Abstract
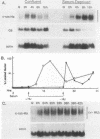
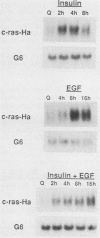
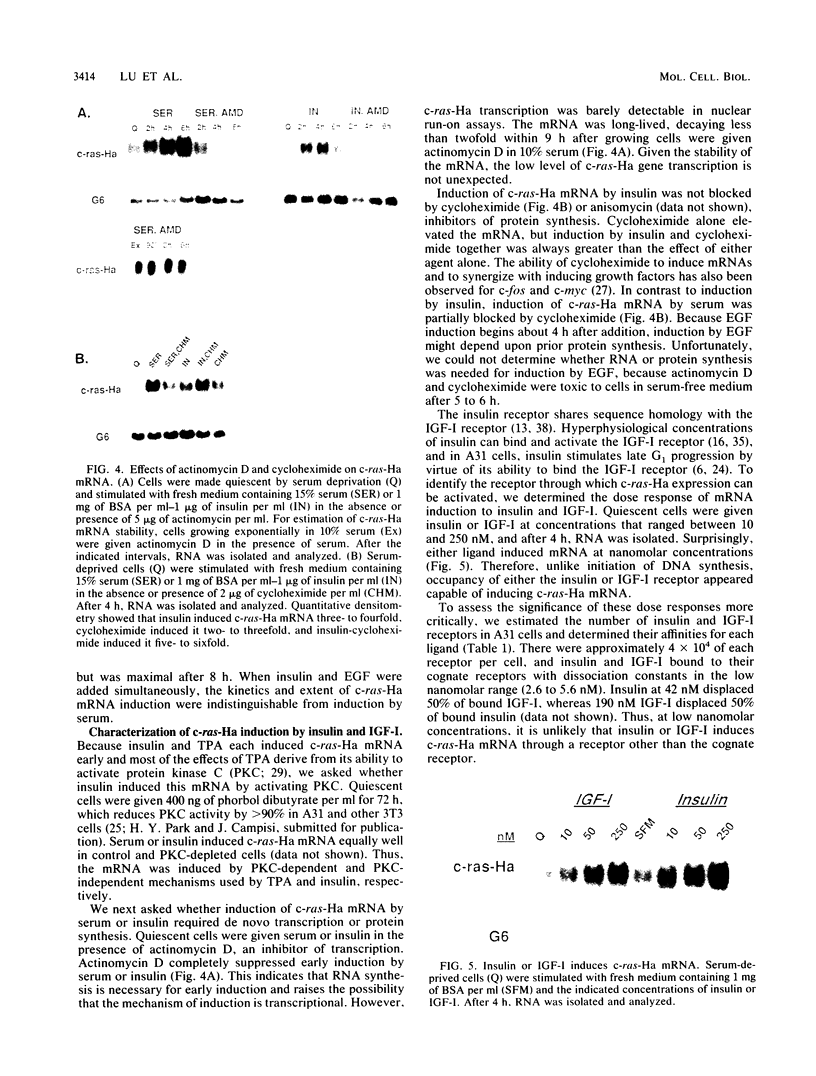
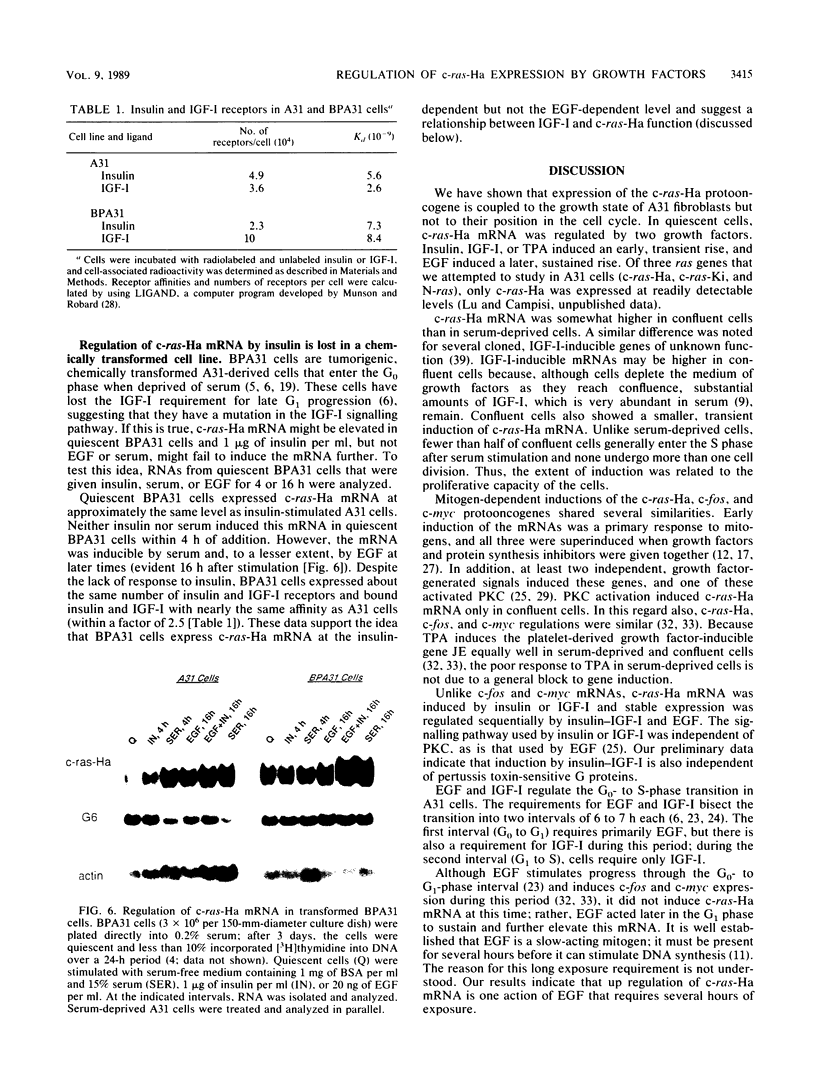
Although much is known about the structure of ras-encoded proteins, little is known about how expression is regulated. In serum-stimulated murine fibroblasts, c-ras-Ha mRNA levels fluctuated with the growth state but not with the position in the cell cycle. Two types of growth factors regulated c-ras-Ha expression: insulin (IN) or insulinlike growth factor I, each apparently acting through its cognate receptor, and epidermal growth factor (EGF). In quiescent cells, IN or insulinlike growth factor I induced c-ras-Ha mRNA three- to fivefold within 4 h, but thereafter the mRNA declined. By contrast, EGF had little effect in 4 h but induced the mRNA after 4 to 6 h. When quiescent cells were given serum or IN and EGF simultaneously, c-ras-Ha mRNA rose steadily, beginning 1 to 2 h after stimulation, and reached a stable five- to sevenfold elevation in 16 h. Thus, c-ras-Ha gene expression was sequentially regulated by two growth factors, one of which (IN) does not induce expression of other growth-regulated protooncogenes. A transformed derivative cell line that does not require IN for G1 progression has lost early IN-dependent but not late serum-dependent regulation. The results support the possibility that c-ras-Ha and IN action are functionally linked.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Campisi J., Medrano E. E. Cell cycle perturbations in normal and transformed fibroblasts caused by detachment from the substratum. J Cell Physiol. 1983 Jan;114(1):53–60. doi: 10.1002/jcp.1041140109. [DOI] [PubMed] [Google Scholar]
- Campisi J., Medrano E. E., Morreo G., Pardee A. B. Restriction point control of cell growth by a labile protein: evidence for increased stability in transformed cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):436–440. doi: 10.1073/pnas.79.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Pardee A. B. Post-transcriptional control of the onset of DNA synthesis by an insulin-like growth factor. Mol Cell Biol. 1984 Sep;4(9):1807–1814. doi: 10.1128/mcb.4.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Levinson A. D. A point mutation in the last intron responsible for increased expression and transforming activity of the c-Ha-ras oncogene. Nature. 1988 Jul 14;334(6178):119–124. doi: 10.1038/334119a0. [DOI] [PubMed] [Google Scholar]
- Das M. Epidermal growth factor: mechanisms of action. Int Rev Cytol. 1982;78:233–256. doi: 10.1016/s0074-7696(08)60107-2. [DOI] [PubMed] [Google Scholar]
- Dean M., Levine R. A., Ran W., Kindy M. S., Sonenshein G. E., Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986 Jul 15;261(20):9161–9166. [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Pardee A. B. S phase synchrony in monolayer CHO cultures. Exp Cell Res. 1976 Jul;100(2):265–275. doi: 10.1016/0014-4827(76)90147-6. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Simon M. I., Teplow D. B., Robishaw J. D., Gilman A. G. Homologies between signal transducing G proteins and ras gene products. Science. 1984 Nov 16;226(4676):860–862. doi: 10.1126/science.6436980. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Van Wyk J. J., O'Keefe E. J., Pledger W. J. Epidermal growth factor (EGF) is required only during the traverse of early G1 in PDGF stimulated density-arrested BALB/c-3T3 cells. Exp Cell Res. 1983 Aug;147(1):202–208. doi: 10.1016/0014-4827(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., DiPaolo J. A. Changes in the uptake of 2-deoxy-D-glucose in BALB-3T3 cells chemically transformed in culture. J Cell Physiol. 1974 Apr;83(2):193–201. doi: 10.1002/jcp.1040830205. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Long L. K., Sorrentino V., Barbacid M. ras gene Amplification and malignant transformation. Mol Cell Biol. 1985 Oct;5(10):2836–2841. doi: 10.1128/mcb.5.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran W., Dean M., Levine R. A., Campisi J. Activation of proto-oncogene expression by growth regulatory signals. Curr Top Microbiol Immunol. 1986;132:313–319. doi: 10.1007/978-3-642-71562-4_46. [DOI] [PubMed] [Google Scholar]
- Ran W., Dean M., Levine R. A., Henkle C., Campisi J. Induction of c-fos and c-myc mRNA by epidermal growth factor or calcium ionophore is cAMP dependent. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8216–8220. doi: 10.1073/pnas.83.21.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts M. H., Levinson A. D. High-level expression of c-H-ras1 fails to fully transform rat-1 cells. Mol Cell Biol. 1988 Apr;8(4):1460–1468. doi: 10.1128/mcb.8.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Maddux B., Wong K. Y., Styne D. M., Van Vliet G., Humbel R. E., Goldfine I. D. Interactions of a monoclonal antibody to the insulin receptor with receptors for insulin-like growth factors. Endocrinology. 1983 May;112(5):1865–1867. doi: 10.1210/endo-112-5-1865. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Thomopoulos P., Roth J., Lovelace E., Pastan I. Insulin receptors in normal and transformed fibroblasts: relationship to growth and transformation. Cell. 1976 Jul;8(3):417–423. doi: 10.1016/0092-8674(76)90154-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Zumstein P., Stiles C. D. Molecular cloning of gene sequences that are regulated by insulin-like growth factor I. J Biol Chem. 1987 Aug 15;262(23):11252–11260. [PubMed] [Google Scholar]