterone and DHT to androstanediol. An A to G substitution was identified in exon 2 that confers a Glu77 Gly (rs41306308) change, this occurred in 4.8% of Caucasian men but was completely absent in Asian men, and the Glu77Gly polymorphism was associated with lower testosterone levels in serum [147]. Furthermore a promoter polymorphism (A to G, rs3763676) of AKR1C3 is more prevalent in Caucasian than Asian men [147] and men with the A allele have a borderline significant decreased risk of PCa [148].
UGT2B15
UGT2B15 is a member of UDP-glucuronosyltransferases (UGTs) family which glucuronidate steroids and many endogenous chemicals, encoded by the UGT2B15 gene located on 14q13–q21.1. It has a high capacity to glucuronidate 3α- androstenediol and a moderate capacity for DHT. A nonsense mutation in codon 85 (aspartate>tyrosine, D85Y, Asp85Tyr) has been identified in the UGT2B15 gene. The 85Y variant associates with a 2-fold increase in activity for 3α- androstenediol and DHT, it is likely to lead to lower androgen exposure compared with 85D. A study found that Asians had a higher 85D allele frequency than Caucasians [149]. Case-control studies are inconclusive, several studies reported the 85D allele increased PCa risk [150-153], but another two studies reported no association with PCa [64,154].
Sex hormone binding globulin
Sex hormone-binding globulin (SHBG) gene is located on 17p12-p13 and encodes a steroid binding protein that is a major regulator of free plasma androgens. It also mediates androgen and estrogen signaling at the cell membrane via cyclic adenosine monophosphate. Most studies found black men who had higher PCa risk had higher plasma SHBG level than white and Asian men [155-157]. Black men were also found to have higher levels of SHBG in their prostate tissue than white men [59]. Interestingly, although the higher risk population have a higher SHBG level, a collaborative analysis of 18 prospective studies found the fifth highest serum SHBG levels had a relative PCa risk reduction of 14% when compared with the fifth lowest [56].
A common polymorphism in the SHBG gene, D356N, encodes for an additional N-glycosylation consensus site, which may reduce its clearance from circulation and alter its binding to membrane receptors [158]. Berndt et al [129] carried out a multicenter study and found the SHBG D356N heterozygotic polymorphism had a higher frequency in white men (17%) than black men (7.8%). The D356N heterozygote is associated with increasing PCa risk in non-Hispanic white but not in black men. Studies carried out in British and US men reported no association between PCa and SHBG polymorphisms [64,159].
Androgen receptor gene (AR gene) polymorphism
AR gene is located at Xq11.2-q12, the open reading frame is separated over eight exons that encode for AR. AR comprises of four functional domains including the amino-terminal transcriptional activation domain, the DNA binding domain, a hinge region, and the carboxyl-terminal ligand binding domain [160]. Expression of AR protein was found to be higher both in benign prostate tissue and PCa tissue in black men compared with white men [161,162]. The amino-terminal transcriptional activation domain, encoded by exon one, includes two high frequency polymorphic repeats, CAG and GGN [163]. AR expression level and function were found to have an inverse association with the length of CAG or GGN repeat in in vitro studies [164,165].
The length of CAG repeats ranges from 8 to 35 repeats in the normal population. Hispanic men have been reported to have the longest average CAG repeat length (23-25). The Chinese population have longer CAG repeats (average between 22-23) than that of the Caucasian population (average between 21-22), and the black population have the shortest average CAG repeats (average between 19-20) [35,97,166-175]. Several studies reported testosterone levels were significantly elevated in men with greater CAG repeat length [167,176,177], but other studies found no correlation between CAG repeat length and serum testosterone levels [178-180]. Studies of polymorphic CAG repeats associating with PCa risk were also inconsistent, several studies found the shorter CAG repeats associated with increasing PCa risk [171,181-188], a meta-analysis reported the association was different in different populations, longer repeat carriers (>/= 20 repeats) had 11% decreased risk in populations from USA, 53% decreased from Europe, and 20% decreased from Asia [189], however recently several projects, including two multiple-center, large-sample studies didn’t find association between CAG repeat length and PCa risk [166-168,170,175,190-194]. A few studies among the East Asian population even observed that longer than average CAG repeat length is more common in PCa cases compared to the controls [195,196]. Instead of studying longer or shorter CAG repeat length, one study focused on some specific CAG repeat numbers, Ding et al [164] reported 17 CAG-repeats was much more common in PCa patients (8.5%) than in the general European and American populations (1.3%).
The biological role of GGN trinucleotide repeats is less clear, polymorphisms in the normal population range from 10 to 31 repeats, present as a (GGT)3GGG(GGT)2(GGC)n motif. Similar to CAG repeat variation in different populations, black men have shorter GGN repeats than white and Asian men [197,198]. Most studies to evaluate the relationship between PCa and GGN repeat length were carried out in Caucasian men. Case-control studies were also controversial among Caucasian and Asian men. A shorter GGN repeat length was found to be associated with PCa risk in several studies [172,199-201]. One study found that PCa risk was higher in American men with 23 GGN repeats than all other repeat numbers [202]. However, more studies found no association between GGN repeats and PCa risk [168,181,182,193,194,203-205]. Unlike the inconclusive results in white and Asian men, the only two studies including black men consistently reported no associations between PCa risk and GGN polymorphism [168,182].
The size or composition of a GGN repeat was reported to have no correlation with the length of the CAG repeat [163], however, there may be interaction between them. When a subgroup with two short repeats (CAG <22; GGN <or =16) was compared with those in which both alleles were long (CAG > or =22; GGN >16), increasing PCa risk was observed in Caucasian men [199], but the result couldn’t be replicated in a later study which found the haplotye with short CAG (<22) and short GGC repeats (<or =17) didn’t increase PCa risk [193].
Other genetic polymorphisms associated with prostate cancer predisposition
Besides these genes related to androgen, some other genetic polymorphisms have also shown population differences and have been implicated for possible association with PCa, as they play important roles in cellular proliferation, differentiation and apoptosis (Table 2). The epidermal growth factor receptor (EGFR) gene is located at 7p12. EGFR, encoded by EGFR gene is a cell surface protein that binds to epidermal growth factor. A dinucleotide (CA)n repeat polymorphism, ranging from 14 to 21 repeats, was suggested to regulate EGFR expression. The frequency of longer alleles is significantly higher in Asian men than Caucasian and African men [206]. Although there is no direct evidence to suggest the polymorphism is associated with PCa, the longer allele with 21 repeats showed an 80% reduction of gene expression compared with the shorter allele with 16 repeats [206,207]. EGFR protein over expression was found in 36% of the prostate tumor samples [208]. Another candidate gene RNASE L is located in the hereditary PCa 1 (HPC1) gene region (1q24-25). The polymorphism Arg462Gln in RNASEL has a higher frequency in Caucasian than African American men and is associated with increasing PCa risk in these men [209,210], but a study among Japanese men reported the Gln462 allele decreased the risk of familial PCa [211]. Homozygous Asp541 in RNASE L is significantly less frequent in Asian than Caucasian and African men [211-214]. The Asp541 allele is associated with decreasing PCa risk in African American men but increasing risk in Japanese men [211,212]. The ELAC homolog-2/hereditary PCa (ELAC2/HPC2) gene at 17p11 is involved in DNA inter strand crosslink repair and mRNA editing, it has a possible role in the regulation of cell cycle progression. The polymorphisms Leu217 and Thr541 in ELAC2 were more prevalent in Caucasian than in Asian and black men [212,215]. A meta-analysis reported the Leu217 allele and Thr541 polymorphisms significantly increased PCa risk in Asian men but moderately affected Caucasian men [215]. Leu217 could also significantly increase PCa risk in African American men [212]. X-ray repair cross-complementing group 1 (XRCC1) is an important DNA repair gene located at 19q13.2-13.3. The polymorphism Arg399Gln correlates with DNA repair activity. Meta-analysis found Gln399 associated with higher PCa risk in Asian men but not Caucasian men [216,217]. E-cadherin (CDH1) gene encoding an adhesion glycoprotein, located at 16q22.1, has a -160C/A polymorphism in the promoter region. The A allele has approximately 68% decreased transcriptional activity compared with the C allele [218]. Most studies showed the A allele increased the risk of PCa among Caucasians [219-223], but did not affect men from African descent [222,223]. Studies in Asian men reported inconclusive results [224-226].
Table 2.
Genetic polymorphisms in non-androgen associated genes with differential racial frequencies
| Gene | Variant | Racial frequency difference | Association with prostate cancer risk | ||
|---|---|---|---|---|---|
|
| |||||
| Asian | White | Black | |||
| EGFR | CA repeat | Longer in Asians than whites and blacks | NA | NA | NA |
| RNASEL | Arg462Gln | More in Caucasians than Asians and African-Americans | IC | IC | IC |
| Asp541Glu | Less Asp allele homozygote in Asians than Caucasians and Africans | IC | IC | IC | |
| ELAC2 | Ser217Leu | More in Caucasians than Asians and blacks | Yes | No | Yes |
| Ala541Thr | More in Caucasians than Asians and blacks | Yes | No | No | |
| XRCC1 | G>A (rs25487) | Similar in Asian and Whites, but higher than African descents | Yes | No | No |
| CDH1 | -160 C/A | Higher in whites and blacks than Asians | IC | IC | No |
| VDR | TaqI | Lowest in Asians | IC | IC | IC |
| ApaI | More in Asians than Caucasians and Africans | IC | IC | IC | |
| Poly(A) | Lowest in Asians | No | No | No | |
| BsmI | Lower in Asians than other populations | IC | IC | IC | |
| FokI | More in Asians than Caucasians and Africans | IC | IC | Yes | |
NA: no report in the population; IC: Inconclusive.
There are several common allelic variants in the vitamin D receptor (VDR) encoding gene VDR, located on chromosome 12q13-q14, including BsmI (rs1544410), ApaI (rs 7975232), TaqI (rs731236), FokI (rs10735810) and a poly(A) in the 3’UTR region. They are in strong linkage disequilibrium with each other in white individuals except FokI. The frequency of the FokI allele and the ApaI A allele is higher in Asians than Caucasians and Africans, whereas the frequency of the BsmI B allele is much lower in the Asian population compared to other populations. The TaqI and poly (A) polymorphisms occur at a similar ratio, with the lowest percentage in Asians [227]. 1,25 (OH) 2D3, the active form of vitamin D, inhibits the proliferation of epithelial cells derived from normal and malignant prostatic tissues [228]. The vitamin D receptor (VDR) is a crucial mediator for the cellular effects of vitamin D and interacts with other cell-signaling pathways that influence cancer development. However, the case-control studies looking at the association between VDR polymorphisms and PCa risk are inconsistent. An earlier meta-analysis, including 26 studies suggested that none of these VDR polymorphisms are related to PCa risk [229], whereas most recent studies reported positive associations [190,230-236]. The study design may be an impormat factor to infleunt the resuts.
With the development of SNP array technology, a genome wide association study (GWAS) emerged for identifying small and moderate risk SNPs. The first two GWAS studies identified a 3.8 Mb interval on chromosome 8q24 as significantly associated with susceptibility to PCa in 2006 [237,238]. Today GWAS have been remarkably successful in identifying dozens of common genetic variants or loci associated with PCa [239-241]. Most of those PCa predisposition SNP loci were initially identified in Western populations and half of them are not associated with PCa risk in the East Asian population [239,240]. Two SNPs located at chromosome 4 have also been reported to show specific ethnical association with PCa risk [242]: rs12500426, which exhibited an association in Europeans but not in Asian or African American men and rs7679673, which was associated with disease in European and Asian populations but not in African American men. A replication study of five PCa loci initially identified in an Asian population (rs13385191, rs12653946, rs1983891, and rs339331, rs9600079) found that one SNP (rs9600079) was not associated with PCa risk in European populations [243].
Conclusions
Most studies for androgen-related genes showed a trend that the alleles leading to higher androgen levels are more common in high risk populations, although a few studies reported the opposite results, such as the A2 allele of CYP17, which potentially increases androgen synthesis and has the highest frequency in Asian men, middle in Caucasian and lowest in African [71,88] and CYP3A5*1, which may decrease testosterone activity but is more prevalent in men of African descent than Caucasian and Asian men [133]. However, the evidences show that the resultant androgen level difference among populations is contradictory. This may be caused by several factors. 1. Androgen and DHT concentration is affected by both androgen synthesis and metabolism, which are controlled by multiple genes, most of them with polymorphisms that play a role in this pathway. Polymorphisms in some genes may be compensated by other genes and, therefore, the total effect on the change in androgen levels is small. 2. Androgen action is determined by cooperation of androgen and AR. Populations with a longer CAG repeat polymorphism of AR, which leads to higher plasma androgen levels to compensate for lower AR transactivity [167,176,177], usually have more genetic polymorphisms leading to lower androgen concentration. These opposing genetic effects may also minimize the population disparity of androgen levels. 3. There are different forms of androgens and the androgen level in plasma and the prostate tissue is not correlated. It is not surprising that previous studies measuring different forms of androgens generate different results. Unfortunately, accurately measuring DHT levels in the prostate tissue, which may be the most effective indicator of androgen activity in the prostate and may be closely associated with the role of androgen in prostate carcinogenesis, is currently difficult. 4. Due to the complexity and limited effects of each of the genetic factors in determining androgen levels and the potential subtle difference among populations of androgen levels, population studies with a large number of individuals from each population are required, but yet have rarely been achieved in previous studies.
Regarding the association between those polymorphisms and PCa risk, case-control studies for most genetic polymorphisms were inconclusive and some SNPs were only found to be associated with disease in a particular population. In addition to the above explanation, which may affect androgen level and PCa risk in complex ways, gene-gene interaction or gene-environment interaction may contribute to these controversial conditions. Some SNPs are found to have no association with PCa individually, but several adjacent loci could increase PCa risk. As a haplotype, some genes on different chromosomes or in different pathways were also reported to interact with and increase PCa risk. An example of this is the SRD5A2 V89L VV genotype, which interacts with VDR FokI TT/CT genotypes in non-Hispanic white men to increase PCa risk [233]. Interestingly, the interactions of genetic polymorphisms with other factors have also presented racial differences, Barnholtz-Sloan et al [136] reported that the CYP3A43 genotype displays a distinct hierarchy of gene-environment and gene-gene interactions. In European American men it is associated with PCa risk in combination with a history of benign prostate hypertrophy, a familial history of PCa and age at consent. However, in African American men, the CYP3A4/CYP3A5 haplotype of this gene is associated with PCa risk in combination with a familial history of PCa, a higher individual proportion of European ancestry and the number of GGC AR repeats.
Inconclusive results may also be due to the majority of previous studies, especially large numbers of case-control studies, having been carried out in white men. Limited case numbers of Asian and African men, result in studies lacking sufficient power to confirm results. Besides the limited case numbers, Kittles et al [124] indicated other characteristics in studies on men of African descent. The African American population was genetically heterogeneous because of its African ancestry and subsequent admixture with European Americans, so strong population stratification happened among African Americans. The results of their study revealed the potential for confusion in association studies including African American men.
In summary, due to the complex nature of the AR pathway, there are many different ways that genetic polymorphisms can contribute to the deregulation of this pathway and PCa risk. Future studies need to include an integrated analysis of the combined effect of these polymorphisms on the AR pathway, as well as androgen metabolism/biosynthesis in addition to more accurate measurements of prostatic DHT levels. Analysis of these polymorphisms also becomes more problematic due to racial disparities in the research data. Future studies should include more African and Asian subjects and take into account all the factors considered when judging the PCa risk. While these are currently difficult to achieve, functional confirmation of those genetic factors in affecting carcinogenic molecular or biological features may help to establish their contribution to PCa development.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010 Aug;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Mao X, Yu Y, Boyd LK, Ren G, Lin D, Chaplin T, Kudahetti SC, Stankiewicz E, Xue L, Beltran L, Gupta M, Oliver RT, Lemoine NR, Berney DM, Young BD, Lu YJ. Distinct genomic alterations in prostate cancers in Chinese and Western populations suggest alternative pathways of prostate carcinogenesis. Cancer Res. 2010 Jul 1;70:5207–12. doi: 10.1158/0008-5472.CAN-09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren G, Liu X, Mao X, Zhang Y, Stankiewicz E, Hylands L, Song R, Berney DM, Clark J, Cooper C, Lu YJ. Identification of frequent BRAF copy number gain and alterations of RAF genes in chinese prostate cancer. Genes Chromosomes Cancer. 2012 Nov;51:1014–23. doi: 10.1002/gcc.21984. [DOI] [PubMed] [Google Scholar]
- 4.Xue L, Mao X, Ren G, Stankiewicz E, Kudahetti SC, Lin D, Beltran L, Berney DM, Lu YJ. Chinese and Western prostate cancers show alternate pathogenetic pathways in association with ERG status. Am J Cancer Res. 2012;2:736–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Cook LS, Goldoft M, Schwartz SM, Weiss NS. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999 Jan;161:152–5. [PubMed] [Google Scholar]
- 6.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991 Jun;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz C, Meier M, Schmid HP. Nutrition, dietary supplements and adenocarcinoma of the prostate. Maturitas. 2011 Dec;70:339–42. doi: 10.1016/j.maturitas.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 9.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009 Apr;89:1155–63. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 10.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007 Mar;16:538–45. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 11.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003 Jul;12:665–8. [PubMed] [Google Scholar]
- 12.Zheng J, Yang B, Huang T, Yu Y, Yang J, Li D. Green tea and black tea consumption and prostate cancer risk: an exploratory meta-analysis of observational studies. Nutr Cancer. 2011;63:663–72. doi: 10.1080/01635581.2011.570895. [DOI] [PubMed] [Google Scholar]
- 13.Arnold M, Razum O, Coebergh JW. Cancer risk diversity in non-western migrants to Europe: An overview of the literature. Eur J Cancer. 2010 Sep;46:2647–59. doi: 10.1016/j.ejca.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 14.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U. S. Cancer Causes Control. 2008 Apr;19:227–56. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar-Apr;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 16.Raymundo EM, Rice KR, Chen Y, Zhao J, Brassell SA. Prostate cancer in Asian Americans: incidence, management and outcomes in an equal access healthcare system. BJU Int. 2011 Apr;107:1216–22. doi: 10.1111/j.1464-410X.2010.09685.x. [DOI] [PubMed] [Google Scholar]
- 17.McCracken M, Olsen M, Chen MS Jr, Jemal A, Thun M, Cokkinides V, Deapen D, Ward E. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007 Jul-Aug;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 18.Zeegers MP, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis. Cancer. 2003 Apr 15;97:1894–903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]
- 19.Bratt O. Hereditary prostate cancer: clinical aspects. J Urol. 2002 Sep;168:906–13. doi: 10.1016/S0022-5347(05)64541-7. [DOI] [PubMed] [Google Scholar]
- 20.Gronberg H, Damber L, Damber JE. Studies of genetic factors in prostate cancer in a twin population. J Urol. 1994 Nov;152:1484–7. doi: 10.1016/s0022-5347(17)32452-7. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000 Jul 13;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 22.Page WF, Braun MM, Partin AW, Caporaso N, Walsh P. Heredity and prostate cancer: a study of World War II veteran twins. Prostate. 1997 Dec 1;33:240–5. doi: 10.1002/(sici)1097-0045(19971201)33:4<240::aid-pros3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Gronberg H. Prostate cancer epidemiology. Lancet. 2003 Mar 8;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 24.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002 Aug 1;52:213–35. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- 25.Brawley OW, Ford LG, Thompson I, Perlman JA, Kramer BS. 5-Alpha-reductase inhibition and prostate cancer prevention. Cancer Epidemiol Biomarkers Prev. 1994 Mar;3:177–82. [PubMed] [Google Scholar]
- 26.Bastus NC, Boyd LK, Mao X, Stankiewicz E, Kudahetti SC, Oliver RT, Berney DM, Lu YJ. Androgen-induced TMPRSS2: ERG fusion in nonmalignant prostate epithelial cells. Cancer Res. 2010 Dec 1;70:9544–8. doi: 10.1158/0008-5472.CAN-10-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani RS, Tomlins SA, Callahan K, Ghosh A, Nyati MK, Varambally S, Palanisamy N, Chinnaiyan AM. Induced chromosomal proximity and gene fusions in prostate cancer. Science. 2009 Nov 27;326:1230. doi: 10.1126/science.1178124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, Zhang J, Rose DW, Fu XD, Glass CK, Rosenfeld MG. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009 Dec 11;139:1069–83. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, Isaacs WB, Bova GS, Liu W, Xu J, Meeker AK, Netto G, De Marzo AM, Nelson WG, Yegnasubramanian S. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010 Aug;42:668–75. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986 Jan;76:45–8. [PubMed] [Google Scholar]
- 31.Litman HJ, Bhasin S, Link CL, Araujo AB, McKinlay JB. Serum androgen levels in black, Hispanic, and white men. J Clin Endocrinol Metab. 2006 Nov;91:4326–34. doi: 10.1210/jc.2006-0037. [DOI] [PubMed] [Google Scholar]
- 32.Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, Hankin J, Teh CZ, Dreon DM, Paffenbarger RS Jr. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev. 1995 Oct-Nov;4:735–41. [PubMed] [Google Scholar]
- 33.Ellis L, Nyborg H. Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids. 1992 Feb;57:72–5. doi: 10.1016/0039-128x(92)90032-5. [DOI] [PubMed] [Google Scholar]
- 34.Kubricht WS 3rd, Williams BJ, Whatley T, Pinckard P, Eastham JA. Serum testosterone levels in African-American and white men undergoing prostate biopsy. Urology. 1999 Dec;54:1035–8. doi: 10.1016/s0090-4295(99)00290-3. [DOI] [PubMed] [Google Scholar]
- 35.Platz EA, Rimm EB, Willett WC, Kantoff PW, Giovannucci E. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000 Dec 20;92:2009–17. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 36.Asbell SO, Raimane KC, Montesano AT, Zeitzer KL, Asbell MD, Vijayakumar S. Prostate-specific antigen and androgens in African-American and white normal subjects and prostate cancer patients. J Natl Med Assoc. 2000 Sep;92:445–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Travis RC, Key TJ, Allen NE, Appleby PN, Roddam AW, Rinaldi S, Egevad L, Gann PH, Rohrmann S, Linseisen J, Pischon T, Boeing H, Johnsen NF, Tjønneland A, Overvad K, Kiemeney L, Bueno-de-Mesquita HB, Bingham S, Khaw KT, Tumino R, Sieri S, Vineis P, Palli D, Quirós JR, Ardanaz E, Chirlaque MD, Larrañaga N, Gonzalez C, Sanchez MJ, Trichopoulou A, Bikou C, Trichopoulos D, Stattin P, Jenab M, Ferrari P, Slimani N, Riboli E, Kaaks R. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007 Sep 15;121:1331–8. doi: 10.1002/ijc.22814. [DOI] [PubMed] [Google Scholar]
- 38.Tsilidis KK, Travis RC, Appleby PN, Allen NE, Lindstrom S, Schumacher FR, Cox D, Hsing AW, Ma J, Severi G, Albanes D, Virtamo J, Boeing H, Bueno-de-Mesquita HB, Johansson M, Quirós JR, Riboli E, Siddiq A, Tjønneland A, Trichopoulos D, Tumino R, Gaziano JM, Giovannucci E, Hunter DJ, Kraft P, Stampfer MJ, Giles GG, Andriole GL, Berndt SI, Chanock SJ, Hayes RB, Key TJ. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006 Jan;15:86–91. doi: 10.1158/1055-9965.EPI-05-0633. [DOI] [PubMed] [Google Scholar]
- 39.Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, Willett WC, Giovannucci E. Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomarkers Prev. 2005 May;14:1262–9. doi: 10.1158/1055-9965.EPI-04-0371. [DOI] [PubMed] [Google Scholar]
- 40.Stattin P, Lumme S, Tenkanen L, Alfthan H, Jellum E, Hallmans G, Thoresen S, Hakulinen T, Luostarinen T, Lehtinen M, Dillner J, Stenman UH, Hakama M. High levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective study. Int J Cancer. 2004 Jan 20;108:418–24. doi: 10.1002/ijc.11572. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Weiss NS, Stanczyk FZ, Lewis SK, DiTommaso D, Etzioni R, Barnett MJ, Goodman GE. Endogenous sex hormones and prostate cancer risk: a case-control study nested within the Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003 Dec;12:1410–6. [PubMed] [Google Scholar]
- 42.Stattin P, Rinaldi S, Stenman UH, Riboli E, Hallmans G, Bergh A, Kaaks R. Plasma prolactin and prostate cancer risk: A prospective study. Int J Cancer. 2001 May 1;92:463–5. doi: 10.1002/ijc.1191. [DOI] [PubMed] [Google Scholar]
- 43.Mohr BA, Feldman HA, Kalish LA, Longcope C, McKinlay JB. Are serum hormones associated with the risk of prostate cancer? Urology. 2001 May;57:930–5. doi: 10.1016/s0090-4295(00)01116-x. [DOI] [PubMed] [Google Scholar]
- 44.Heikkilä R, Aho K, Heliövaara M, Hakama M, Marniemi J, Reunanen A, Knekt P. Serum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal study. Cancer. 1999 Jul 15;86:312–5. [PubMed] [Google Scholar]
- 45.Dorgan JF, Albanes D, Virtamo J, Heinonen OP, Chandler DW, Galmarini M, McShane LM, Barrett MJ, Tangrea J, Taylor PR. Relationships of serum androgens and estrogens to prostate cancer risk: results from a prospective study in Finland. Cancer Epidemiol Biomarkers Prev. 1998 Dec;7:1069–74. [PubMed] [Google Scholar]
- 46.Vatten LJ, Ursin G, Ross RK, Stanczyk FZ, Lobo RA, Harvei S, Jellum E. Androgens in serum and the risk of prostate cancer: a nested case-control study from the Janus serum bank in Norway. Cancer Epidemiol Biomarkers Prev. 1997 Nov;6:967–9. [PubMed] [Google Scholar]
- 47.Guess HA, Friedman GD, Sadler MC, Stanczyk FZ, Vogelman JH, Imperato-McGinley J, Lobo RA, Orentreich N. 5 alpha-reductase activity and prostate cancer: a case-control study using stored sera. Cancer Epidemiol Biomarkers Prev. 1997 Jan;6:21–4. [PubMed] [Google Scholar]
- 48.Nomura AM, Stemmermann GN, Chyou PH, Henderson BE, Stanczyk FZ. Serum androgens and prostate cancer. Cancer Epidemiol Biomarkers Prev. 1996 Aug;5:621–5. [PubMed] [Google Scholar]
- 49.Corder EH, Friedman GD, Vogelman JH, Orentreich N. Seasonal variation in vitamin D, vitamin D-binding protein, and dehydroepiandrosterone: risk of prostate cancer in black and white men. Cancer Epidemiol Biomarkers Prev. 1995 Sep;4:655–9. [PubMed] [Google Scholar]
- 50.Carter HB, Pearson JD, Metter EJ, Chan DW, Andres R, Fozard JL, Rosner W, Walsh PC. Longitudinal evaluation of serum androgen levels in men with and without prostate cancer. Prostate. 1995 Jul;27:25–31. doi: 10.1002/pros.2990270106. [DOI] [PubMed] [Google Scholar]
- 51.Hsing AW, Comstock GW. Serological precursors of cancer: serum hormones and risk of subsequent prostate cancer. Cancer Epidemiol Biomarkers Prev. 1993 Jan-Feb;2:27–32. [PubMed] [Google Scholar]
- 52.Comstock GW, Gordon GB, Hsing AW. The relationship of serum dehydroepiandrosterone and its sulfate to subsequent cancer of the prostate. Cancer Epidemiol Biomarkers Prev. 1993 May-Jun;2:219–21. [PubMed] [Google Scholar]
- 53.Nomura A, Heilbrun LK, Stemmermann GN, Judd HL. Prediagnostic serum hormones and the risk of prostate cancer. Cancer Res. 1988 Jun 15;48:3515–7. [PubMed] [Google Scholar]
- 54.Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, Metter EJ. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005 Sep;14:2257–60. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 55.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996 Aug 21;88:1118–26. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 56.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008 Feb 6;100:170–83. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsing AW, Chu LW, Stanczyk FZ. Androgen and prostate cancer: is the hypothesis dead? Cancer Epidemiol Biomarkers Prev. 2008 Oct;17:2525–30. doi: 10.1158/1055-9965.EPI-08-0448. [DOI] [PubMed] [Google Scholar]
- 58.Marks LS, Mostaghel EA, Nelson PS. Prostate tissue androgens: history and current clinical relevance. Urology. 2008 Aug;72:247–54. doi: 10.1016/j.urology.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohler JL, Gaston KE, Moore DT, Schell MJ, Cohen BL, Weaver C, Petrusz P. Racial differences in prostate androgen levels in men with clinically localized prostate cancer. J Urol. 2004 Jun;171:2277–80. doi: 10.1097/01.ju.0000127739.88383.79. [DOI] [PubMed] [Google Scholar]
- 60.Piras I, Falchi A, Moral P, Melis A, Giovannoni L, Paoli G, Calò C, Vona G, Varesi L. Frequencies of promoter pentanucleotide (TTTTA)n of CYP11A gene in European and North African populations. Genet Test. 2008 Mar;12:93–6. doi: 10.1089/gte.2007.0060. [DOI] [PubMed] [Google Scholar]
- 61.Kumazawa T, Tsuchiya N, Wang L, Sato K, Kamoto T, Ogawa O, Nakamura A, Kato T, Habuchi T. Microsatellite polymorphism of steroid hormone synthesis gene CYP11A1 is associated with advanced prostate cancer. Int J Cancer. 2004 May 20;110:140–4. doi: 10.1002/ijc.20070. [DOI] [PubMed] [Google Scholar]
- 62.Tang L, Yao S, Till C, Goodman PJ, Tangen CM, Wu Y, Kristal AR, Platz EA, Neuhouser ML, Stanczyk FZ, Reichardt JK, Santella RM, Hsing A, Hoque A, Lippman SM, Thompson IM, Ambrosone CB. Repeat polymorphisms in estrogen metabolism genes and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Carcinogenesis. 2011 Oct;32:1500–6. doi: 10.1093/carcin/bgr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Celhar T, Gersak K, Ovcak Z, Sedmak B, Mlinaric-Rascan I. The presence of the CYP11A1 (TTTTA)6 allele increases the risk of biochemical relapse in organ confined and low-grade prostate cancer. Cancer Genet Cytogenet. 2008 Nov;187:28–33. doi: 10.1016/j.cancergencyto.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Cunningham JM, Hebbring SJ, McDonnell SK, Cicek MS, Christensen GB, Wang L, Jacobsen SJ, Cerhan JR, Blute ML, Schaid DJ, Thibodeau SN. Evaluation of genetic variations in the androgen and estrogen metabolic pathways as risk factors for sporadic and familial prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007 May;16:969–78. doi: 10.1158/1055-9965.EPI-06-0767. [DOI] [PubMed] [Google Scholar]
- 65.Douglas JA, Zuhlke KA, Beebe-Dimmer J, Levin AM, Gruber SB, Wood DP, Cooney KA. Identifying susceptibility genes for prostate cancer--a family-based association study of polymorphisms in CYP17, CYP19, CYP11A1, and LH-beta. Cancer Epidemiol Biomarkers Prev. 2005 Aug;14:2035–9. doi: 10.1158/1055-9965.EPI-05-0170. [DOI] [PubMed] [Google Scholar]
- 66.Cicek MS, Liu X, Casey G, Witte JS. Role of androgen metabolism genes CYP1B1, PSA/KLK3, and CYP11alpha in prostate cancer risk and aggressiveness. Cancer Epidemiol Biomarkers Prev. 2005 Sep;14:2173–7. doi: 10.1158/1055-9965.EPI-05-0215. [DOI] [PubMed] [Google Scholar]
- 67.Picado-Leonard J, Miller WL. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA. 1987 Oct;6:439–48. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- 68.Waterman MR, Keeney DS. Genes involved in androgen biosynthesis and the male phenotype. Horm Res. 1992;38:217–21. doi: 10.1159/000182546. [DOI] [PubMed] [Google Scholar]
- 69.Ross RK, Pike MC, Coetzee GA, Reichardt JK, Yu MC, Feigelson H, Stanczyk FZ, Kolonel LN, Henderson BE. Androgen metabolism and prostate cancer: establishing a model of genetic susceptibility. Cancer Res. 1998 Oct 15;58:4497–504. [PubMed] [Google Scholar]
- 70.Nedelcheva Kristensen V, Haraldsen EK, Anderson KB, Lønning PE, Erikstein B, Kåresen R, Gabrielsen OS, Børresen-Dale AL. CYP17 and breast cancer risk: the polymorphism in the 5’ flanking area of the gene does not influence binding to Sp-1. Cancer Res. 1999 Jun 15;59:2825–8. [PubMed] [Google Scholar]
- 71.Ntais C, Polycarpou A, Ioannidis JP. Association of the CYP17 gene polymorphism with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003 Feb;12:120–6. [PubMed] [Google Scholar]
- 72.Lunn RM, Bell DA, Mohler JL, Taylor JA. Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and steroid reductase (SRD5A2) Carcinogenesis. 1999 Sep;20:1727–31. doi: 10.1093/carcin/20.9.1727. [DOI] [PubMed] [Google Scholar]
- 73.Gsur A, Bernhofer G, Hinteregger S, Haidinger G, Schatzl G, Madersbacher S, Marberger M, Vutuc C, Micksche M. A polymorphism in the CYP17 gene is associated with prostate cancer risk. Int J Cancer. 2000 Aug 1;87:434–7. doi: 10.1002/1097-0215(20000801)87:3<434::aid-ijc19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 74.Yamada Y, Watanabe M, Murata M, Yamanaka M, Kubota Y, Ito H, Katoh T, Kawamura J, Yatani R, Shiraishi T. Impact of genetic polymorphisms of 17-hydroxylase cytochrome P-450 (CYP17) and steroid 5alpha-reductase type II (SRD5A2) genes on prostate-cancer risk among the Japanese population. Int J Cancer. 2001 Jun 1;92:683–6. doi: 10.1002/1097-0215(20010601)92:5<683::aid-ijc1255>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 75.Haiman CA, Stampfer MJ, Giovannucci E, Ma J, Decalo NE, Kantoff PW, Hunter DJ. The relationship between a polymorphism in CYP17 with plasma hormone levels and prostate cancer. Cancer Epidemiol Biomarkers Prev. 2001 Jul;10:743–8. [PubMed] [Google Scholar]
- 76.Kittles RA, Panguluri RK, Chen W, Massac A, Ahaghotu C, Jackson A, Ukoli F, Adams-Campbell L, Isaacs W, Dunston GM. Cyp17 promoter variant associated with prostate cancer aggressiveness in African Americans. Cancer Epidemiol Biomarkers Prev. 2001 Sep;10:943–7. [PubMed] [Google Scholar]
- 77.Stanford JL, Noonan EA, Iwasaki L, Kolb S, Chadwick RB, Feng Z, Ostrander EA. A polymorphism in the CYP17 gene and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002 Mar;11:243–7. [PubMed] [Google Scholar]
- 78.Sarma AV, Dunn RL, Lange LA, Ray A, Wang Y, Lange EM, Cooney KA. Genetic polymorphisms in CYP17, CYP3A4, CYP19A1, SRD5A2, IGF-1, and IGFBP-3 and prostate cancer risk in African-American men: the Flint Men’s Health Study. Prostate. 2008 Feb 15;68:296–305. doi: 10.1002/pros.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sobti RC, Gupta L, Thakur H, Seth A, Singh SK, Kaur P. CYP17 gene polymorphism and its association in north Indian prostate cancer patients. Anticancer Res. 2009 May;29:1659–63. [PubMed] [Google Scholar]
- 80.Souiden Y, Mahdouani M, Chaieb K, Elkamel R, Mahdouani K. CYP17 gene polymorphism and prostate cancer susceptibility in a Tunisian population. Cancer Epidemiol. 2011 Oct;35:480–4. doi: 10.1016/j.canep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Chang B, Zheng SL, Isaacs SD, Wiley KE, Carpten JD, Hawkins GA, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu J. Linkage and association of CYP17 gene in hereditary and sporadic prostate cancer. Int J Cancer. 2001 Nov 20;95:354–9. doi: 10.1002/1097-0215(20011120)95:6<354::aid-ijc1062>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 82.Latil AG, Azzouzi R, Cancel GS, Guillaume EC, Cochan-Priollet B, Berthon PL, Cussenot O. Prostate carcinoma risk and allelic variants of genes involved in androgen biosynthesis and metabolism pathways. Cancer. 2001 Sep 1;92:1130–7. doi: 10.1002/1097-0142(20010901)92:5<1130::aid-cncr1430>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 83.dos Santos A, Ribeiro ML, Mesquita JC, Carvalho-Salles AB, Hackel C. No association of the 5’ promoter region polymorphism of CYP17 gene with prostate cancer risk. Prostate Cancer Prostatic Dis. 2002;5:28–31. doi: 10.1038/sj.pcan.4500550. [DOI] [PubMed] [Google Scholar]
- 84.Severi G, Hayes VM, Tesoriero AA, Southey MC, Hoang HN, Padilla EJ, Morris HA, English DR, Sutherland RL, Boyle P, Hopper JL, Giles GG. The rs743572 common variant in the promoter of CYP17A1 is not associated with prostate cancer risk or circulating hormonal levels. BJU Int. 2008 Feb;101:492–6. doi: 10.1111/j.1464-410X.2007.07272.x. [DOI] [PubMed] [Google Scholar]
- 85.Wadelius M, Andersson AO, Johansson JE, Wadelius C, Rane E. Prostate cancer associated with CYP17 genotype. Pharmacogenetics. 1999 Oct;9:635–9. [PubMed] [Google Scholar]
- 86.Habuchi T, Liqing Z, Suzuki T, Sasaki R, Tsuchiya N, Tachiki H, Shimoda N, Satoh S, Sato K, Kakehi Y, Kamoto T, Ogawa O, Kato T. Increased risk of prostate cancer and benign prostatic hyperplasia associated with a CYP17 gene polymorphism with a gene dosage effect. Cancer Res. 2000 Oct 15;60:5710–3. [PubMed] [Google Scholar]
- 87.Onen IH, Ekmekci A, Eroglu M, Polat F, Biri H. The association of 5alpha-reductase II (SRD5A2) and 17 hydroxylase (CYP17) gene polymorphisms with prostate cancer patients in the Turkish population. DNA Cell Biol. 2007 Feb;26:100–7. doi: 10.1089/dna.2006.0534. [DOI] [PubMed] [Google Scholar]
- 88.Wang F, Zou YF, Feng XL, Su H, Huang F. CYP17 gene polymorphisms and prostate cancer risk: A meta-analysis based on 38 independent studies. Prostate. 2011 Aug 1;71:1167–77. doi: 10.1002/pros.21332. [DOI] [PubMed] [Google Scholar]
- 89.Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin Invest. 1993 Aug;92:903–10. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ross RK, Bernstein L, Lobo RA, Shimizu H, Stanczyk FZ, Pike MC, Henderson BE. 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992 Apr 11;339:887–9. doi: 10.1016/0140-6736(92)90927-u. [DOI] [PubMed] [Google Scholar]
- 91.Lookingbill DP, Demers LM, Wang C, Leung A, Rittmaster RS, Santen RJ. Clinical and biochemical parameters of androgen action in normal healthy Caucasian versus Chinese subjects. J Clin Endocrinol Metab. 1991 Jun;72:1242–8. doi: 10.1210/jcem-72-6-1242. [DOI] [PubMed] [Google Scholar]
- 92.Makridakis NM, Ross RK, Pike MC, Crocitto LE, Kolonel LN, Pearce CL, Henderson BE, Reichardt JK. Association of mis-sense substitution in SRD5A2 gene with prostate cancer in African-American and Hispanic men in Los Angeles, USA. Lancet. 1999 Sep 18;354:975–8. doi: 10.1016/S0140-6736(98)11282-5. [DOI] [PubMed] [Google Scholar]
- 93.Li X, Huang Y, Fu X, Chen C, Zhang D, Yan L, Xie Y, Mao Y, Li Y. Meta-analysis of three polymorphisms in the steroid-5-alpha-reductase, alpha polypeptide 2 gene (SRD5A2) and risk of prostate cancer. Mutagenesis. 2011 May;26:371–83. doi: 10.1093/mutage/geq103. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Coates RJ, Gwinn M, Khoury MJ. Steroid 5-{alpha}-reductase Type 2 (SRD5a2) gene polymorphisms and risk of prostate cancer: a HuGE review. Am J Epidemiol. 2010 Jan 1;171:1–13. doi: 10.1093/aje/kwp318. [DOI] [PubMed] [Google Scholar]
- 95.Ntais C, Polycarpou A, Ioannidis JP. SRD5A2 gene polymorphisms and the risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003 Jul;12:618–24. [PubMed] [Google Scholar]
- 96.Makridakis N, Ross RK, Pike MC, Chang L, Stanczyk FZ, Kolonel LN, Shi CY, Yu MC, Henderson BE, Reichardt JK. A prevalent missense substitution that modulates activity of prostatic steroid 5alpha-reductase. Cancer Res. 1997 Mar 15;57:1020–2. [PubMed] [Google Scholar]
- 97.Giwercman C, Giwercman A, Pedersen HS, Toft G, Lundin K, Bonde JP, Lundberg Giwercman Y. Polymorphisms in genes regulating androgen activity among prostate cancer low-risk Inuit men and high-risk Scandinavians. Int J Androl. 2008 Feb;31:25–30. doi: 10.1111/j.1365-2605.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 98.Hayes VM, Severi G, Padilla EJ, Morris HA, Tilley WD, Southey MC, English DR, Sutherland RL, Hopper JL, Boyle P, Giles GG. 5alpha-Reductase type 2 gene variant associations with prostate cancer risk, circulating hormone levels and androgenetic alopecia. Int J Cancer. 2007 Feb 15;120:776–80. doi: 10.1002/ijc.22408. [DOI] [PubMed] [Google Scholar]
- 99.Hsing AW, Chen C, Chokkalingam AP, Gao YT, Dightman DA, Nguyen HT, Deng J, Cheng J, Sesterhenn IA, Mostofi FK, Stanczyk FZ, Reichardt JK. Polymorphic markers in the SRD5A2 gene and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2001 Oct;10:1077–82. [PubMed] [Google Scholar]
- 100.Wang C, Tao W, Chen Q, Hu H, Wen XY, Han R. SRD5A2 V89L polymorphism and prostate cancer risk: a meta-analysis. Prostate. 2010 Feb 1;70:170–8. doi: 10.1002/pros.21050. [DOI] [PubMed] [Google Scholar]
- 101.Davis DL, Russell DW. Unusual length polymorphism in human steroid 5 alpha-reductase type 2 gene (SRD5A2) Hum Mol Genet. 1993 Jun;2:820. doi: 10.1093/hmg/2.6.820. [DOI] [PubMed] [Google Scholar]
- 102.Reichardt JK, Makridakis N, Henderson BE, Yu MC, Pike MC, Ross RK. Genetic variability of the human SRD5A2 gene: implications for prostate cancer risk. Cancer Res. 1995 Sep 15;55:3973–5. [PubMed] [Google Scholar]
- 103.Sobti RC, Gupta L, Singh SK, Seth A, Kaur P, Thakur H. Role of hormonal genes and risk of prostate cancer: gene-gene interactions in a North Indian population. Cancer Genet Cytogenet. 2008 Sep;185:78–85. doi: 10.1016/j.cancergencyto.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 104.Simard J, Durocher F, Mébarki F, Turgeon C, Sanchez R, Labrie Y, Couet J, Trudel C, Rhéaume E, Morel Y, Luu-The V, Labrie F. Molecular biology and genetics of the 3 beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. J Endocrinol. 1996 Sep;150(Suppl):S189–207. [PubMed] [Google Scholar]
- 105.Wang L, Salavaggione E, Pelleymounter L, Eckloff B, Wieben E, Weinshilboum R. Human 3beta-hydroxysteroid dehydrogenase types 1 and 2: Gene sequence variation and functional genomics. J Steroid Biochem Mol Biol. 2007 Oct;107:88–99. doi: 10.1016/j.jsbmb.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang BL, Zheng SL, Hawkins GA, Isaacs SD, Wiley KE, Turner A, Carpten JD, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu J. Joint effect of HSD3B1 and HSD3B2 genes is associated with hereditary and sporadic prostate cancer susceptibility. Cancer Res. 2002 Mar 15;62:1784–9. [PubMed] [Google Scholar]
- 107.Mononen N, Seppälä EH, Duggal P, Autio V, Ikonen T, Ellonen P, Saharinen J, Saarela J, Vihinen M, Tammela TL, Kallioniemi O, Bailey-Wilson JE, Schleutker J. Profiling genetic variation along the androgen biosynthesis and metabolism pathways implicates several single nucleotide polymorphisms and their combinations as prostate cancer risk factors. Cancer Res. 2006 Jan 15;66:743–7. doi: 10.1158/0008-5472.CAN-05-1723. [DOI] [PubMed] [Google Scholar]
- 108.Verreault H, Dufort I, Simard J, Labrie F, Luu- The V. Dinucleotide repeat polymorphisms in the HSD3B2 gene. Hum Mol Genet. 1994 Feb;3:384. doi: 10.1093/hmg/3.2.384. [DOI] [PubMed] [Google Scholar]
- 109.Neslund-Dudas C, Bock CH, Monaghan K, Nock NL, Yang JJ, Rundle A, Tang D, Rybicki BA. SRD5A2 and HSD3B2 polymorphisms are associated with prostate cancer risk and aggressiveness. Prostate. 2007 Nov 1;67:1654–63. doi: 10.1002/pros.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Devgan SA, Henderson BE, Yu MC, Shi CY, Pike MC, Ross RK, Reichardt JK. Genetic variation of 3 beta-hydroxysteroid dehydrogenase type II in three racial/ethnic groups: implications for prostate cancer risk. Prostate. 1997 Sep 15;33:9–12. doi: 10.1002/(sici)1097-0045(19970915)33:1<9::aid-pros2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 111.Beuten J, Gelfond JA, Franke JL, Weldon KS, Crandall AC, Johnson-Pais TL, Thompson IM, Leach RJ. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009 Jun;18:1869–80. doi: 10.1158/1055-9965.EPI-09-0076. [DOI] [PubMed] [Google Scholar]
- 112.Eriksson AL, Lorentzon M, Vandenput L, Labrie F, Lindersson M, Syvänen AC, Orwoll ES, Cummings SR, Zmuda JM, Ljunggren O, Karlsson MK, Mellström D, Ohlsson C. Genetic variations in sex steroid-related genes as predictors of serum estrogen levels in men. J Clin Endocrinol Metab. 2009 Mar;94:1033–41. doi: 10.1210/jc.2008-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Travis RC, Schumacher F, Hirschhorn JN, Kraft P, Allen NE, Albanes D, Berglund G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Calle EE, Chanock S, Dunning AM, Hayes R, Feigelson HS, Gaziano JM, Giovannucci E, Haiman CA, Henderson BE, Kaaks R, Kolonel LN, Ma J, Rodriguez L, Riboli E, Stampfer M, Stram DO, Thun MJ, Tjønneland A, Trichopoulos D, Vineis P, Virtamo J, Le Marchand L, Hunter DJ. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2009 Oct;18:2734–44. doi: 10.1158/1055-9965.EPI-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sonoda T, Suzuki H, Mori M, Tsukamoto T, Yokomizo A, Naito S, Fujimoto K, Hirao Y, Miyanaga N, Akaza H. Polymorphisms in estrogen related genes may modify the protective effect of isoflavones against prostate cancer risk in Japanese men. Eur J Cancer Prev. 2010 Mar;19:131–7. doi: 10.1097/CEJ.0b013e328333fbe2. [DOI] [PubMed] [Google Scholar]
- 115.Huang YC, Chen M, Lin MW, Chung MY, Chang YH, Huang WJ, Wu TT, Hsu JM, Yang S, Chen YM. CYP19 TCT tri-nucleotide Del/Del genotype is a susceptibility marker for prostate cancer in a Taiwanese population. Urology. 2007 May;69:996–1000. doi: 10.1016/j.urology.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki K, Nakazato H, Matsui H, Koike H, Okugi H, Ohtake N, Takei T, Nakata S, Hasumi M, Yamanaka H. Association of the genetic polymorphism of the CYP19 intron 4 [TTTA] n repeat with familial prostate cancer risk in a Japanese population. Anticancer Res. 2003 Nov-Dec;23:4941–6. [PubMed] [Google Scholar]
- 117.Onsory K, Sobti RC, Al-Badran AI, Watanabe M, Shiraishi T, Krishan A, Mohan H, Kaur P. Hormone receptor-related gene polymorphisms and prostate cancer risk in North Indian population. Mol Cell Biochem. 2008 Jul;314:25–35. doi: 10.1007/s11010-008-9761-1. [DOI] [PubMed] [Google Scholar]
- 118.Modugno F, Weissfeld JL, Trump DL, Zmuda JM, Shea P, Cauley JA, Ferrell RE. Allelic variants of aromatase and the androgen and estrogen receptors: toward a multigenic model of prostate cancer risk. Clin Cancer Res. 2001 Oct;7:3092–6. [PubMed] [Google Scholar]
- 119.Gellner K, Eiselt R, Hustert E, Arnold H, Koch I, Haberl M, Deglmann CJ, Burk O, Buntefuss D, Escher S, Bishop C, Koebe HG, Brinkmann U, Klenk HP, Kleine K, Meyer UA, Wojnowski L. Genomic organization of the human CYP3A locus: identification of a new, inducible CYP3A gene. Pharmacogenetics. 2001 Mar;11:111–21. doi: 10.1097/00008571-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 120.Rebbeck TR. More about: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 2000 Jan 5;92:76. doi: 10.1093/jnci/92.1.76. [DOI] [PubMed] [Google Scholar]
- 121.Walker AH, Jaffe JM, Gunasegaram S, Cummings SA, Huang CS, Chern HD, Olopade OI, Weber BL, Rebbeck TR. Characterization of an allelic variant in the nifedipine-specific element of CYP3A4: ethnic distribution and implications for prostate cancer risk. Mutations in brief no. 191. Online. Hum Mutat. 1998;12:289. [PubMed] [Google Scholar]
- 122.Paris PL, Kupelian PA, Hall JM, Williams TL, Levin H, Klein EA, Casey G, Witte JS. Association between a CYP3A4 genetic variant and clinical presentation in African-American prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1999 Oct;8:901–5. [PubMed] [Google Scholar]
- 123.Bangsi D, Zhou J, Sun Y, Patel NP, Darga LL, Heilbrun LK, Powell IJ, Severson RK, Everson RB. Impact of a genetic variant in CYP3A4 on risk and clinical presentation of prostate cancer among white and African-American men. Urol Oncol. 2006 Jan-Feb;24:21–7. doi: 10.1016/j.urolonc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 124.Kittles RA, Chen W, Panguluri RK, Ahaghotu C, Jackson A, Adebamowo CA, Griffin R, Williams T, Ukoli F, Adams-Campbell L, Kwagyan J, Isaacs W, Freeman V, Dunston GM. CYP3A4-V and prostate cancer in African Americans: causal or confounding association because of population stratification? Hum Genet. 2002 Jun;110:553–60. doi: 10.1007/s00439-002-0731-5. [DOI] [PubMed] [Google Scholar]
- 125.Plummer SJ, Conti DV, Paris PL, Curran AP, Casey G, Witte JS. CYP3A4 and CYP3A5 genotypes, haplotypes, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003 Sep;12:928–32. [PubMed] [Google Scholar]
- 126.Fernandez P, Zeigler-Johnson CM, Spangler E, van der Merwe A, Jalloh M, Gueye SM, Rebbeck TR. Androgen Metabolism Gene Polymorphisms, Associations with Prostate Cancer Risk and Pathological Characteristics: A Comparative Analysis between South African and Senegalese Men. Prostate Cancer. 2012;2012:798634. doi: 10.1155/2012/798634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeigler-Johnson C, Friebel T, Walker AH, Wang Y, Spangler E, Panossian S, Patacsil M, Aplenc R, Wein AJ, Malkowicz SB, Rebbeck TR. CYP3A4, CYP3A5, and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res. 2004 Nov 15;64:8461–7. doi: 10.1158/0008-5472.CAN-04-1651. [DOI] [PubMed] [Google Scholar]
- 128.Rebbeck TR, Jaffe JM, Walker AH, Wein AJ, Malkowicz SB. Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 1998 Aug 19;90:1225–9. doi: 10.1093/jnci/90.16.1225. [DOI] [PubMed] [Google Scholar]
- 129.Berndt SI, Chatterjee N, Huang WY, Chanock SJ, Welch R, Crawford ED, Hayes RB. Variant in sex hormone-binding globulin gene and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007 Jan;16:165–8. doi: 10.1158/1055-9965.EPI-06-0689. [DOI] [PubMed] [Google Scholar]
- 130.Powell IJ, Zhou J, Sun Y, Sakr WA, Patel NP, Heilbrun LK, Everson RB. CYP3A4 genetic variant and disease-free survival among white and black men after radical prostatectomy. J Urol. 2004 Nov;172:1848–52. doi: 10.1097/01.ju.0000142779.76603.be. [DOI] [PubMed] [Google Scholar]
- 131.Nam RK, Zhang WW, Trachtenberg J, Jewett MA, Emami M, Vesprini D, Chu W, Ho M, Sweet J, Evans A, Toi A, Pollak M, Narod SA. Comprehensive assessment of candidate genes and serological markers for the detection of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2003 Dec;12:1429–37. [PubMed] [Google Scholar]
- 132.Nam RK, Zhang WW, Trachtenberg J, Jewett MA, Emami M, Vesprini D, Chu W, Ho M, Sweet J, Evans A, Toi A, Pollak M, Narod SA. Cytochrome P450 3A5 is highly expressed in normal prostate cells but absent in prostate cancer. Endocr Relat Cancer. 2007 Sep;14:645–54. doi: 10.1677/ERC-07-0078. [DOI] [PubMed] [Google Scholar]
- 133.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001 Apr;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 134.Wojnowski L, Hustert E, Klein K, Goldammer M, Haberl M, Kirchheiner J, Koch I, Klattig J, Zanger U, Brockmöller J. Re: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J Natl Cancer Inst. 2002 Apr 17;94:630–1. doi: 10.1093/jnci/94.8.630. author reply 1-2. [DOI] [PubMed] [Google Scholar]
- 135.Zhenhua L, Tsuchiya N, Narita S, Inoue T, Horikawa Y, Kakinuma H, Kato T, Ogawa O, Habuchi T. CYP3A5 gene polymorphism and risk of prostate cancer in a Japanese population. Cancer Lett. 2005 Jul 28;225:237–43. doi: 10.1016/j.canlet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 136.Barnholtz-Sloan JS, Guan X, Zeigler-Johnson C, Meropol NJ, Rebbeck TR. Decision tree-based modeling of androgen pathway genes and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2011 Jun;20:1146–55. doi: 10.1158/1055-9965.EPI-10-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vaarala MH, Mattila H, Ohtonen P, Tammela TL, Paavonen TK, Schleutker J. The interaction of CYP3A5 polymorphisms along the androgen metabolism pathway in prostate cancer. Int J Cancer. 2008 Jun 1;122:2511–6. doi: 10.1002/ijc.23425. [DOI] [PubMed] [Google Scholar]
- 138.Domanski TL, Finta C, Halpert JR, Zaphiropoulos PG. cDNA cloning and initial characterization of CYP3A43, a novel human cytochrome P450. Mol Pharmacol. 2001 Feb;59:386–92. doi: 10.1124/mol.59.2.386. [DOI] [PubMed] [Google Scholar]
- 139.Stone A, Ratnasinghe LD, Emerson GL, Modali R, Lehman T, Runnells G, Carroll A, Carter W, Barnhart S, Rasheed AA, Greene G, Johnson DE, Ambrosone CB, Kadlubar FF, Lang NP. CYP3A43 Pro(340)Ala polymorphism and prostate cancer risk in African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005 May;14:1257–61. doi: 10.1158/1055-9965.EPI-04-0534. [DOI] [PubMed] [Google Scholar]
- 140.Siemes C, Visser LE, de Jong FH, Coebergh JW, Uitterlinden AG, Hofman A, Stricker BH, van Schaik RH. Cytochrome P450 3A gene variation, steroid hormone serum levels and prostate cancer--The Rotterdam Study. Steroids. 2010 Dec;75:1024–32. doi: 10.1016/j.steroids.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 141.Kraft P, Pharoah P, Chanock SJ, Albanes D, Kolonel LN, Hayes RB, Altshuler D, Andriole G, Berg C, Boeing H, Burtt NP, Bueno-de-Mesquita B, Calle EE, Cann H, Canzian F, Chen YC, Crawford DE, Dunning AM, Feigelson HS, Freedman ML, Gaziano JM, Giovannucci E, Gonzalez CA, Haiman CA, Hallmans G, Henderson BE, Hirschhorn JN, Hunter DJ, Kaaks R, Key T, Le Marchand L, Ma J, Overvad K, Palli D, Pike MC, Riboli E, Rodriguez C, Setiawan WV, Stampfer MJ, Stram DO, Thomas G, Thun MJ, Travis R, Trichopoulou A, Virtamo J, Wacholder S. Genetic variation in the HSD17B1 gene and risk of prostate cancer. PLoS Genet. 2005 Nov;1:e68. doi: 10.1371/journal.pgen.0010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun T, Oh WK, Jacobus S, Regan M, Pomerantz M, Freedman ML, Lee GS, Kantoff PW. The impact of common genetic variations in genes of the sex hormone metabolic pathways on steroid hormone levels and prostate cancer aggressiveness. Cancer Prev Res (Phila) 2011 Dec;4:2044–50. doi: 10.1158/1940-6207.CAPR-11-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Margiotti K, Kim E, Pearce CL, Spera E, Novelli G, Reichardt JK. Association of the G289S single nucleotide polymorphism in the HSD17B3 gene with prostate cancer in Italian men. Prostate. 2002 Sep 15;53:65–8. doi: 10.1002/pros.10134. [DOI] [PubMed] [Google Scholar]
- 144.Lindström S, Zheng SL, Wiklund F, Jonsson BA, Adami HO, Bälter KA, Brookes AJ, Sun J, Chang BL, Liu W, Li G, Isaacs WB, Adolfsson J, Grönberg H, Xu J. Systematic replication study of reported genetic associations in prostate cancer: Strong support for genetic variation in the androgen pathway. Prostate. 2006 Dec 1;66:1729–43. doi: 10.1002/pros.20489. [DOI] [PubMed] [Google Scholar]
- 145.Rasiah KK, Gardiner-Garden M, Padilla EJ, Möller G, Kench JG, Alles MC, Eggleton SA, Stricker PD, Adamski J, Sutherland RL, Henshall SM, Hayes VM. HSD17B4 overexpression, an independent biomarker of poor patient outcome in prostate cancer. Mol Cell Endocrinol. 2009 Mar 25;301:89–96. doi: 10.1016/j.mce.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 146.Ross RW, Oh WK, Xie W, Pomerantz M, Nakabayashi M, Sartor O, Taplin ME, Regan MM, Kantoff PW, Freedman M. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 2008 Feb 20;26:842–7. doi: 10.1200/JCO.2007.13.6804. [DOI] [PubMed] [Google Scholar]
- 147.Jakobsson J, Palonek E, Lorentzon M, Ohlsson C, Rane A, Ekstrom L. A novel polymorphism in the 17beta-hydroxysteroid dehydrogenase type 5 (aldo-keto reductase 1C3) gene is associated with lower serum testosterone levels in caucasian men. Pharmacogenomics J. 2007 Aug;7:282–9. doi: 10.1038/sj.tpj.6500419. [DOI] [PubMed] [Google Scholar]
- 148.Schulze JJ, Karypidis H, Ekstrom L. Basal and Regulatory Promoter Studies of the AKR1C3 Gene in Relation to Prostate Cancer. Front Pharmacol. 2012;3:151. doi: 10.3389/fphar.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lampe JW, Bigler J, Bush AC, Potter JD. Prevalence of polymorphisms in the human UDP-glucuronosyltransferase 2B family: UGT2B4(D458E), UGT2B7(H268Y), and UGT2B15(D85Y) Cancer Epidemiol Biomarkers Prev. 2000 Mar;9:329–33. [PubMed] [Google Scholar]
- 150.Okugi H, Nakazato H, Matsui H, Ohtake N, Nakata S, Suzuki K. Association of the polymorphisms of genes involved in androgen metabolism and signaling pathways with familial prostate cancer risk in a Japanese population. Cancer Detect Prev. 2006;30:262–8. doi: 10.1016/j.cdp.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 151.Park J, Chen L, Shade K, Lazarus P, Seigne J, Patterson S, Helal M, Pow-Sang J. Asp85tyr polymorphism in the udp-glucuronosyltransferase (UGT) 2B15 gene and the risk of prostate cancer. J Urol. 2004 Jun;171:2484–8. doi: 10.1097/01.ju.0000117748.44313.43. [DOI] [PubMed] [Google Scholar]
- 152.Hajdinjak T, Zagradisnik B. Prostate cancer and polymorphism D85Y in gene for dihydrotestosterone degrading enzyme UGT2B15: Frequency of DD homozygotes increases with Gleason Score. Prostate. 2004 Jun 1;59:436–9. doi: 10.1002/pros.20024. [DOI] [PubMed] [Google Scholar]
- 153.MacLeod SL, Nowell S, Plaxco J, Lang NP. An allele-specific polymerase chain reaction method for the determination of the D85Y polymorphism in the human UDP-glucuronosyltransferase 2B15 gene in a case-control study of prostate cancer. Ann Surg Oncol. 2000 Dec;7:777–82. doi: 10.1007/s10434-000-0777-3. [DOI] [PubMed] [Google Scholar]
- 154.Gsur A, Preyer M, Haidinger G, Schatzl G, Madersbacher S, Marberger M, Vutuc C, Micksche M. A polymorphism in the UDP-Glucuronosyltransferase 2B15 gene (D85Y) is not associated with prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2002 May;11:497–8. [PubMed] [Google Scholar]
- 155.Heald AH, Ivison F, Anderson SG, Cruickshank K, Laing I, Gibson JM. Significant ethnic variation in total and free testosterone concentration. Clin Endocrinol (Oxf) 2003 Mar;58:262–6. doi: 10.1046/j.1365-2265.2003.01653.x. [DOI] [PubMed] [Google Scholar]
- 156.Abdelrahaman E, Raghavan S, Baker L, Weinrich M, Winters SJ. Racial difference in circulating sex hormone-binding globulin levels in prepubertal boys. Metabolism. 2005 Jan;54:91–6. doi: 10.1016/j.metabol.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 157.Winters SJ, Brufsky A, Weissfeld J, Trump DL, Dyky MA, Hadeed V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism. 2001 Oct;50:1242–7. doi: 10.1053/meta.2001.26714. [DOI] [PubMed] [Google Scholar]
- 158.Power SG, Bocchinfuso WP, Pallesen M, Warmels-Rodenhiser S, Van Baelen H, Hammond GL. Molecular analyses of a human sex hormone-binding globulin variant: evidence for an additional carbohydrate chain. J Clin Endocrinol Metab. 1992 Oct;75:1066–70. doi: 10.1210/jcem.75.4.1400872. [DOI] [PubMed] [Google Scholar]
- 159.Low YL, Taylor JI, Grace PB, Mulligan AA, Welch AA, Scollen S, Dunning AM, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. Phytoestrogen exposure, polymorphisms in COMT, CYP19, ESR1, and SHBG genes, and their associations with prostate cancer risk. Nutr Cancer. 2006;56:31–9. doi: 10.1207/s15327914nc5601_5. [DOI] [PubMed] [Google Scholar]
- 160.Janne OA, Palvimo JJ, Kallio P, Mehto M. Androgen receptor and mechanism of androgen action. Ann Med. 1993 Feb;25:83–9. doi: 10.3109/07853899309147863. [DOI] [PubMed] [Google Scholar]
- 161.Gaston KE, Kim D, Singh S, Ford OH 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol. 2003 Sep;170:990–3. doi: 10.1097/01.ju.0000079761.56154.e5. [DOI] [PubMed] [Google Scholar]
- 162.Olapade-Olaopa EO, Muronda CA, MacKay EH, Danso AP, Sandhu DP, Terry TR, Habib FK. Androgen receptor protein expression in prostatic tissues in Black and Caucasian men. Prostate. 2004 Jun 1;59:460–8. doi: 10.1002/pros.20014. [DOI] [PubMed] [Google Scholar]
- 163.Lumbroso R, Beitel LK, Vasiliou DM, Trifiro MA, Pinsky L. Codon-usage variants in the polymorphic (GGN)n trinucleotide repeat of the human androgen receptor gene. Hum Genet. 1997 Nov;101:43–6. doi: 10.1007/s004390050583. [DOI] [PubMed] [Google Scholar]
- 164.Ding D, Xu L, Menon M, Reddy GP, Barrack ER. Effect of a short CAG (glutamine) repeat on human androgen receptor function. Prostate. 2004 Jan 1;58:23–32. doi: 10.1002/pros.10316. [DOI] [PubMed] [Google Scholar]
- 165.Ding D, Xu L, Menon M, Reddy GP, Barrack ER. Effect of GGC (glycine) repeat length polymorphism in the human androgen receptor on androgen action. Prostate. 2005 Feb 1;62:133–9. doi: 10.1002/pros.20128. [DOI] [PubMed] [Google Scholar]
- 166.Price DK, Chau CH, Till C, Goodman PJ, Baum CE, Ockers SB, English BC, Minasian L, Parnes HL, Hsing AW, Reichardt JK, Hoque A, Tangen CM, Kristal AR, Thompson IM, Figg WD. Androgen receptor CAG repeat length and association with prostate cancer risk: results from the prostate cancer prevention trial. J Urol. 2010 Dec;184:2297–302. doi: 10.1016/j.juro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lindström S, Ma J, Altshuler D, Giovannucci E, Riboli E, Albanes D, Allen NE, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Chanock SJ, Dunning AM, Feigelson HS, Gaziano JM, Haiman CA, Hayes RB, Henderson BE, Hunter DJ, Kaaks R, Kolonel LN, Le Marchand L, Martínez C, Overvad K, Siddiq A, Stampfer M, Stattin P, Stram DO, Thun MJ, Trichopoulos D, Tumino R, Virtamo J, Weinstein SJ, Yeager M, Kraft P, Freedman ML. A large study of androgen receptor germline variants and their relation to sex hormone levels and prostate cancer risk. Results from the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. J Clin Endocrinol Metab. 2010 Sep;95:E121–7. doi: 10.1210/jc.2009-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lange EM, Sarma AV, Ray A, Wang Y, Ho LA, Anderson SA, Cunningham JM, Cooney KA. The androgen receptor CAG and GGN repeat polymorphisms and prostate cancer susceptibility in African-American men: results from the Flint Men’s Health Study. J Hum Genet. 2008;53:220–6. doi: 10.1007/s10038-007-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Brien TG, Guo Y, Visvanathan K, Sciulli J, McLaine M, Helzlsouer KJ, Watkins-Bruner D. Differences in ornithine decarboxylase and androgen receptor allele frequencies among ethnic groups. Mol Carcinog. 2004 Oct;41:120–3. doi: 10.1002/mc.20047. [DOI] [PubMed] [Google Scholar]
- 170.Huang SP, Chou YH, Chang WS, Wu MT, Yu CC, Wu T, Lee YH, Huang JK, Wu WJ, Huang CH. Androgen receptor gene polymorphism and prostate cancer in Taiwan. J Formos Med Assoc. 2003 Oct;102:680–6. [PubMed] [Google Scholar]
- 171.Balic I, Graham ST, Troyer DA, Higgins BA, Pollock BH, Johnson-Pais TL, Thompson IM, Leach RJ. Androgen receptor length polymorphism associated with prostate cancer risk in Hispanic men. J Urol. 2002 Nov;168:2245–8. doi: 10.1016/S0022-5347(05)64364-9. [DOI] [PubMed] [Google Scholar]
- 172.Hsing AW, Gao YT, Wu G, Wang X, Deng J, Chen YL, Sesterhenn IA, Mostofi FK, Benichou J, Chang C. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Res. 2000 Sep 15;60:5111–6. [PubMed] [Google Scholar]
- 173.Sartor O, Zheng Q, Eastham JA. Androgen receptor gene CAG repeat length varies in a race-specific fashion in men without prostate cancer. Urology. 1999 Feb;53:378–80. doi: 10.1016/s0090-4295(98)00481-6. [DOI] [PubMed] [Google Scholar]
- 174.Irvine RA, Yu MC, Ross RK, Coetzee GA. The CAG and GGC microsatellites of the androgen receptor gene are in linkage disequilibrium in men with prostate cancer. Cancer Res. 1995 May 1;55:1937–40. [PubMed] [Google Scholar]
- 175.Santos ML, Sarkis AS, Nishimoto IN, Nagai MA. Androgen receptor CAG repeat polymorphism in prostate cancer from a Brazilian population. Cancer Detect Prev. 2003;27:321–6. doi: 10.1016/s0361-090x(03)00106-5. [DOI] [PubMed] [Google Scholar]
- 176.Krithivas K, Yurgalevitch SM, Mohr BA, Wilcox CJ, Batter SJ, Brown M, Longcope C, McKinlay JB, Kantoff PW. Evidence that the CAG repeat in the androgen receptor gene is associated with the age-related decline in serum androgen levels in men. J Endocrinol. 1999 Jul;162:137–42. doi: 10.1677/joe.0.1620137. [DOI] [PubMed] [Google Scholar]
- 177.Crabbe P, Bogaert V, De Bacquer D, Goemaere S, Zmierczak H, Kaufman JM. Part of the interindividual variation in serum testosterone levels in healthy men reflects differences in androgen sensitivity and feedback set point: contribution of the androgen receptor polyglutamine tract polymorphism. J Clin Endocrinol Metab. 2007 Sep;92:3604–10. doi: 10.1210/jc.2007-0117. [DOI] [PubMed] [Google Scholar]
- 178.Van Pottelbergh I, Lumbroso S, Goemaere S, Sultan C, Kaufman JM. Lack of influence of the androgen receptor gene CAG-repeat polymorphism on sex steroid status and bone metabolism in elderly men. Clin Endocrinol (Oxf) 2001 Nov;55:659–66. doi: 10.1046/j.1365-2265.2001.01403.x. [DOI] [PubMed] [Google Scholar]
- 179.Zitzmann M, Brune M, Kornmann B, Gromoll J, von Eckardstein S, von Eckardstein A, Nieschlag E. The CAG repeat polymorphism in the AR gene affects high density lipoprotein cholesterol and arterial vasoreactivity. J Clin Endocrinol Metab. 2001 Oct;86:4867–73. doi: 10.1210/jcem.86.10.7889. [DOI] [PubMed] [Google Scholar]
- 180.Alevizaki M, Cimponeriu AT, Garofallaki M, Sarika HL, Alevizaki CC, Papamichael C, Philippou G, Anastasiou EA, Lekakis JP, Mavrikakis M. The androgen receptor gene CAG polymorphism is associated with the severity of coronary artery disease in men. Clin Endocrinol (Oxf) 2003 Dec;59:749–55. doi: 10.1046/j.1365-2265.2003.01917.x. [DOI] [PubMed] [Google Scholar]
- 181.Silva Neto B, Koff WJ, Biolchi V, Brenner C, Biolo KD, Spritzer PM, Brum IS. Polymorphic CAG and GGC repeat lengths in the androgen receptor gene and prostate cancer risk: analysis of a Brazilian population. Cancer Invest. 2008 Feb;26:74–80. doi: 10.1080/07357900701638251. [DOI] [PubMed] [Google Scholar]
- 182.Akinloye O, Gromoll J, Simoni M. Variation in CAG and GGN repeat lengths and CAG/GGN haplotype in androgen receptor gene polymorphism and prostate carcinoma in Nigerians. Br J Biomed Sci. 2011;68:138–42. doi: 10.1080/09674845.2011.11730341. [DOI] [PubMed] [Google Scholar]
- 183.Krishnaswamy V, Kumarasamy T, Venkatesan V, Shroff S, Jayanth VR, Paul SF. South Indian men with reduced CAG repeat length in the androgen receptor gene have an increased risk of prostate cancer. J Hum Genet. 2006;51:254–7. doi: 10.1007/s10038-005-0346-5. [DOI] [PubMed] [Google Scholar]
- 184.Ashtiani ZO, Hasheminasab SM, Ayati M, Goulian BS, Modarressi MH. Are GSTM1, GSTT1 and CAG repeat length of androgen receptor gene polymorphisms associated with risk of prostate cancer in Iranian patients? Pathol Oncol Res. 2011 Jun;17:269–75. doi: 10.1007/s12253-010-9309-z. [DOI] [PubMed] [Google Scholar]
- 185.Mishra D, Thangaraj K, Mandhani A, Kumar A, Mittal R. Is reduced CAG repeat length in androgen receptor gene associated with risk of prostate cancer in Indian population? Clin Genet. 2005 Jul;68:55–60. doi: 10.1111/j.1399-0004.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 186.Liu JH, Li HW, Tong M, Li M, Na YQ. [Genetic risk factors of prostate cancer in Han nationality population in Northern China and a preliminary study of the reason of racial difference in prevalence of prostate cancer] . Zhonghua Yi Xue Za Zhi. 2004 Mar 2;84:364–8. [PubMed] [Google Scholar]
- 187.Andersson P, Varenhorst E, Soderkvist P. Androgen receptor and vitamin D receptor gene polymorphisms and prostate cancer risk. Eur J Cancer. 2006 Nov;42:2833–7. doi: 10.1016/j.ejca.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 188.Nicolaiew N, Cancel-Tassin G, Azzouzi AR, Grand BL, Mangin P, Cormier L, Fournier G, Giordanella JP, Pouchard M, Escary JL, Valeri A, Cussenot O. Association between estrogen and androgen receptor genes and prostate cancer risk. Eur J Endocrinol. 2009 Jan;160:101–6. doi: 10.1530/EJE-08-0321. [DOI] [PubMed] [Google Scholar]
- 189.Gu M, Dong X, Zhang X, Niu W. The CAG repeat polymorphism of androgen receptor gene and prostate cancer: a meta-analysis. Mol Biol Rep. 2012 Mar;39:2615–24. doi: 10.1007/s11033-011-1014-9. [DOI] [PubMed] [Google Scholar]
- 190.Patiño-García B, Arroyo C, Rangel-Villalobos H, Soto-Vega E, Velarde-Félix JS, Gabilondo F, Sandoval-Ramirez L, Figuera LE. Association between polymorphisms of the androgen and vitamin D receptor genes with prostate cancer risk in a Mexican population. Rev Invest Clin. 2007 Jan-Feb;59:25–31. [PubMed] [Google Scholar]
- 191.Gilligan T, Manola J, Sartor O, Weinrich SP, Moul JW, Kantoff PW. Absence of a correlation of androgen receptor gene CAG repeat length and prostate cancer risk in an African-American population. Clin Prostate Cancer. 2004 Sep;3:98–103. doi: 10.3816/cgc.2004.n.019. [DOI] [PubMed] [Google Scholar]
- 192.Gsur A, Preyer M, Haidinger G, Zidek T, Madersbacher S, Schatzl G, Marberger M, Vutuc C, Micksche M. Polymorphic CAG repeats in the androgen receptor gene, prostate-specific antigen polymorphism and prostate cancer risk. Carcinogenesis. 2002 Oct;23:1647–51. doi: 10.1093/carcin/23.10.1647. [DOI] [PubMed] [Google Scholar]
- 193.Chen C, Lamharzi N, Weiss NS, Etzioni R, Dightman DA, Barnett M, DiTommaso D, Goodman G. Androgen receptor polymorphisms and the incidence of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2002 Oct;11:1033–40. [PubMed] [Google Scholar]
- 194.Salinas CA, Austin MA, Ostrander EO, Stanford JL. Polymorphisms in the androgen receptor and the prostate-specific antigen genes and prostate cancer risk. Prostate. 2005 Sep 15;65:58–65. doi: 10.1002/pros.20230. [DOI] [PubMed] [Google Scholar]
- 195.Li C, Grönberg H, Matsuyama H, Weber G, Nordenskjöld M, Naito K, Bergh A, Bergerheim U, Damber JE, Larsson C, Ekman P. Difference between Swedish and Japanese men in the association between AR CAG repeats and prostate cancer suggesting a susceptibility-modifying locus overlapping the androgen receptor gene. Int J Mol Med. 2003 Apr;11:529–33. [PubMed] [Google Scholar]
- 196.Das K, Cheah PY, Lim PL, Zain YB, Stephanie FC, Zhao Y, Cheng C, Lau W. Shorter CAG repeats in androgen receptor and non-GG genotypes in prostate-specific antigen loci are associated with decreased risk of benign prostatic hyperplasia and prostate cancer. Cancer Lett. 2008 Sep 18;268:340–7. doi: 10.1016/j.canlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 197.Kittles RA, Young D, Weinrich S, Hudson J, Argyropoulos G, Ukoli F, Adams-Campbell L, Dunston GM. Extent of linkage disequilibrium between the androgen receptor gene CAG and GGC repeats in human populations: implications for prostate cancer risk. Hum Genet. 2001 Sep;109:253–61. doi: 10.1007/s004390100576. [DOI] [PubMed] [Google Scholar]
- 198.Esteban E, Rodon N, Via M, Gonzalez-Perez E, Santamaria J, Dugoujon JM, Chennawi FE, Melhaoui M, Cherkaoui M, Vona G, Harich N, Moral P. Androgen receptor CAG and GGC polymorphisms in Mediterraneans: repeat dynamics and population relationships. J Hum Genet. 2006;51:129–36. doi: 10.1007/s10038-005-0336-7. [DOI] [PubMed] [Google Scholar]
- 199.Stanford JL, Just JJ, Gibbs M, Wicklund KG, Neal CL, Blumenstein BA, Ostrander EA. Polymorphic repeats in the androgen receptor gene: molecular markers of prostate cancer risk. Cancer Res. 1997 Mar 15;57:1194–8. [PubMed] [Google Scholar]
- 200.Chang BL, Zheng SL, Hawkins GA, Isaacs SD, Wiley KE, Turner A, Carpten JD, Bleecker ER, Walsh PC, Trent JM, Meyers DA, Isaacs WB, Xu J. Polymorphic GGC repeats in the androgen receptor gene are associated with hereditary and sporadic prostate cancer risk. Hum Genet. 2002 Feb;110:122–9. doi: 10.1007/s00439-001-0662-6. [DOI] [PubMed] [Google Scholar]
- 201.Zeegers MP, Kiemeney LA, Nieder AM, Ostrer H. How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev. 2004 Nov;13:1765–71. [PubMed] [Google Scholar]
- 202.Platz EA, Giovannucci E, Dahl DM, Krithivas K, Hennekens CH, Brown M, Stampfer MJ, Kantoff PW. The androgen receptor gene GGN microsatellite and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 1998 May;7:379–84. [PubMed] [Google Scholar]
- 203.Correa-Cerro L, Wöhr G, Häussler J, Berthon P, Drelon E, Mangin P, Fournier G, Cussenot O, Kraus P, Just W, Paiss T, Cantú JM, Vogel W. (CAG)nCAA and GGN repeats in the human androgen receptor gene are not associated with prostate cancer in a French-German population. Eur J Hum Genet. 1999 Apr;7:357–62. doi: 10.1038/sj.ejhg.5200298. [DOI] [PubMed] [Google Scholar]
- 204.Vijayalakshmi K, Thangaraj K, Rajender S, Vettriselvi V, Venkatesan P, Shroff S, Vishwanathan KN, Paul SF. GGN repeat length and GGN/CAG haplotype variations in the androgen receptor gene and prostate cancer risk in south Indian men. J Hum Genet. 2006;51:998–1005. doi: 10.1007/s10038-006-0051-z. [DOI] [PubMed] [Google Scholar]
- 205.Miller EA, Stanford JL, Hsu L, Noonan E, Ostrander EA. Polymorphic repeats in the androgen receptor gene in high-risk sibships. Prostate. 2001 Aug 1;48:200–5. doi: 10.1002/pros.1098. [DOI] [PubMed] [Google Scholar]
- 206.Liu W, Innocenti F, Chen P, Das S, Cook EH Jr, Ratain MJ. Interethnic difference in the allelic distribution of human epidermal growth factor receptor intron 1 polymorphism. Clin Cancer Res. 2003 Mar;9:1009–12. [PubMed] [Google Scholar]
- 207.Gebhardt F, Zanker KS, Brandt B. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999 May 7;274:13176–80. doi: 10.1074/jbc.274.19.13176. [DOI] [PubMed] [Google Scholar]
- 208.Peraldo-Neia C, Migliardi G, Mello-Grand M, Montemurro F, Segir R, Pignochino Y, Cavalloni G, Torchio B, Mosso L, Chiorino G, Aglietta M. Epidermal Growth Factor Receptor (EGFR) mutation analysis, gene expression profiling and EGFR protein expression in primary prostate cancer. BMC Cancer. 2011;11:31. doi: 10.1186/1471-2407-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Casey G, Neville PJ, Plummer SJ, Xiang Y, Krumroy LM, Klein EA, Catalona WJ, Nupponen N, Carpten JD, Trent JM, Silverman RH, Witte JS. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet. 2002 Dec;32:581–3. doi: 10.1038/ng1021. [DOI] [PubMed] [Google Scholar]
- 210.Rennert H, Zeigler-Johnson CM, Addya K, Finley MJ, Walker AH, Spangler E, Leonard DG, Wein A, Malkowicz SB, Rebbeck TR. Association of susceptibility alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with prostate cancer severity in European American and African American men. Cancer Epidemiol Biomarkers Prev. 2005 Apr;14:949–57. doi: 10.1158/1055-9965.EPI-04-0637. [DOI] [PubMed] [Google Scholar]
- 211.Nakazato H, Suzuki K, Matsui H, Ohtake N, Nakata S, Yamanaka H. Role of genetic polymorphisms of the RNASEL gene on familial prostate cancer risk in a Japanese population. Br J Cancer. 2003 Aug 18;89:691–6. doi: 10.1038/sj.bjc.6601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Robbins CM, Hernandez W, Ahaghotu C, Bennett J, Hoke G, Mason T, Pettaway CA, Vijayakumar S, Weinrich S, Furbert-Harris P, Dunston G, Powell IJ, Carpten JD, Kittles RA. Association of HPC2/ELAC2 and RNASEL non-synonymous variants with prostate cancer risk in African American familial and sporadic cases. Prostate. 2008 Dec 1;68:1790–7. doi: 10.1002/pros.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wei B, Xu Z, Ruan J, Zhu M, Jin K, Zhou D, Yan Z, Xuan F, Zhou H, Huang X, Zhang J, Lu P, Shao J. RNASEL Asp541Glu and Arg462Gln polymorphisms in prostate cancer risk: evidences from a meta-analysis. Mol Biol Rep. 2012 Mar;39:2347–53. doi: 10.1007/s11033-011-0985-x. [DOI] [PubMed] [Google Scholar]
- 214.Li H, Tai BC. RNASEL gene polymorphisms and the risk of prostate cancer: a meta-analysis. Clin Cancer Res. 2006 Oct 1;12:5713–9. doi: 10.1158/1078-0432.CCR-05-2799. [DOI] [PubMed] [Google Scholar]
- 215.Xu B, Tong N, Li JM, Zhang ZD, Wu HF. ELAC2 polymorphisms and prostate cancer risk: a meta-analysis based on 18 case-control studies. Prostate Cancer Prostatic Dis. 2010 Sep;13:270–7. doi: 10.1038/pcan.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Geng J, Zhang Q, Zhu C, Wang J, Chen L. XRCC1 genetic polymorphism Arg399Gln and prostate cancer risk: a meta-analysis. Urology. 2009 Sep;74:648–53. doi: 10.1016/j.urology.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 217.Robbins CM, Hernandez W, Ahaghotu C, Bennett J, Hoke G, Mason T, Pettaway CA, Vijayakumar S, Weinrich S, Furbert-Harris P, Dunston G, Powell IJ, Carpten JD, Kittles RA. XRCC1 Arg- 399Gln and Arg194Trp polymorphisms in prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis. 2011 Sep;14:225–31. [Google Scholar]
- 218.Li LC, Chui RM, Sasaki M, Nakajima K, Perinchery G, Au HC, Nojima D, Carroll P, Dahiya R. A single nucleotide polymorphism in the E-cadherin gene promoter alters transcriptional activities. Cancer Res. 2000 Feb 15;60:873–6. [PubMed] [Google Scholar]
- 219.Lindström S, Wiklund F, Jonsson BA, Adami HO, Bälter K, Brookes AJ, Xu J, Zheng SL, Isaacs WB, Adolfsson J, Grönberg H. Comprehensive genetic evaluation of common E-cadherin sequence variants and prostate cancer risk: strong confirmation of functional promoter SNP. Hum Genet. 2005 Dec;118:339–47. doi: 10.1007/s00439-005-0060-6. [DOI] [PubMed] [Google Scholar]
- 220.Hajdinjak T, Toplak N. E-cadherin polymorphism--160 C/A and prostate cancer. Int J Cancer. 2004 Apr 10;109:480–1. doi: 10.1002/ijc.11705. [DOI] [PubMed] [Google Scholar]
- 221.Verhage BA, van Houwelingen K, Ruijter TE, Kiemeney LA, Schalken JA. Single-nucleotide polymorphism in the E-cadherin gene promoter modifies the risk of prostate cancer. Int J Cancer. 2002 Aug 20;100:683–5. doi: 10.1002/ijc.10541. [DOI] [PubMed] [Google Scholar]
- 222.Pookot D, Li LC, Tabatabai ZL, Tanaka Y, Greene KL, Dahiya R. The E-cadherin -160 C/A polymorphism and prostate cancer risk in white and black American men. J Urol. 2006 Aug;176:793–6. doi: 10.1016/j.juro.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 223.Bonilla C, Mason T, Long L, Ahaghotu C, Chen W, Zhao A, Coulibaly A, Bennett F, Aiken W, Tullock T, Coard K, Freeman V, Kittles RA. E-cadherin polymorphisms and haplotypes influence risk for prostate cancer. Prostate. 2006 Apr 1;66:546–56. doi: 10.1002/pros.20374. [DOI] [PubMed] [Google Scholar]
- 224.Goto T, Nakano M, Ito S, Ehara H, Yamamoto N, Deguchi T. Significance of an E-cadherin gene promoter polymorphism for risk and disease severity of prostate cancer in a Japanese population. Urology. 2007 Jul;70:127–30. doi: 10.1016/j.urology.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 225.Kamoto T, Isogawa Y, Shimizu Y, Minamiguchi S, Kinoshita H, Kakehi Y, Mitsumori K, Yamamoto S, Habuchi T, Kato T, Ogawa O. Association of a genetic polymorphism of the E-cadherin gene with prostate cancer in a Japanese population. Jpn J Clin Oncol. 2005 Mar;35:158–61. doi: 10.1093/jjco/hyi040. [DOI] [PubMed] [Google Scholar]
- 226.Tsukino H, Kuroda Y, Imai H, Nakao H, Qiu D, Komiya Y, Inatomi H, Hamasaki T, Kohshi K, Osada Y, Katoh T. Lack of evidence for the association of E-cadherin gene polymorphism with increased risk or progression of prostate cancer. Urol Int. 2004;72:203–7. doi: 10.1159/000077115. [DOI] [PubMed] [Google Scholar]
- 227.Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009 Sep;29:3511–36. [PubMed] [Google Scholar]
- 228.Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D. Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res. 1994 Feb 1;54:805–10. [PubMed] [Google Scholar]
- 229.Berndt SI, Dodson JL, Huang WY, Nicodemus KK. A systematic review of vitamin D receptor gene polymorphisms and prostate cancer risk. J Urol. 2006 May;175:1613–23. doi: 10.1016/S0022-5347(05)00958-4. [DOI] [PubMed] [Google Scholar]
- 230.Szendroi A, Speer G, Tabak A, Kosa JP, Nyirady P, Majoros A, Romics I, Lakatos P. The role of vitamin D, estrogen, calcium sensing receptor genotypes and serum calcium in the pathogenesis of prostate cancer. Can J Urol. 2011 Jun;18:5710–6. [PubMed] [Google Scholar]
- 231.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009 Jul;30:1170–80. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 232.Bai Y, Yu Y, Yu B, Ge J, Ji J, Lu H, Wei J, Weng Z, Tao Z, Lu J. Association of vitamin D receptor polymorphisms with the risk of prostate cancer in the Han population of Southern China. BMC Med Genet. 2009;10:125. doi: 10.1186/1471-2350-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Torkko KC, van Bokhoven A, Mai P, Beuten J, Balic I, Byers TE, Hokanson JE, Norris JM, Barón AE, Lucia MS, Thompson IM, Leach RJ. VDR and SRD5A2 polymorphisms combine to increase risk for prostate cancer in both non-Hispanic White and Hispanic White men. Clin Cancer Res. 2008 May 15;14:3223–9. doi: 10.1158/1078-0432.CCR-07-4894. [DOI] [PubMed] [Google Scholar]
- 234.Onen IH, Ekmekci A, Eroglu M, Konac E, Yesil S, Biri H. Association of genetic polymorphisms in vitamin D receptor gene and susceptibility to sporadic prostate cancer. Exp Biol Med (Maywood) 2008 Dec;233:1608–14. doi: 10.3181/0803-RM-110. [DOI] [PubMed] [Google Scholar]
- 235.Li H, Stampfer MJ, Hollis JB, Mucci LA, Gaziano JM, Hunter D, Giovannucci EL, Ma J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007 Mar;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Holick CN, Stanford JL, Kwon EM, Ostrander EA, Nejentsev S, Peters U. Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007 Oct;16:1990–9. doi: 10.1158/1055-9965.EPI-07-0487. [DOI] [PubMed] [Google Scholar]
- 237.Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, Penney K, Steen RG, Ardlie K, John EM, Oakley-Girvan I, Whittemore AS, Cooney KA, Ingles SA, Altshuler D, Henderson BE, Reich D. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006 Sep 19;103:14068–73. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Amundadottir LT, Sulem P, Gudmundsson J, Helgason A, Baker A, Agnarsson BA, Sigurdsson A, Benediktsdottir KR, Cazier JB, Sainz J, Jakobsdottir M, Kostic J, Magnusdottir DN, Ghosh S, Agnarsson K, Birgisdottir B, Le Roux L, Olafsdottir A, Blondal T, Andresdottir M, Gretarsdottir OS, Bergthorsson JT, Gudbjartsson D, Gylfason A, Thorleifsson G, Manolescu A, Kristjansson K, Geirsson G, Isaksson H, Douglas J, Johansson JE, Bälter K, Wiklund F, Montie JE, Yu X, Suarez BK, Ober C, Cooney KA, Gronberg H, Catalona WJ, Einarsson GV, Barkardottir RB, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006 Jun;38:652–8. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 239.Boyd LK, Mao X, Lu YJ. The complexity of prostate cancer: genomic alterations and heterogeneity. Nat Rev Urol. 2012 Nov;9:652–64. doi: 10.1038/nrurol.2012.185. [DOI] [PubMed] [Google Scholar]
- 240.Akamatsu S, Takata R, Haiman CA, Takahashi A, Inoue T, Kubo M, Furihata M, Kamatani N, Inazawa J, Chen GK, Le Marchand L, Kolonel LN, Katoh T, Yamano Y, Yamakado M, Takahashi H, Yamada H, Egawa S, Fujioka T, Henderson BE, Habuchi T, Ogawa O, Nakamura Y, Nakagawa H. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in Japanese. Nat Genet. 2012 Apr;44:426–9. S1. doi: 10.1038/ng.1104. [DOI] [PubMed] [Google Scholar]
- 241.Xu J, Mo Z, Ye D, Wang M, Liu F, Jin G, Xu C, Wang X, Shao Q, Chen Z, Tao Z, Qi J, Zhou F, Wang Z, Fu Y, He D, Wei Q, Guo J, Wu D, Gao X, Yuan J, Wang G, Xu Y, Wang G, Yao H, Dong P, Jiao Y, Shen M, Yang J, Ou-Yang J, Jiang H, Zhu Y, Ren S, Zhang Z, Yin C, Gao X, Dai B, Hu Z, Yang Y, Wu Q, Chen H, Peng P, Zheng Y, Zheng X, Xiang Y, Long J, Gong J, Na R, Lin X, Yu H, Wang Z, Tao S, Feng J, Sun J, Liu W, Hsing A, Rao J, Ding Q, Wiklund F, Gronberg H, Shu XO, Zheng W, Shen H, Jin L, Shi R, Lu D, Zhang X, Sun J, Zheng SL, Sun Y. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat Genet. 2012 Nov;44:1231–5. doi: 10.1038/ng.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, Muir K, Hopper JL, Henderson BE, Haiman CA, Schleutker J, Hamdy FC, Neal DE, Donovan JL, Stanford JL, Ostrander EA, Ingles SA, John EM, Thibodeau SN, Schaid D, Park JY, Spurdle A, Clements J, Dickinson JL, Maier C, Vogel W, Dörk T, Rebbeck TR, Cooney KA, Cannon-Albright L, Chappuis PO, Hutter P, Zeegers M, Kaneva R, Zhang HW, Lu YJ, Foulkes WD, English DR, Leongamornlert DA, Tymrakiewicz M, Morrison J, Ardern-Jones AT, Hall AL, O’Brien LT, Wilkinson RA, Saunders EJ, Page EC, Sawyer EJ, Edwards SM, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As N, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper CS, Southey MC, Lophatananon A, Liu JF, Kolonel LN, Le Marchand L, Wahlfors T, Tammela TL, Auvinen A, Lewis SJ, Cox A, FitzGerald LM, Koopmeiners JS, Karyadi DM, Kwon EM, Stern MC, Corral R, Joshi AD, Shahabi A, McDonnell SK, Sellers TA, Pow-Sang J, Chambers S, Aitken J, Gardiner RA, Batra J, Kedda MA, Lose F, Polanowski A, Patterson B, Serth J, Meyer A, Luedeke M, Stefflova K, Ray AM, Lange EM, Farnham J, Khan H, Slavov C, Mitkova A, Cao G, Easton DF UK Genetic Prostate Cancer Study Collaborators/British Association of Urological Surgeons’ Section of Oncology; UK ProtecT Study Collaborators; PRACTICAL Consortium. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009 Oct;41:1116–21. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jemal A, Center MM, DeSantis C, Ward EM. Replication of five prostate cancer loci identified in an Asian population--results from the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) Cancer Epidemiol Biomarkers Prev. 2012 Jan;21:212–6. doi: 10.1158/1055-9965.EPI-11-0870-T. [DOI] [PMC free article] [PubMed] [Google Scholar]