Abstract
Testosterone (T) and its metabolites may underlie some beneficial effects for anxiety and cognition, but the mechanisms for these effects are unclear. T is reduced to dihydrotestosterone (DHT), which can be converted to 5α-androstane,3α,17β-diol (3α-diol) and/or 5α-androstane-3β,17β-diol (3β-diol). Additionally, T can be converted to androstenedione, and then to androsterone. These metabolites bind with varying affinity to androgen receptors (ARs; T and DHT), estrogen receptors (ERβ; 3α-diol, 3β-diol), or GABAA/benzodiazepine receptors (GBRs; 3α-diol, androsterone). Three experiments were performed to investigate the hypothesis that reduced anxiety-like and enhanced cognitive performance may be due in part to actions of T metabolites at ERβ. Experiment 1: Gonadectomized (GDX) wildtype and ERβ knockout mice (βERKO) were subcutaneously (SC) administered 3α-diol, 3β-diol, androsterone, or oil vehicle at weekly intervals, and tested in anxiety tasks (open field, elevated plus maze, light–dark transition) or for cognitive performance in the object recognition task. Experiment 2: GDX rats were administered SC 3α-diol, 3β-diol, androsterone, or oil vehicle, and tested in the same tasks. Experiment 3: GDX rats were androsterone- or vehicle-primed and administered an antagonist of ARs (flutamide), ERs (tamoxifen), or GBRs (flumazenil), or vehicle and then tested in the elevated plus maze. Both rats and wildtype mice, but not βERKO mice, consistently had reduced anxiety and improved performance in the object recognition task. Androsterone was only effective at reducing anxiety-like behavior in the elevated plus maze and this effect was modestly reduced by flumazenil administration. Thus, actions at ERβ may be required for T’s anxiety-reducing and cognitive-enhancing effects.
Keywords: Anxiety, Cognition, 3α-diol, 3β-diol, Androsterone, Estrogen receptor beta
Introduction
Variations in the levels of testosterone (T) and of its metabolites may influence affect and /or cognition in men. Men who have higher endogenous levels of T have a lower incidence of depression (Earls, 1987). Conversely, young hypogonadal men, with low endogenous T and dihydrotestosterone (DHT) levels, are more likely to be diagnosed with an anxiety or depressive disorder, and exhibit decreased performance in some cognitive tasks (Howell and Shalet, 2001; Kaminetsky, 2005). Treatment for these men includes T replacement, which can increase positive, and decrease negative, mood and improve cognition (Zitzmann, 2006). A similar pattern is observed in aging men, where T levels, along with age, negatively correlate with anxiety, and with decreased performance in visuospatial tasks (Janowsky, 2006; Janowsky et al., 1994; Li et al., 2002). Administration of T to these men reinstates their affective and cognitive performance (Alexander et al., 1998; Delhez et al., 2003; Janowsky, 2006). Together these data implicate androgens as having anxiolytic and cognitive-enhancing effects among men.
In animal models of androgen-deficiency, androgens can mediate both affective and cognitive behavior. Removing rats’ primary source of endogenous androgens through androgen extirpation (i.e. castration or gonadectomy- GDX) results in increased anxiety-like behavior, and detriments in cognitive performance (Ceccarelli et al., 2001; Frye and Seliga, 2001; Edinger et al., 2004). Replacing their extirpated androgens decreases anxiety-like behavior in the open field, elevated plus maze, and defensive freezing tasks (Aikey et al., 2002; Edinger et al., 2004; Frye and Edinger, 2004) and enhances cognitive performance in the inhibitory avoidance (Edinger and Frye, 2004a) and conditioned contextual fear (Edinger et al., 2004) tasks. Administration of androgens to intact rodents can also decrease anxiety-like behavior and enhance learning and memory. When given T (Bitran et al., 1993) or anabolic steroids (Bitran et al., 1993; Rojas-Ortiz et al., 2006), intact male rats exhibited decreased anxiety-like behavior in the elevated plusmaze and Vogel paradigms. Together, these data fromanimal models demonstrate that androgens modulate affective and cognitive processes.
One possible mechanism by which T may influence hippocampally- mediated processes, such as affect and cognition, is through actions of its metabolites at estrogen receptors (ERs,; Frye et al., 1996; Pak et al., 2005). Estrogens and androgens have actions in the hippocampus to increase plasticity (Mukai et al., 2006). There are two isoforms of ER, estrogen receptor alpha and beta (referred to as ERα and ERβ). Studies indicate that ERβ, which is found in the hippocampus (Zhang et al., 2002), facilitates cell proliferation and learning and memory (Walf and Frye, 2008; Walf and Frye, 2007a; Rissman et al., 2002). Administration of selective ER modulators (SERMs) to female rats, which specifically target ERβ, but not ERα, enhances cognition, and reduces anxiety-like, and depressive behaviors of ovx rats or wildtype mice, but not those that are ERβ knockouts (βERKO; Rhodes and Frye, 2006; Walf and Frye, 2005; Walf et al., 2008). Although T and its 5α-reduced metabolite, DHT, do not bind to ERs in physiological concentrations, T can be aromatized to 17β-estradiol (E2; MacLusky et al., 1987), which binds to both ERα and ERβ, and has anxiolytic-like and (reviewed in Walf and Frye, 2006), cognitive-enhancing effects in rodents (Frye and Rhodes, 2002; Frye et al., 2007; Rhodes and Frye, 2006; Walf et al., 2006; Luine, 2008). Alternatively, T is either metabolized by 5α-reductase to dihydrotestosterone (DHT), which is then converted to 5α-androstane-3α,17β-diol (3α-diol) and 5α-androstane-3β,17β-diol (3β-diol), or T can also be converted to androstenedione and then further reduced to androsterone (Brown et al., 1994; Frye et al., 2007; Luttge, 1979). Administration of 3α-diol alone decreases anxiety-like behavior in tasks, such as the open field and elevated plus maze, and increases cognitive performance in the inhibitory avoidance, conditioned fear, and place learning tasks, similar to that observed with T and DHT administration (Edinger and Frye, 2004a; 2005; 2007; Edinger et al., 2004; Frye and Lacey, 2001; Frye et al., 2001). Blocking formation of 3α-diol through administration of indomethacin can reverse these beneficial effects of androgens on affect and cognition (Frye and Walf, 2004). 3α-diol may bind to ERβ and has a high affinity for GABA/benzodiazepine receptors (GBRs), while 3β-diol can bind with high affinity to ERβ and is a negative modulator of GBRs (Handa et al., 2008; Pak et al., 2005; Edinger and Frye, 2007; Frye et al., 2007; Gee, 1988; Masonis and McCarthy, 1995). In particular, 3β-diol not only has a high affinity for ERβ, but, once bound, activates transcription with high potency (Lund et al., 2005). In addition, 3α-diol-replaced GDX rats infused intracerebroventricularly with antisense oligonucleotides (AS-ODNS) targeted against ERβ, but not ERα, had poorer inhibitory avoidance performance compared to rats infused with vehicle (Edinger and Frye, 2007). Together, these findings suggest that androgens’ affective and cognitive effects may be mediated through actions at ERβ.
In order to assess whether ERβ is a potential target for androgens’ anxiety-reducing and/or cognitive-enhancing effects, three experiments were conducted. In Experiment 1, GDX βERKO and age-matched wildtype (WT) mice were administered sesame oil vehicle or T metabolites that bind to ERβ (3β-diol- Handa et al., 2008), GBRs (androsterone- McMahon and France, 2003) or both (3α-diol- Edinger and Frye, 2007). Anxiety behavior was assessed using the open field, elevated plus maze, and light–dark transition task, and cognition was measured using the object recognition task. In Experiment 2, the effects of these androgen treatments were assessed in GDX rats in the same battery of tests as were the mice in Experiment 1. Finally, Experiment 3 was designed to elucidate whether some of the anxiolytic effects that androsterone exerts are via GBRs, ERs, and/or androgen receptors (ARs). As such, GDX rats were administered either a GBR (flumazenil; Hoffman and Warren, 1993), ER (tamoxifen; MacGregor and Jordan, 1998), or AR (flutamide; Simard et al., 1986) antagonist in conjunction with an injection of androsterone or sesame oil vehicle, and then tested in the elevated plus maze paradigm. We hypothesized that, if ERβ is a substrate for androgens’ beneficial effects on affective and/or cognitive behavior, then administration of 3α-diol or 3β-diol, but not androsterone, will reduce anxiety-like behavior, in the open field, elevated plus maze, and light–dark transition, and improve cognitive performance in the object recognition task of GDX rats and WT, but not βERKO, mice. Furthermore, if androsterone does not have some anxiolytic effects, then it will have limited task-specific effects on reducing anxiety-like behavior.
Materials and methods
These methods were pre-approved by the Institutional Animal Care and Use Committee at the University at Albany-SUNY.
Animals and housing
Experiment 1—Mouse strain and genotyping
Male mice (N=167), approximately 55 days of age, were either WT (n=51) or βERKO (n=116), derived from breeder pairs purchased from Jackson Laboratories (Bar Harbor, ME) and bred onto a C57BL/6 background. Mice were group-housed (3–5 per cage) in a temperature-controlled room (21±1 °C) in the Laboratory Animal Care Facility. Mice were maintained on a 12/12-hour reversed light cycle (lights off at 8:00 am) with continuous access to Purina Rodent Chow and tap water in their home cages.
Genotype of mice cannot be determined based on phenotypic characteristics. As such, genomic DNA was isolated from tails and analyzed by PCR in our laboratory and/or the Molecular Core Facility at SUNY Albany to determine genotype of our experimental mice. A brief description of the PCR procedure utilized is as follows. DNA was denatured at 94 °C for 3 min, followed by 35 cycles of amplification: 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s and a final primer extension step at 72 °C for 2 min. Specific primers, obtained from Integrated DNA Technologies (Coralville, IL), used: ESR2-1, which lies upstream of insertion site in exon 2 (5′-GTTGTGCCAGCCCTGTTACT-3′), ESR2-1, which lies downstream of the insertion site in exon 2 (5′-TCACAGGACCAGACACCGTA-3′), and ESR2-3, a neo gene-specific primer (5′-GCAGCCTCTGTTCCACATACAC-3′). Bands of approximately 106 and 160 base pairs were amplified for WT and βERKO mice, respectively (Walf et al., 2008).
Experiments 2 and 3—Rats
Subjects (N=103; N=75) were male Long–Evans rats, approximately 55 days old, obtained from our in-house breeding colony (original stock from Taconic Farms, Germantown, NY). Rats were group-housed (3–4 per cage) in polycarbonate cages (45×24×21 cm) in the Laboratory Animal Care Facility of The Life Sciences Research Building at The University at Albany-SUNY in a controlled temperature room (21±1 °C) that was maintained on a 12:12 reverse light cycle (lights off at 8:00 am). Rats had continuous access to Purina Rodent Chow and tap water in their home cages.
Surgery
Young adult mice were GDX under sodium pentobarbital (80 mg/kg or to effect; Fort Dodge Animal Health, Fort Dodge, IA) at least 3–6weeks before behavioral testing. Young adult rats were GDX under xylazine (12 mg/kg; Bayer Corp., Shawnee Mission, KS) and ketamine (60 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia at least 3–6weeks before behavioral testing.
Screening procedure in mice
Prior to inclusion in the study, each mouse was examined for general health and normative responses to external stimuli (as described in Crawley et al., 2007). Observers who were blind to the genotypes of mice performed the behavioral evaluations, which included: the general appearance of mice (clean fur, whiskers, posture, gait, muscle tone), normative behavior (i.e. fur grooming, nestbuilding with Nestlet cotton squares provided in home cage, huddling with cage mates, ability to cage climb, paw withdrawal when gently pulled), and reflexes (blinking to cotton swab placed close to eyes, ear twitch when cotton swab is gently placed on ear). All mice were included in the study as no differences were noted between theWT or βERKO mice for these measures.
Handling procedure in mice
Mice underwent a 5-day habituation/handling protocol, as per published methods (Frye et al., 2006), to accustom mice to steroid injections, handling by the investigator, and behavioral testing. Briefly, in the following order, mice were exposed to: handling, cage transfer, placement in a scale, travel on a cart in while in their homecages, subcutaneous (SC) injections with 0.2 cm3 vegetable oil SC, and placement in novel environment for 5 min.
Androgen administration
For Experiments 1 and 2, mice and rats were randomly-assigned to androgens with high affinity for GBRs (3α-diol or androsterone), androgens with high affinity for ERβ (3α-diol or 3β-diol) or vehicle. Rats and mice were administer sesame oil vehicle, 3α-diol, 3β-diol or androsterone acutely through SC injections (1 mg/kg) 1 h before testing in the anxiety tasks, and immediately following training in object recognition. These regimens have been used previously to produce physiological androgen levels (Edinger and Frye, 2004a; Fernandez-Guasti and Martinez-Mota, 2007). Crystalline androgens were obtained from Sigma (St. Louis, MO) and dissolved in sesame oil vehicle. For Experiment 3, animals were randomly- assigned to receive either androsterone or vehicle SC, in conjunction with an injection of intraperitoneal flumazenil (GBR antagonist; 15 mg/kg in 0.2% methyl-cellulose), flutamide SC (AR antagonist; 50 mg/kg in oil and 5% ethanol), tamoxifen SC (ER antagonist; 15 mg/kg; in oil), or vehicle SC. Antagonists were obtained from Sigma (St. Louis, MO). Antagonist injections were given prior to androsterone/vehicle injections, 1 h before testing.
Procedure
Mice were tested once per week to assess behavior in the tasks described below. The order in which mice were tested through behavioral tasks was the same for all animals and was as indicated below. Rats were tested once in a single behavioral task (described below). Behavioral data were collected by trained observers and simultaneously video-recorded with a video-camera and/or videotracking system (Any-maze — Stoelting, Inc., Wood Dale, IL).
Behavioral testing
Open field—mice
Motor behavior of mice was assessed in a 39×39×30 cm activity monitor (AccuScan Instruments, Inc., Columbus, OH), as per previous methods (Frye and Walf, 2004). The activity chamber had a grid floor with a total of 16 equal squares delineated. An observer recorded the number of entries made by the mouse into the 12 peripheral or 4 central squares for 5 min, the number of interruptions in light beams in a horizontal plane were automatically recorded. Increased central entries are indicative of reduced anxiety-like behavior (Frye and Walf, 2004).
Open field—rats
The open field (76×57×35 cm) has a 48-square grid floor and was situated in a brightly-lit room. As per previously published methods, the number of central and peripheral squares (which were summed for total) that each rat entered during the 5 min test were recorded (Frye et al., 2000). Total entries made are an index of general motor behavior and an increased number of central entries is an index of anxiety.
Elevated plus maze—mice
The matte black stainless steel elevated plus maze (Columbus Instruments, Inc., Columbus, OH) has 2 open arms, which are 30 cm in length and 5 cm in width, and 2 closed arms, which are the same size but enclosed by 14.5 cm high walls. The arms of the maze are elevated 47.5 cm off the ground. Micewere placed in the center of the maze and the duration of open and closed arm entries (max latency=300 s) was measured. The time spent in the 2 open (5×40 cm) or closed (5×40×20 cm) arms was recorded for 5 min. Increased time spent on the open arms indicates decreased anxiety and the total arm entries made is an index of general motor behavior (Frye and Edinger, 2004, 2006; Walf and Frye, 2007b).
Elevated plus maze—rats
The elevated plus maze was situated in a brightly-lit room and consisted of four arms (2 open without walls and 2 enclosed by 30 cm high walls) 49 cm long and 10 cmwide, elevated 50 cm off the ground. Rats were placed at the junction of the open and closed arms and the number of entries and time spent on the open and closed arms were recorded (as per Frye et al., 2000; Walf and Frye 2007b). Total armentries made in the plus maze are an index of general motor behavior and an increase in time spent on the open arms indicates decreased anxiety.
Light–dark transition task—mice
Mice are placed in the dark side of the chamber (24.5 cm×23.5 cm×35 cm) and allowed to move between the two chambers. Time spent in the light side of the box was recorded for 5 min (Frye et al., 2006).
Light–dark transition task—rats
Rats were placed on the side of a two-chambered box (30×40×40 cm) with white walls and floor and illuminated by a 40-watt light from above; the other side of the box was painted black and had a lid so it was not illuminated. The time spent on the light side of this chamber during 5 min compared to the dark side was recorded (Walf and Frye, 2005). Increased time in the light side is indicative of decreased anxiety.
Object recognition—mice
The object recognition task, a test of non-spatial reference memory, was conducted in an open field box (46×57×30 cm), constructed of white laminate and located in a quiet room with bright lighting. Small, plastic, washable toys that have distinct circular (oranges, lemons, apples) or cone-like shapes (buoys, bottles) were utilized as target stimuli. A digital camera mounted on the ceiling above the testing chamber and connected to a computer with a videotracking system, objectively monitored and quantified animals’ movements (Any-maze — Stoelting, Inc., Wood Dale, IL). Testing, which takes advantage of the natural affinity of rodents for novelty, was conducted as described previously (Walf et al., 2008; Frye and Walf, 2008). Briefly, during training, each mouse was placed in the open field box for 180 s and allowed to explore two identical objects (placed approximately 15 cm from the northeast and northwest walls). After 180 s, each mouse was removed from the box, immediately injected with vehicle or androgens and returned to its individual holding cage. One familiar object (identical to that which was used in training) and one novel object were placed in the same location of the box as was used during training. Whether the novel object was placed in the northeast or northwest location was counterbalanced within and between groups. The time spent exploring each object was recorded during both the training and testing phases using Any-maze video-tracking system. Object exploration was scored when the mouse was sniffing, or touching the object and was a body’s length or less away from the object (i.e. less than 3 cm) while facing the object and/or oriented towards it. All mice investigated both objects during training for a similar duration. Four hours later, the time mice spent exploring the objects were recorded during testing. An increased percentage of time spent exploring the displaced object compared to the total amount of time spent exploring both objects during testing (duration spent with novel object / (duration spent with novel object+duration spent with familiar object)×100) was considered an index of enhanced performance in this task. Inclusion criteria are that animals had to investigate the objects. This was operationalized using a criteria setup in the Anymaze behavioral tracking program for when animals investigate both objects for greater than 0.5 s during the training period. Chance levels of performance are when mice spend 50% of the total exploration time investigating each object.
Object recognition—rats
The object recognition task was done as described above in mice and previously in rats (Walf et al., 2006). This taskwas conducted in an open field box (76×57×35 cm), constructed of white laminate and located in a quiet room with bright lighting. The same target stimuli and methods of data collectionwere utilized. Each ratwas placed in the open field box for 180 s and allowed to explore two identical objects (placed approximately 15 cm from the northeast and northwestwalls). After 180 s, each rat was removed from the box, immediately injected with vehicle or androgens and returned to its individual holding cage. One familiar object (identical to that which was used in training) and one novel object were placed in the same location of the box as was used during training. Whether the novel object was placed in the northeast or northwest location was counterbalanced within and between groups. The time spent exploring each object was recorded during both the training and testing phases using Any-maze videotracking system. Object exploration was scored when the rat was sniffing, or touching the object and was a body’s length or less away from the object while facing the object and/or oriented towards it. All rats investigated both objects during training. Four hours later, the time rats spent exploring the objects were recorded during testing.
Statistical analyses
For Experiment 1, two-way analyses of variance (ANOVAs) tests, with Fisher’s post hoc tests, as appropriate, were utilized to determine effects of genotype (WT vs. βERKO) and androgen condition (vehicle, 3α-diol, 3β-diol, or androsterone) on dependent measures. For Experiment Two, one-way ANOVAs, with Fisher’s post hoc tests, as appropriate, were used to evaluate the effects of androgen condition in a given task. Two-way ANOVAs, with Fisher’s post hoc tests, were used to evaluate effects of androgen condition (vehicle, 3α-diol, 3β-diol, or androsterone) by antagonist (flumazenil, flutamide, tamoxifen, or vehicle; androsterone or vehicle) on behavioral measures. The α level for statistical significance was a p-value of <0.05 and a trend was considered when p<0.10.
Results
Experiment 1
Open field
There was a main effect of genotype for central entries (F3,159=3.17, p<0.05) due to 3α-diol or 3β-diol increasing the number of central entries into the open field of WT, but not βERKO, mice (Fig. 1). The main effects of genotype (F1,159=40.93, p<0.01) was due to WT mice making more total entries than βERKO mice (Table 1).
Fig. 1.
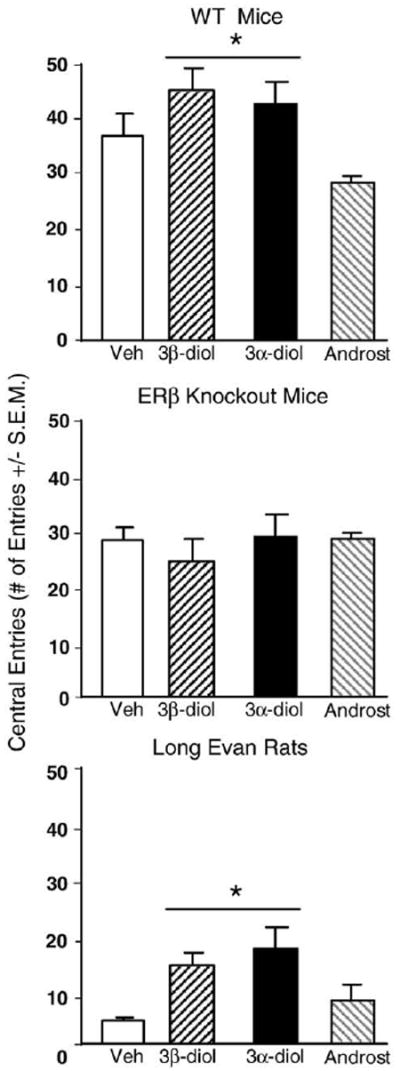
Represents mean central entries in the open field for: wildtype (WT) mice (top) administered acute vehicle control (white bar; n=15), 3β-diol (horizontally-striped bar; n=11), 3α-diol (black bar; n=13), or androsterone (androst; gray-stripe bar; n=12); to ERβ receptor knockout (βERKO) mice (middle) administered acute vehicle control (white bar; n=21), 3β-diol (horizontally-striped bar; n=34), 3α-diol (black bar; n=34), or androsterone (androst; gray-stripe bar; n=29); and to rats (bottom) administered acute vehicle control (white bar; n=22), 3β-diol (horizontally-striped bar; n=26), 3α-diol (black bar; n=25), or androsterone (androst; gray-stripe bar; n=25). Rats and WT mice administered 3α-diol or 3β-diol had significantly more central entries than animals administered androsterone or vehicle. * Denotes significant difference (p<.05).
Table 1.
Mice: Behavior (mean±sem) of vehicle-administered WT (n=15) or estrogen receptor β knockout (βERKO; n=21) mice, 3β-diol-administered WT (n=11) or βERKO (n=34) mice, 3α-diol-administered WT (n=13) or βERKO (n=34) mice or androsterone (Androst) administered WT (n=12) or βERKO (n=29)
| Condition | OF-total entries | EPM-total open arm entries | EPM-% open arm time | EPM-% open arm entries | |
|---|---|---|---|---|---|
| Wildtype mice* | Vehicle | 94.7±6.8 | 3.2±0.5 | 1.0± 0.4 | 6.9±2.5 |
| 3β-diol | 112.3±11.2 | 3.9±0.4 | 8.0±2.5** | 26.6±4.1** | |
| 3α-diol | 107.4±6.4 | 5.0±0.5 | 5.1±1.2** | 21.3±3.6** | |
| Androsterone | 102.8±8.4 | 4.2±0.6 | 5.4±1.5** | 20.1±3.3** | |
| βERKO mice | Vehicle | 146.9±8.8 | 7.7±1.2 | 2.1±0.4 | 13.5±2.4 |
| 3β-diol | 162.4±4.6 | 9.6±1.4 | 2.3±0.6 | 11.1±1.9 | |
| 3α-diol | 164.5±10.5 | 10±1.3 | 2.5±0.8 | 10.1±2.4 | |
| Androsterone | 131.6±4.1 | 10.8±1.2 | 4.8±0.8 | 15.9±2.1 | |
| Rats | Vehicle | 119.9±4.9 | 7.7±0.8 | 0.4±0.2 | 5.1±2.7 |
| 3β-diol | 118.1 ±3.9 | 9.6±1.4 | 4.2±1.4** | 17.4±4.1** | |
| 3α-diol | 121.9±5.9 | 8.1±0.6 | 3.2±0.8** | 16.9±3.3** | |
| Androsterone | 126.6±4.9 | 7.8±0.5 | 4.5±1.1** | 20.1±3.3** |
Rats: behavior (mean±sem) of vehicle administered (n=22), 3β-diol-administered (n=26), 3α-diol-administered (n=25) or Androst administered (n=25).
Denotes significance <0.05 compared to βERKO knockout mice.
Denotes significance <0.05 compared to vehicle administration.
Elevated plus maze
Androgen condition and genotype interacted (F3,159=4.09, p<0.01) insofar as 3α-diol or 3β-diol to WT, but not βERKO, mice increased the duration of time (s) spent on the open arms compared to vehicle administration. However, androsterone increased the time spent on the open arms of the elevated plus maze among bothWT and βERKOs (Fig. 2) compared to vehicle.
Fig. 2.
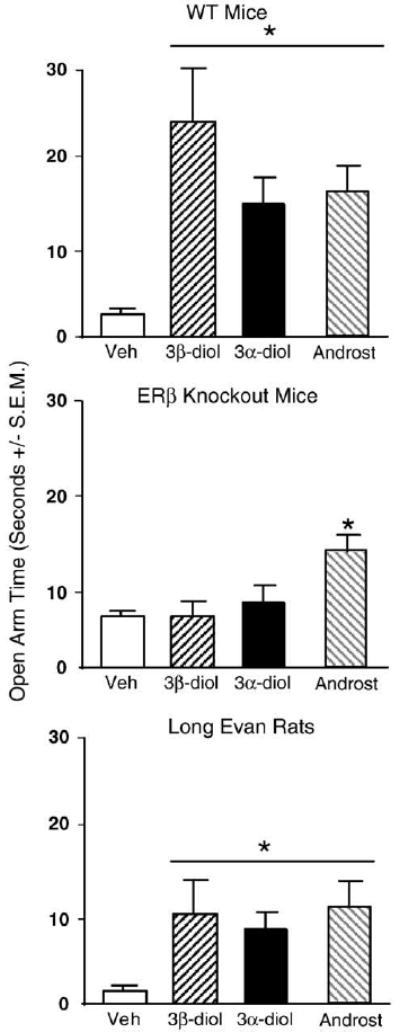
Represents mean time spent on the open arm in the elevated plus maze for: wildtype (WT) mice (top) administered acute vehicle control (white bar; n=15), 3β-diol (horizontally-striped bar; n=11), 3α-diol (black bar; n=13), or androsterone (androst; gray-stripe bar; n=12); to ERβ receptor knockout (βERKO) mice (middle) administered acute vehicle control (white bar; n=21), 3β-diol (horizontally-striped bar; n=34), 3α-diol (black bar; n=34), or androsterone (androst; gray-stripe bar; n=29); and to rats (bottom) administered acute vehicle control (white bar; n=22), 3β-diol (horizontally-striped bar; n=26), 3α-diol (black bar; n=25), or androsterone (androst; gray-stripe bar; n=25). Rats administered 3α-diol, 3β-diol, or androsterone spent significantly more time on the open arm than those given vehicle. * Denotes significant difference (p<0.05).
There was a main effect of genotype (F1,159=6.49, p<0.05; Table 1), but not androgen condition, for the number of total entries in the elevated plus maze. WT mice made more total entries than did βERKO mice. Additionally, 3α-diol, 3β-diol, or androsterone treatment, compared to vehicle, significantly increased the percent of open entries (Table 1) made by the WT, but not βERKO animals (F1,159=5.560, p<0.01). A similar pattern was observed for percent open arm time (Table 1), where WT animals who received 3β-diol or 3α-diol spent a significantly higher percentage of their time on the open arm (F1,159=4.095, p<0.01).
Light dark transition-mice
Androgen condition and genotype interacted (F3,159=3.83, p=0.01), such that WT, but not βERKO, mice administered 3α-diol or 3β-diol spent a significantly longer duration of time (in seconds) in the white chamber compared to mice administered vehicle (Fig. 3).
Fig. 3.
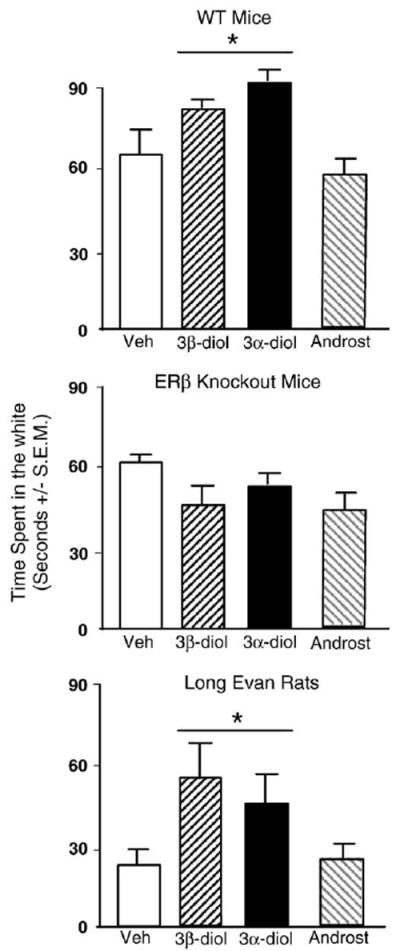
Represents mean time spent on the light side in the light–dark task for: wildtype (WT) mice (top) administered acute vehicle control (white bar; n=15), 3β-diol (horizontally-striped bar; n=11), 3α-diol (black bar; n=13), or androsterone (androst; gray-stripe bar; n=12); and to ERβ receptor knockout (βERKO) mice (middle) administered acute vehicle control (white bar; n=21), 3β-diol (horizontally-striped bar; n=34), 3α-diol (black bar; n=34), or androsterone (androst; gray-stripe bar; n=29); and to rats (bottom) administered vehicle control (white bar; n=22), 3β-diol (horizontally-striped bar; n=26), 3α-diol (black bar; n=25), or androsterone (androst; gray-stripe bar; n=25). Rats andWT, but not βERKO, mice administered 3α-diol or 3β-diol displayed significantly less anxiety-like behavior than those that received vehicle or androsterone. * denotes significant difference (p<0.05).
Object recognition
Androgen and genotype interacted (F3,120=3.07, p<0.05), where WT, but not βERKO mice administered 3α-diol or 3β-diol compared to vehicle or androsterone, had an increased percentage of time spent with the novel object (Fig. 4).
Fig. 4.
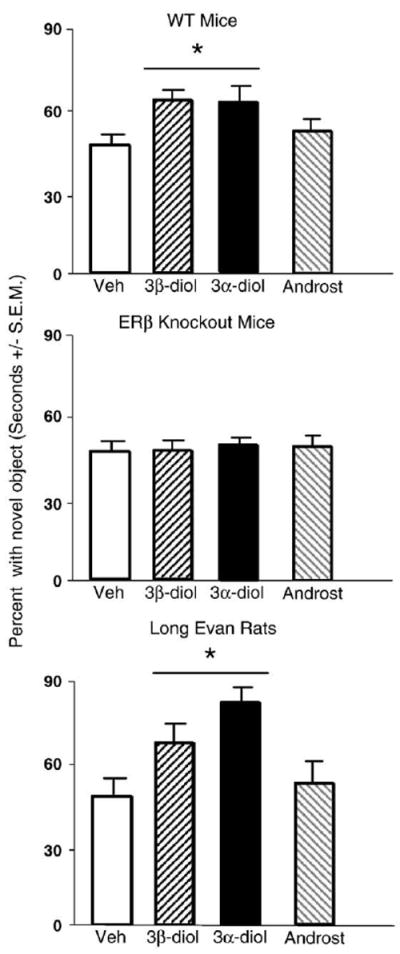
Represents mean percentage of time with novel object for: wildtype (WT) mice (top) administered acute vehicle control (white bar; n=15), 3β-diol (horizontally-striped bar; n=11), 3α-diol (black bar; n=13), or androsterone (androst; gray-stripe bar; n=12); to ERβ receptor knockout (βERKO) mice (middle) administered acute vehicle control (white bar; n=21), 3β-diol (horizontally-striped bar; n=34), 3α-diol (black bar; n=34), or androsterone (androst; gray-stripe bar; n=29); and to rats (bottom) administered vehicle control (white bar; n=22), 3β-diol (horizontally-striped bar; n=26), 3α-diol (black bar; n=25), or androsterone (androst; gray-stripe bar; n=25). 3α-diol and 3β-diol, but not vehicle or androsterone, significantly increased the percentage of time rats and WT, but not βERKO, mice spent exploring the novel object. * Denotes significant difference (p<0.05).
Experiment 2
Open field
There was an increase in central entries in the open field (Fig. 1, bottom) for rats administered 3α-diol or 3β-diol (F3,99=5.82, p<0.01) compared to those administered vehicle or androsterone. There was no significant effect of androgen administration for the number of total entries in the open field.
Elevated plus maze
Administration of 3α-diol, 3β-diol, or androsterone significantly increased amount of time spent on the open arms of the elevated plus maze (F3,94=3.00, p<0.05; Fig. 2, bottom) compared to vehicle administration. There was no effect of androgen treatment on total open arm entries compared to that of vehicle administration. Additionally, compared to rats administered vehicle, rats administered 3β-diol, 3α-diol, or androsterone made significantly greater percentage of entries (Table 1) onto the open arms (F3,93=3.501, p<0.05), as well as spent a significantly greater percentage of time in the elevated plus maze (Table 1) out on the open arm (F3,93=3.243, p<0.05).
Light dark transition-rats
A significant inxcrease in time spent in the light chamber of the light dark transition box was observed in rats administered 3α-diol or 3β-diol compared to androsterone or vehicle (F3,100=2.58, p=0.05; Fig. 3, bottom).
Object recognition
Administration of 3α-diol or 3β-diol to rats increased percentage of time spent investigating the novel object in the object recognition task (F3,105=3.3, p<0.05; Fig. 4, bottom), compared to androsterone or vehicle administration.
Experiment 3
Elevated plus maze
There was a main effect for androsterone treatment, such that rats administered androsterone, compared to vehicle, had increased open arm time (F1,59=7.005, p<0.01; Fig. 5), number of open arm entries (F1,59=8.332, p<0.01), percentage of time in the plus maze on the open arms (F1,59=6.834, p<0.05; Fig. 5), but not for percentage of open arm entries (Fig. 5). Additionally, in open arm entries, there was a main effect for number of open arm entries (F3,59=3.183, p<0.05) where flumazenil administration resulted in significantly fewer open arm entries (1.0+0.3) compared to flutamide administration (1.9+0.5).
Fig. 5.
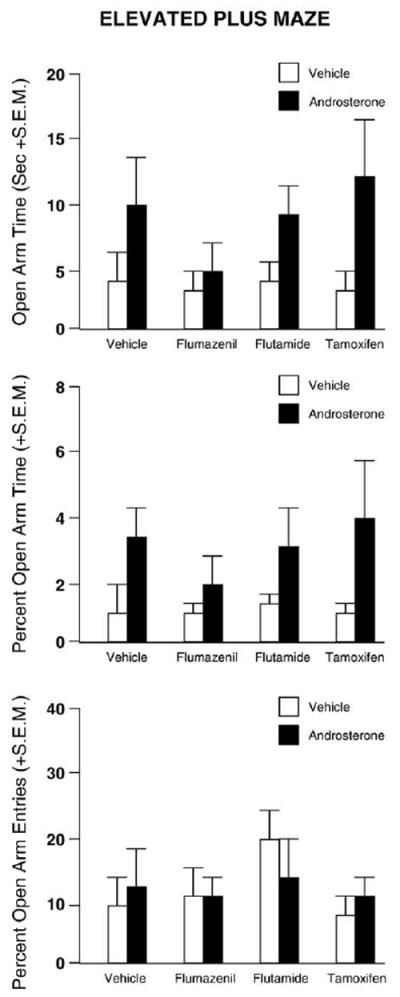
Represents mean time spent out on the open arm, percentage of time on the open arm, and percentage of open arm entries for the elevated plus maze for acute coadministration of vehicle (white bars) or androsterone (black bars), with flumazenil (vehicle, n=10; androsterone n=7), flutamide (vehicle, n=10; androsterone, n=10), tamoxifen (vehicle, n=10; androsterone, n=9), or vehicle (vehicle, n=9; androsterone, n=10) in the elevated plus maze task. There was a significant main effect for animals administered androsterone spending significantly more time (top) and a greater percentage of time (middle) on the open arm than vehicle treated animals. Therewas no effect for percentage of open arm entries (bottom). * Denotes significant difference (p<0.05).
Discussion
The findings supported the hypothesis that actions of androgens at ERβ may underlie their cognitive-enhancing and anxiolytic effects. Mice deficient in ERβ did not demonstrate the reductions in anxietylike behavior in the elevated plus maze, open field, or light–dark transition; nor did they benefit from the cognitive enhancements observed in the WT animals that were administered 3α-diol or 3β- diol. Among GDX rats, 3α-diol and 3β-diol, which can bind to ERβ with high affinity, significantly decreased anxiety in the elevated plus maze, open field, and light–dark transition tasks compared to vehicle administration. 3α-diol and 3β-diol significantly increased performance in the object recognition task over administration of vehicle and androsterone, which does not bind to ERβ. Experiment 3 demonstrated that androsterone can have anxiolytic effects in the elevated plus maze. Despite the addition of antagonists for ARs, ERs, and GBRs, there was a main effect for androsterone administration in decreasing anxiety-like behavior. However, flumazenil did significantly block this effect for open arm entries. Thus, these data suggest that one potential target of androgen metabolites may be ERβ for improved performance in the open field, elevated plus maze, light– dark transition task, and object recognition task.
Our findings are congruent with past research showing that androgens have cognitive-enhancing effects. GDX can produce cognitive deficits in rodents, which can be reversed with T-replacement (Frye and Seliga, 2001), or replacement of its metabolites (Edinger and Frye, 2004a). Given that different metabolites bind with varying affinity to different substrates to produce these effects, it is possible that androgens’ cognitive-enhancing effects work through a combination of actions of its metabolites at ARs, GBRs, and/or ERβ (Edinger and Frye, 2007). However, the cumulative results obtained from experiments one and two implicate actions at ERβ as having more robust effects on cognition, which is supported by additional research that utilized ER AS-ODNs for both ERβ and ERα. When administered directly into the hippocampus, along with 3α-diol, ASODNs for ERβ and not ERα decreased cognitive performance in GDX rats (Edinger and Frye, 2007). The present findings extend this previous data by illustrating that administration of 3β-diol alone, which cannot be back-converted to T or aromatized to E2, is effective at enhancing cognitive performance. In addition, administration of androgens to WT, but not βERKO mice, was effective at enhancing cognitive performance. Together, these findings show that actions at ERβ are, in part, responsible for mediating androgen’s cognitive-enhancing effects.
The present findings are consistent with past research illustrating the importance of T and its metabolites in mediating anxiolysis in rodents. GDX increases anxiety of male rats, an effect that can be reversed through administration of T (Fernandez-Guasti and Martinez-Mota, 2003; Frye and Seliga, 2001), and of T’s metabolites (Bitran et al., 1993; Edinger and Frye, 2004b). Additionally, studies have found DHT and 3α-diol to be just as effective, if not more so, than sole administration of T, at decreasing anxiety in GDX rats (Edinger and Frye, 2004a; Edinger and Frye, 2004b). Additionally, work by Fernández-Guasti and Martínez-Mota, (2005) demonstrated, that although androsterone does bind to GBRs, it does not always produce strong anxiolytic effects, either chronically or acutely. Experiment 3 demonstrated that androsterone can have anxiolytic effects. Our results are congruent with previous findings from other laboratories demonstrating that androsterone does have modest effects on reducing anxiety-like behavior (Aikey et al., 2002). Despite the addition of antagonists for ARs, ERs, and GBRs, there was amain effect for androsterone to decrease anxiety-like behavior in the elevated plus maze, as defined by increasing open armtime, percent open arm time, and open arm entries. However, flumazenil did block this effect for open arm entries. Similarly, flumazenil did not significantly alter defensive burying time when testosterone propionate, which is capable of being metabolized into androsterone and 3α-diol, was concurrently administered (Fernández-Guasti and Martínez-Mota, 2005). This suggests that, although androsterone can modestly reduce anxiety-like behavior in a task-specificmanner, likely through its activation of GBRs (Fernandez-Guasti and Martinez-Mota, 2005), which are a common target for benzodiazepines prescribed for anxiety (reviewed in Nemeroff, 2003), these effects may not be as robust as those produced via ERβ’s activation, and may require more chronic administration to produce a consistent significant anxiolytic effect (Aikey et al., 2002). The current data extend these previous findings by implicating ERβ as a potential site for androgens’ anxiolytic effects. Administration of 3β-diol, which cannot be backconverted to T, or be aromatized into E2, resulted in decreased anxiety-like effects similar to those seen when T is administered alone. In addition, administration of androgens to βERKO mice, which lack functional ERβ, was not effective at reducing anxiety-like behavior. Together, this evidence suggests that the 5α-reduced metabolites of T mediate anxiety-like behavior via ERβ.
It is important to consider actions at intra-cellular receptors (ARs and ERs) versus rapid actions at membrane receptors (GBRs) when considering these results. T has been known to act via both classical and non-classical signaling mechanisms (Walker and Cheng, 2005). Both T and DHT have a high affinity for ARs (Roselli et al., 1987). When ARs are blocked by administration of an AR antagonist, flutamide, the beneficial effects of DHT are not seen (Edinger and Frye, 2006). However, unlike T and DHT, 3α-diol and 3β-diol do not bind readily to ARs (Roselli et al., 1987 Although it has long been thought that androgens non-classical membrane actions occurred through aromatization to E2, current research suggests that T and/or its metabolites may act directly at membrane receptors. For example, 3α-diol has a high affinity for GBRs (Handa et al., 2008; Pak et al., 2005), and actions at GBRs can decrease anxiety-like behavior (reviewed in Nemeroff, 2003). However, in the current experiment, administration of 3β-diol, which does not bind to GBRs, and instead binds with high affinity to ERβ, resulted in decreased anxiety-like behavior, at levels similar to that of animals administered 3α-diol alone. In future studies, it will be important to examine these effects at genomic and non-genomic targets. Until recently, much of the research done to determine that 3β-diol works through ERβ has been done on cell cultures. Little behavioral research using animal models exists that can confirm these results. However, our research findings are analogous to those found using the animal and human derived cell cultures. Using mouse hippocampus-derived neuronal cell lines (HT-22), it has been determined that 3β-diol can bind to ERβ, and not to AR, and that it is this binding that is responsible for direct regulation by androgens in the neuronal cell types that lack ARs (Pak et al., 2005). Similar findings utilizing human neuroblastoma-derived cell line SK-N-SH demonstrated that DHT and 3β-diol can stimulate arginine vasopressin (AVP) through ERβ. AVP is a neuropeptide that can assist in the regulation of stress and anxiety (Pak et al., 2007). Evidence still exists that supports our findings on the importance of 3α-diol, and 3β-diol binding to, and exerting their effects through ERβ, and not an AR. Despite these caveats, past findings support our current results demonstrating that 3β-diol can reduce anxiety-like behavior and increase cognitive performance.
In conclusion, these results suggest that T’s anxiolytic and cognitive-enhancing effects may be mediated largely through its metabolites, 3α-diol and 3β-diol, and may require actions at ERβ. This is supported by findings that GDX rats or WT mice administered 3α- diol and 3β-diol exhibited improved anxiety and cognition. We also found that, in mice lacking functional ERβ, there were no affective or cognitive improvements with administration of 3α-diol or 3β-diol. Determining the precise route and mechanisms in which testosterone and/or its metabolites exert their beneficial effects have potential implications in regards to the ever increasing incidence of prostate cancer, proportion of aging individuals, and the continued use of Tenhancing steroids within the young and aging population.
Acknowledgments
This research was supported, in part, by grants from the Karo Bio Research Foundation, National Science Foundation (IBN03-16083), and National Institute of Mental Health (MH0676980), as well as an intramural faculty research award grant to CAF. Technical assistance provided by Fabiola Estrada, Mary Beth Gillepsie, Mary Unger, and Jari Willing is greatly appreciated.
References
- Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Horm Behav. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. Androgen-behavior correlations in hypogonadal men and eugonadal men. II. Cognitive abilities. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellog CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Adler GH, Sharma M, Hochberg RB, MacLusky NJ. Androgen treatment decreases estrogen receptor binding in the ventromedial nucleus of the rat brain a quantitative in vitro autoradiographic analysis. Mol Cell Neurosci. 1994;5:549–555. doi: 10.1006/mcne.1994.1067. [DOI] [PubMed] [Google Scholar]
- Ceccarelli I, Scaramuzzino A, Aloisi AM. Effects of gonadal hormones and persistent pain on non-spatial working memory in male and female rats. Behav Brain Res. 2001;123(1):65–76. doi: 10.1016/s0166-4328(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Delhez M, Hansenne M, Legros JJ. Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinology. 2003;28:863–874. doi: 10.1016/s0306-4530(02)00102-6. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5:1–23. [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Behav Neurosci. 2004a:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2004b;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5α-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav. 2006;50:216–222. doi: 10.1016/j.yhbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Androgens effects to enhance learning and memory maybe mediated in part by actions at estrogen receptor-β in the hippocampus. Neurobiol Learn Mem. 2007;87:78–85. doi: 10.1016/j.nlm.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Orchidectomy sensitizes male rats to the action of diazepam on burying behavior latency: role of testosterone. Pharmacol Biochem Behav. 2003;75:473–479. doi: 10.1016/s0091-3057(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30(8):762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti, Martinez-Mota Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2007;30:762–770. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Frye CA, Van Keuren KR, Rao PN, Erskine MS. Progesterone and 3α-androstanediol conjugated to bovine serum albumin affects estrous behavior when applied to the MBH and POA. Behav Neurosci. 1996;110:603–612. doi: 10.1037//0735-7044.110.3.603. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α, 5α-THP. Pharmocol Biochem Behav. 2000;67:587–597. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci. 2001;1:172–182. doi: 10.3758/cabn.1.2.172. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3α-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:731–750. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone’s metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78(3):473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone systemically or to the amygdala can have anxiety, fear, and pain reducing effects in ovariectomized rats. Behav Neurosci. 2004;118:306–313. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, Pfaff DW, Rhodes ME. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006;186(3):312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2007;33:1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW. Steroid modulation of the GABA/benzodiazepine receptor-linked chloride ionophore. Mol Neurobiol. 1988;2:291–317. doi: 10.1007/BF02935636. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EJ, Warren EW. Flumazenil: a benzodiazepine antagonist. Clin Pharm. 1993;12:699–701. [PubMed] [Google Scholar]
- Howell S, Shalet S. Testosterone deficiency and replacement. Horm Res. 2001;56:86–92. doi: 10.1159/000048142. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Kaminetsky JC. Benefits of a new testosterone gel formulation for hypogonadal men. Clin Cornerstone. 2005;7:8–12. doi: 10.1016/s1098-3597(05)80091-2. [DOI] [PubMed] [Google Scholar]
- Li JY, Zhu JC, Dou JT, Bai WJ, Deng SM, Li M, Huang W, Jin H. Effects of androgen supplementation therapy on partial androgen deficiency in the aging male: a preliminary study. Aging Male. 2002;5(1):47–51. [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5α-Dihydrotestosterone and it’s metabolite 5α-androstan-3β,17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2005;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttge WG. Endocrine control of mammalian male sexual behavior: an analysis of the potential role of testosterone metabolites. In: Beyer C, editor. Comprehensive Endocrinology Endocrine Control of Sexual Behavior. Raven Press; New York: 1979. pp. 341–364. [Google Scholar]
- MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakie PS. Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 1987;50:459–474. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Masonis AE, McCarthy MP. Direct effects of the anabolic/androgenic steroids, stanozolol and 17α-methyltestosterone, on benzodiazepine binding to the gamma-aminobutyric acid(a) receptor. Neurosci Lett. 1995;189:35–38. doi: 10.1016/0304-3940(95)11445-3. [DOI] [PubMed] [Google Scholar]
- MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- McMahon LR, France CP. Discriminative stimulus effects of positive GABAA modulators and other anxiolytics, sedatives, and anticonvulsants in untreated and diazepam-treated monkeys. J Pharmacol Exp Ther. 2003;304:109–120. doi: 10.1124/jpet.102.040931. [DOI] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol Bull. 2003;37:133–146. [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5α-androstane-3β, 17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WCJ, Hinds LR, Handa RJ. Estrogen Receptor-β mediates dihydrotestosterone-induced stimulation of arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148:3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in mice. Proc Natl Acad Sci U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Ortiz YA, Rundle-Gonzalez V, Rivera-Ramos I, Jorge JC. Modulation of elevated plus maze behavior after chronic exposure to the anabolic steroid 17α-methyltestosterone in adult mice. Horm Behav. 2006;49:123–128. doi: 10.1016/j.yhbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Time-course and steroid specificity of aromatase induction in rat hypothalamus-preoptic area. Biol Reprod. 1987;37:628–633. doi: 10.1095/biolreprod37.3.628. [DOI] [PubMed] [Google Scholar]
- Simard J, Lythy I, Guay J, Belanger A, Labrie F. Characteristics of interaction of the antiandrogen flutamide with the androgen receptor in various target tissues. Mol Cell Endocrinol. 1986;44:261–270. doi: 10.1016/0303-7207(86)90132-2. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30(9):1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav. 2007a;86:407–414. doi: 10.1016/j.pbb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007b;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Rapid and estrogen receptor beta mediated actions in the hippocampus mediate some functional effects of estrogen. Steroids. 2008;73:997–1007. doi: 10.1016/j.steroids.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130(1):15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, DeZhou S, Su BY. Distribution and differences of estrogen receptor β immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- Zitzmann M. Testosterone and the brain. Aging Male. 2006;9:195–199. doi: 10.1080/13685530601040679. [DOI] [PubMed] [Google Scholar]


