Abstract
Lactation is associated with an increased demand for calcium and is accompanied by a remarkable cycle of bone loss and recovery that helps to supply calcium and phosphorus for milk production. Bone loss is the result of increased bone resorption that is due, in part, to increased levels of PTHrP and decreased levels of estrogen. However, the regulation of bone turnover during this time is not fully understood. In the 1960’s and 1970’s many observations were made to suggest that osteocytes could resorb bone and increase the size of their lacunae. This concept became know as osteocytic osteolysis and studies suggested that it occurred in response to parathyroid hormone and/or an increased systemic demand for calcium. However, this concept fell out of favor in the late 1970’s when it was established that osteoclasts were the principal bone-resorbing cells. Given that lactation is associated with increased PTHrP levels and negative calcium balance, we recently examined whether osteocytes contribute to bone loss during this time. Our findings suggest that osteocytes can remodel their perilacunar and pericanalicular matrix and that they participate in the liberation of skeletal calcium stores during reproductive cycles. These findings raise new questions about the role of osteocytes in coordinating bone and mineral metabolism during lactation as well as the recovery of bone mass after weaning. It is also interesting to consider whether osteocyte lacunar and canalicular remodeling contributes more broadly to the maintenance of skeletal and mineral homeostasis.
Introduction
As detailed in this issue of the Journal, over the last decade, the osteocyte has emerged as an incredibly versatile cell that clearly contributes to the regulation of bone turnover and mineral metabolism (1–5). One of the hallmarks of these cells is the extensive network of dendritic processes that extends from each cell to make contact with other osteocytes, periosteal and endosteal lining cells, and cells in the bone marrow (1). Osteocytes and their dendritic processes reside within a series of interconnected lacunae and canaliculi within the mineralized bone tissue that are in close communication with the vascular space. As a result, the lacunar-canalicular network represents an enormous area of contact between bone mineral and the extracellular fluid (ECF), one larger than the periosteal, endosteal and trabecular surfaces combined (1, 6).
Milk production during lactation requires a great deal of calcium and is associated with rapid skeletal turnover and maternal bone loss (7–9). The enormous surface area of the osteocyte canalicular network potentially makes these cells an ideal site for calcium mobilization from the skeleton in order to meet the demands of milk production. Previous literature from the 1970’s suggested that osteocytes could remove bone mineral directly in response to increased demand for calcium, but this idea fell out of favor in the 1980’s and 1990’s (10–13). Recent evidence suggests that osteocytes can, indeed, directly remove bone during lactation and replace it after milk production ceases (14). The purpose of this review will be to highlight changes in bone metabolism during lactation, to consider the older evidence for “osteocytic osteolysis”, to review the new data supporting osteocyte perilacunar and pericanalicular remodeling during and after lactation, and to consider a potentially wider role for osteocytes in coordinating the skeletal adaptations to lactation.
Lactation is Associated with Reversible Bone Loss
Milk provides all the energy and nutrients, including calcium and phosphorus, required for the rapid skeletal growth that occurs in the neonatal period. Nursing humans secrete between 300 mg to 400 mg of calcium into milk each day (8, 15). The extra demand for calcium for milk production stresses maternal calcium homeostasis and, as a result, lactation is associated with a series of adaptations to calcium and bone metabolism. Suckling induces hyperphagia and prolactin secretion, which stimulates the absorption of calcium by the gastrointestinal tract (16, 17). Therefore, some of the extra calcium comes from the diet (8, 18). During lactation, the kidneys retain calcium, and urinary calcium excretion declines to very low levels (8, 15). Thus, some calcium is reclaimed from the urine. Finally, lactation is associated with significant bone loss and it has been assumed that much of the calcium that is used for milk production comes from the skeleton (8, 15).
Bone loss during lactation is impressive both in its magnitude and its rapidity. Bone mineral density (BMD) declines between 5–8% over 6 months of full time nursing in humans. This decline occurs at an estimated rate of between 1–3% per month, which, by comparison, approximates the yearly rate of BMD decline following menopause (8). The decrease in BMD correlates with the amount of milk produced; women nursing twins and triplets lose more bone than women nursing just one baby (19, 20). Rodents, which typically nurse many more offspring than humans, loose up to 20–30% of their bone mass over 3 weeks of lactation (15, 21). In mice, lactation is associated with changes in bone microarchitecture and bone mineralization as well as with changes in bone density (22, 23).
Bone loss during lactation is associated with increased bone turnover. Biochemical markers of bone resorption have been reported to be 2–3-fold elevated in mice and humans, and markers of bone formation have been demonstrated to be elevated in nursing humans. In rodents, histomorphometry data reveal that osteoclast numbers and activity are increased in lactating as compared to nulliparous females. Likewise, osteoblast numbers and bone formation rates are elevated, although not enough to offset the increased rates of bone resorption resulting in net bone loss (8, 15, 21, 22).
The mechanisms that drive bone loss during lactation are only partly understood. It appears to be precipitated, in part, by suckling-induced, hypothalamic suppression of circulating estrogen levels combined with elevated levels of circulating parathyroid hormone-related protein (PTHrP) secreted from the lactating breast. Suckling stimulates afferent nerves in the breast that connect through the brainstem to inhibit GnRH production (18, 24). Suppression of GnRH secretion, in turn, leads to hypogonadotropic hypogonadism and a decline in circulating levels of estradiol. Estrogen deficiency is well known to cause elevations in bone turnover and bone loss, so it is reasonable to assume that estrogen deficiency contributes to lactational bone loss (25). In fact, human studies have found a correlation between the degree of bone lost and the duration of amenorrhea post-partum (8, 26). Furthermore, estrogen levels correlate with rates of bone resorption and pharmacologic estrogen replacement mitigates bone loss in lactating mice (21). The lactating breast secretes PTHrP both into the systemic circulation and into milk (8, 15, 21, 27). Many studies have now documented elevated circulating PTHrP levels during lactation (8, 15, 21, 27). Plasma levels of PTHrP correlate directly with biochemical markers of bone resorption and inversely with changes in bone mass in lactating mice (21). Sowers and colleagues demonstrated a correlation between bone loss and elevated circulating PTHrP levels in nursing women as well (28). Finally, selective disruption of the PTHrP gene in mammary epithelial cells at the onset of lactation reduced circulating levels of PTHrP, reduced rates of bone turnover and reduced bone loss by 50% in mice (27). While changes in estrogen and PTHrP levels clearly contribute to the effects of lactation, they appear not to be the entire story since experimentally lowering estrogen levels and increasing PTHrP levels in mice does not fully reproduce the degree of bone loss observed during lactation (29). Therefore, other factors must also contribute to the skeletal response to milk production.
A key aspect of the bone loss associated with lactation is its rapid and complete reversibility after weaning (8, 22). In rodents, skeletal mass is almost completely restored 4 weeks after the end of lactation. In humans, bone mineral density is restored to baseline within 6–12 months after nursing has stopped (8, 30–32). In mice and rats, the transition from bone loss to bone accrual at weaning is associated with a rapid decline in osteoclast numbers and activity. Weaning of pups at mid-lactation is associated with a sudden decrease in the RANKL/OPG ratio and a wave of coordinated osteoclast apoptosis (22, 33). However, osteoblast numbers and bone formation rates, which are already elevated during lactation, are maintained or significantly increased after weaning (34, 35). These changes in osteoclast and osteoblast activity result in a period of relatively unopposed bone formation and the rapid restoration of bone mass. Recent detailed micro-CT studies employing sophisticated finite element analyses suggest that bone microarchitecture may not return fully to baseline at all skeletal sites after the first reproductive cycle (23). Nonetheless, the pattern of bone loss and recovery supports multiple rounds of pregnancy and lactation, and repeated nursing does not predict future osteoporosis in women (8, 9).
Osteocytic Osteolysis
It was initially suggested that osteocytes could remove bone over 100 years ago (36, 37), but Baud and colleagues recorded the first detailed morphological studies demonstrating periosteocytic osteolysis in the early 1960’s. These investigators performed ultrastructural studies of bone and demonstrated enlarged osteocyte lacunae with irregular borders, rough walls and varying degrees of perilacunar demineralization (38). The frequency of enlarged lacunae was increased by the administration of PTH and decreased by the administration of calcitonin. Krempian and colleagues also used electron microscopy and reported that the acute or chronic administration of PTH to rats “activated” osteocytes as evidenced by widening of the lacunae with lysis of the lacunar wall as well as an increase in the rough endoplasmic reticulum, the number of lysosomes and cytoskeletal changes within the cells (39). A series of subsequent studies using histological and microradiographic techniques supported the idea that the administration of PTH resulted in the enlargement of osteocyte lacunae (10, 40, 41). In one study, continuous infusion of PTH into rats for 4 weeks, using osmotic mini-pumps, caused osteocyte lacunae in cortical bone to enlarge and induced the expression of acid phosphatase in a subset of osteocytes (41). Enlarged osteocyte lacunae have also been noted in bone biopsies from human patients with primary hyperparathyroidism (42). Therefore, multiple older studies suggested that PTH signaling could induce osteocytic osteolysis.
Osteocyte bone remodeling has also been reported in association with other conditions. Restricting calcium intake has been shown to increase osteocyte lacunar size and enlarged osteocyte lacunae have been reported in patients with renal osteodystrophy. Both of these conditions are associated with secondary hyperparathyroidism (10, 43, 44). Microgravity associated with spaceflight has been reported to cause osteocytic osteolysis in rats and monkeys (45, 46). The enlargement of osteocyte lacunae has been reported in bone near skeletal metastases, which might possibly represent the local effects of PTHrP secreted from the tumor cells (47). Osteocytic osteolysis has been reported in hibernating bats, squirrels and hamsters (48–50). Snakes appear to activate osteocytic bone resorption during winter months and during reproductive cycles (51). As these studies indicate, there is a considerable body of evidence to suggest that increased calcium demand appears to trigger osteocytes to resorb bone.
Fewer studies have examined whether osteocytes can form bone. Baylink first showed direct evidence for periosteocytic mineralization. He treated rats with daily tetracycline for 17 days and found labeling within the osteocyte lacunar and canalicular spaces, suggesting that osteocytes had deposited new bone tissue (52). Osteocyte matrix deposition was also suggested by a study that documented osteopontin deposition within osteocyte lacunae in multiple concentric rings suggesting that osteocytes had undergone a cyclical process of bone formation (53). Finally, Zallone and colleagues studied osteocyte bone deposition in laying hens that had been deprived of dietary calcium and then repleted, which induces avid bone formation (54, 55). Using 3H-proline pulses, histology and tetracycline labeling, these investigators documented that osteocyte lacunae were sites of active matrix deposition and mineralization (54, 55).
Despite the evidence cited above, skepticism persists over the concept that osteocytes can remove and replace bone, and not all studies have found evidence for enlarged osteocyte lacunae. Weisbrode et al did not find conclusive evidence for osteocytic osteolysis in thyroparathyroidectomized rats placed on a low calcium diet and given PTH (56). In addition, Sissons and colleagues failed to document periosteocytic osteolysis in virgin rats fed a low-calcium diet, although they noted that osteocytes newly formed after the commencement of calcium restriction had slightly larger lacunae (57). In addition, some of the original proponents of osteocytic osteolysis also believed in another concept called “bone flow”. This theory suggested that bone formation occurred primarily at bone surfaces, but that bone resorption occurred primarily in the interior as a result of osteocytic osteolysis (10, 58). The idea was that bone tissue flowed from the surface towards the osteocytes. The osteocytic osteolysis/bone flow theory attempted to explain the relatively low numbers of osteoclasts on the bone surface and assumed that these were insufficient to explain normal bone turnover (10, 58). However, in 1977, Parfitt argued that bone flow did not occur and that the resorbing capacity of osteoclasts was sufficient to explain physiological levels of bone remodeling (13). He dismissed observations of osteocyte perilacunar enlargement as artifacts of tissue preparation and the difficulty of accounting for differences in osteocyte orientation and variability in the stage of osteocyte maturation using 2-dimensional histological and radiographic techniques (13). The concept that osteocytes could resorb bone was further called into question when it was found that isolated osteocytes, unlike osteoclasts, did not form resorption pits on dentin slices in culture (59). Given that the concept of bone flow was debunked and given the general acceptance of osteoclasts as the principal bone-resorbing cell, interest and research in osteocytic osteolysis declined precipitously after the 1970’s.
Reversible Osteocytic Perilacunar and Pericanalicular Remodeling During Lactation
Osteocytic osteolysis was often reported in situations of increased calcium demand or excess parathyroid function. Given that lactation is associated with negative calcium balance and the activation of skeletal PTHR1 signaling, it seemed to us that it might be a likely situation in which physiologic osteocytic bone remodeling might occur. Previous studies on this subject were mixed. Kwiecinski and colleagues found that osteocyte lacunae became enlarged in lactating brown bats, but Rasmussen et al reported that osteocytic osteolysis did not occur in lactating rats maintained on a low calcium diet (60). Therefore, in collaboration with the Bonewald and Pajevich laboratories, we re-examined whether osteocytic osteolysis occurred in lactating mice.
Using backscatter electron microscopy and acid-etched scanning electron microscopy, we first examined the size of osteocyte lacunae in virgin, lactating, and post-lactation recovered mice (14). As shown in Fig. 1, the size of osteocyte lacunae in both the tibia and vertebrae, but not the calvarium, increased in lactating mice as compared to virgin mice. Furthermore, we found that 7 days after the end of lactation, during the recovery phase of bone accrual, the size of the lacunae had decreased back to the baseline of virgin mice. The return of the lacunar size to baseline was accompanied by dual fluorochrome labeling of the lacunar space demonstrating bone formation in these sites (Fig. 2). These changes were not non-specific responses to increased bone turnover since they did not occur in the setting of tail-suspension, even though this maneuver caused bone loss equivalent to that observed during lactation. In a related study, we found a reversible decrease in trabecular central tissue mineralization during lactation using micro-CT-based digital topological analysis, a finding consistent with removal of bone mineral by osteocytes (23). Histochemical staining and gene array studies demonstrated that lactation is associated with the reversible activation, in osteocytes, of genes typically associated with osteoclastic bone resorption. These include tartrate resistant acid phosphatase (TRAP), cathepsin K, carbonic anhydrase, matrix metallopeptidase 13 (MMP13), and several subunits of the H+-ATPase (Fig 3). A recent study from Tang and colleagues confirmed that MMP13 contributes to osteocyte perilacunar and pericanalicular remodeling in cortical bone during lactation (61). We also found that infusing virgin mice with PTHrP 1–36 for 11 days using an osmotic minipump, induced an increase in the size of the osteocyte lacunae and induced the expression of TRAP expression in osteocytes (Fig. 3) (14, 29). Finally, we found that conditional disruption of the type 1 PTH/PTHrP receptor (PTHR1) gene in osteocytes using the DMP1-Cre transgenic mouse completely blocked the increase in lacunar size and the induction of TRAP activity normally observed during lactation (Fig. 4) (14). Therefore, we concluded that lactation is associated with reversible periosteocytic bone remodeling due, in part, to PTHrP-PTHR1 signaling, which activates a bone-resorption program in osteocytes that employs some of the same acid-protease mediators typically used by osteoclasts.
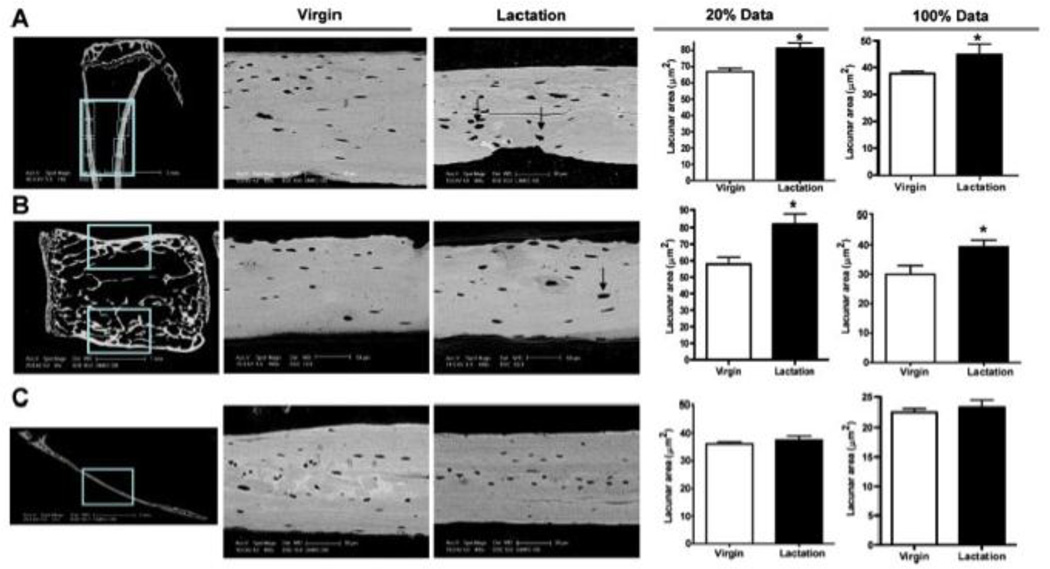
Figure 1. Lactation induces osteocytic lacunar enlargement.
Osteocyte lacunar area as measured by back scatter electron microscopy increases in the tibiae (A) and lumbar vertebrae (B), but not in the calvarium (C). Each panel shows the location of the measurements, representative images and the quantification of the size of the largest 20% of the lacunae and all (100%) of the lacunae in virgin mice as compared to lactating mice. Note that the lacunae are significantly larger in lactating mice in both the tibiae and vertebral bodies, but not in the calvarium. (reproduced from Qing et al, J Bone Miner Res 27:1018–1029, 2012 with permission)
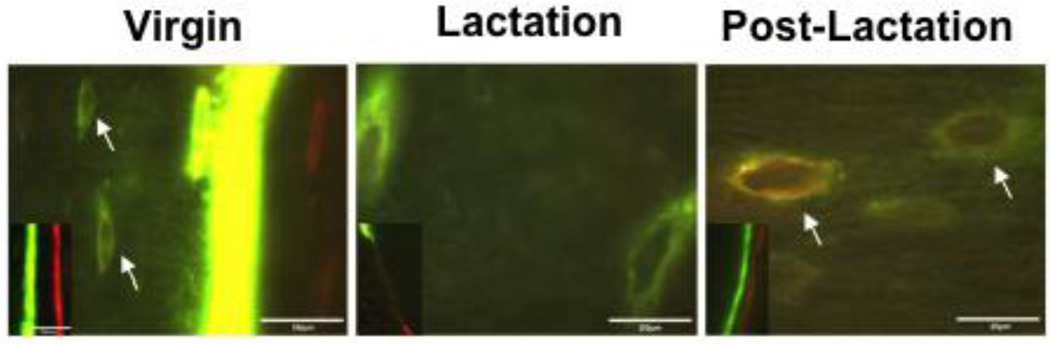
Figure 2. Osteocytes Deposit Mineral during the Recovery Period Post-Lactation.
Double fluorochrome labeling of bone from virgin, lactating and post-lactation mice. Distinct double labels can be found at the surface (insets) of virgin and post-lactating mice. Only an intermittent single label is seen at the surface of lactating bone given the rapid turnover. In virgin mice, some label was taken up in osteocyte lacunae near the mineralization front (white arrows, left panel). However, osteocyte lacunae distant from the mineralization front were labeled with both fluorochromes in post-lactating mice, suggesting active mineralization around osteocytes in the period of recovery from lactation. (adapted from Qing et al, J Bone Miner Res 27:1018–1029, 2012 with permission)
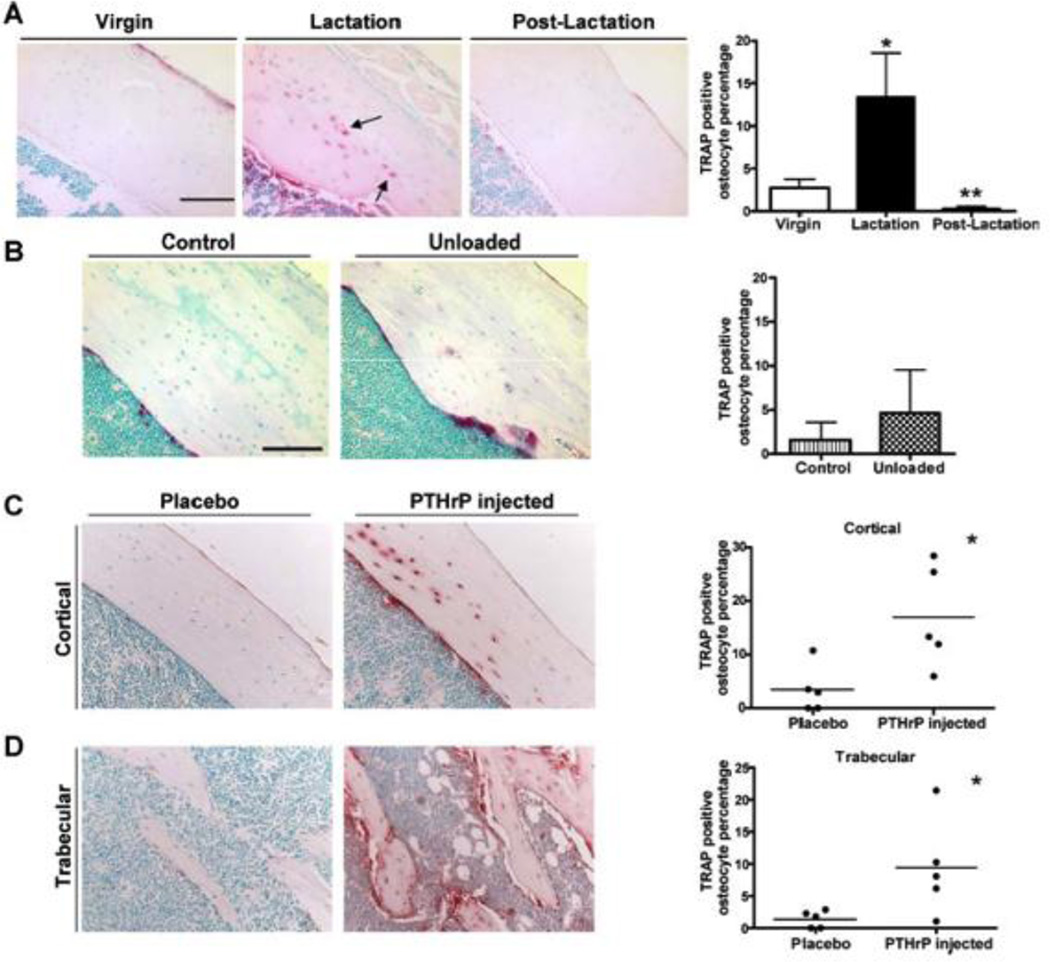
Figure 3. PTHrP Signaling Induces TRAP Expression in Osteocytes during Lactation.
A) TRAP staining is positive in osteocytes in the tibia during lactation but is lost in osteocytes 7 days after pups are weaned. Graph shows the percentage of TRAP-positive osteocytes at each stage. B) Unloading the skeleton does not induce TRAP staining despite rapid bone loss. C) Infusion of PTHrP into virgin mice induces TRAP staining in osteocytes from both cortical and trabecular bone, reproducing the effects of lactation. (reproduced from Qing et al, J Bone Miner Res 27:1018–1029, 2012 with permission)
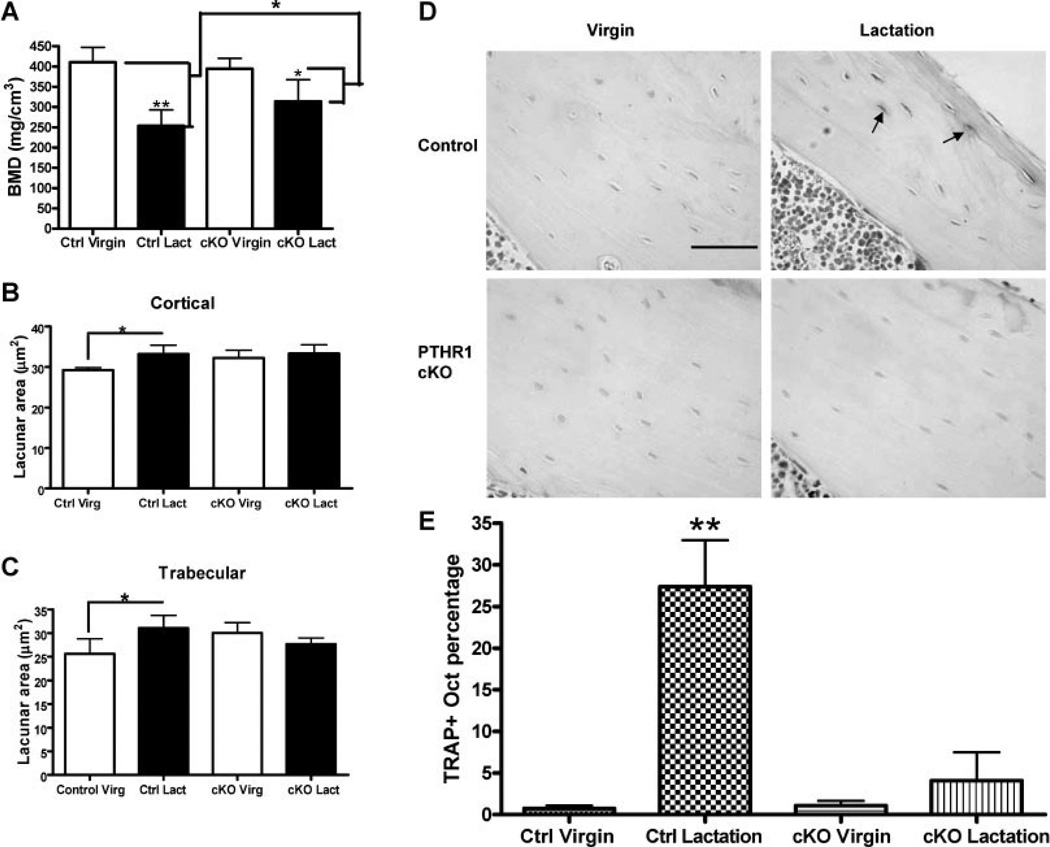
Figure 4. Osteocyte remodeling during lactation is blocked by osteocyte-specific disruption of the PTHR1 gene (PTHR1 CKO).
(A) BMD as measured by micro-CT in virgin and lactating PTHR1 CKO mice. Bone loss during lactation is attenuated by disruption of the PTHR1 in osteocytes. (B&C) Measurement of osteocyte lacunar area in cortical bone (B) and trabecular bone (C) of virgin and lactating control and PTHR1 CKO mice. Loss of the PTHR1 in osteocytes prevents the increase in lacunar size that occurs during lactation in controls. (D) TRAP staining in osteocytes in virgin and lactating control and PTHR1 CKO mice. (E) Quantification of the numbers of TRAP-positive osteocytes in virgin and lactating, control and PTHR1 CKO mice. In controls, many osteocytes become TRAP positive during lactation, but activation of TRAP expression is prevented in lactating PTHR1 CKO mice. (reproduced from Qing et al, J Bone Miner Res 27:1018–1029, 2012 with permission)
Other Potential Functions of Osteocytes During Lactation
Our study in lactating mice clearly demonstrates that osteocytes remodel their perilacunar and pericanalicular spaces, removing bone mineral during lactation and replacing it during the recovery period after weaning. These observations support the idea that osteocytes can directly resorb bone and they raise questions about whether this process might be involved more generally in calcium and bone metabolism. The very large surface area of the lacunar-canalicular network makes the osteocyte an attractive candidate for mediating mineral exchange between bone and the systemic circulation. A key question is whether the changes in lactation represent an exaggeration of a process that contributes to mineral homeostasis on a minute-to-minute basis, or whether they represent a specialized process that becomes engaged only during lactation? Further studies that measure the quantitative contribution of osteocytic remodeling to calcium fluxes in virgin and lactating mice will be needed to answer this question.
One possibility is that periostecytic remodeling is part of an integrated response by which the osteocyte activates surface osteoclastic activity as well as perilacunar and canalicular bone resorption. Recently it has become appreciated that osteocytes are an important source of RANKL production in bone and are particularly critical for maintaining osteoclast activity in the bone remodeling cycle (62–64). In addition, it has been shown that PTHR1 signaling can induce osteocyte RANKL production (62, 65). Thus, it is possible that during lactation, PTHrP-PTHR1 signaling stimulates osteocytes to both remodel their immediate environment and to activate osteoclast-mediated, surface bone resorption by upregulating RANKL production. Given that changes in the mineral content around osteocytes and/or the increase in the size of the osteocyte lacunae are likely to change mechanical-biochemical coupling by the osteocyte, it is even possible that periosteocytic bone remodeling may be necessary for the full response of surface bone resorption during lactation. Consistent with this possibility, bone loss during lactation was significantly reduced in the DMP1-Cre;PTHR1lox/lox mice, although, at present, it is not clear if this is due to a reduction in surface bone activity, the loss of osteocyte remodeling or both (14).
Osteocytes, through their production of sclerostin, are also an important regulator of osteoblast activity (1, 2). We have very little understanding of the regulation of the transition from bone loss to bone recovery triggered by the cessation of milk production. Studies have shown that weaning triggers a wave of osteoclast apoptosis associated with alterations in the local RANKL/OPG ratio (22, 33). However, unlike many other circumstances, the decrease in osteoclast activity at weaning is not accompanied by a decrease in bone formation (9). In contrast, osteoblast numbers and activity are maintained or increased, resulting in relatively unopposed bone formation. It is certainly possible that osteocytes may orchestrate this rapid switch from net bone resorption to net bone formation.
Finally, skeletal growth requires phosphate in addition to calcium, and milk must provide neonates with adequate amounts of this mineral as well. Very little is known about phosphate metabolism during lactation, but presumably there is an efflux of phosphate from the skeleton associated with the bone loss that occurs during this period. Since osteocytes are key players in coordinating skeletal mineralization with renal phosphate handling by secreting FGF23, it is very likely that these cells are actively involved in controlling phosphate metabolism during lactation (4, 5). However, the adaptations in the osteocyte-FGF23-renal phosphate axis during lactation have yet to be defined.
Conclusions
Mammalian reproduction requires mothers to provide nutrition to their offspring for prolonged periods after birth. Initially, these needs are met by milk production. Since the neonatal period is the most rapid phase of skeletal growth, milk must provide all the calcium and phosphorus required to make new bone. As part of the adaptations that help provide calcium and phosphorus for milk production, lactating mothers mobilize skeletal stores, which they then replace after lactation ends. This cycle of bone loss and recovery requires extensive and coordinated changes in the activity of bone cells. Traditionally, the changes in bone mass during reproductive cycles have been described in terms of the changes in osteoclast and osteoblast activity. However, recent studies have shown that osteocytes are also likely to be important to the adaptations of the skeleton to lactation. We have found that osteocytes can reversibly remodel perilacunar and pericanalicular bone, actively removing mineralized tissue during lactation and actively replacing it during the recovery period after weaning. These findings are consistent with the old concept of osteocytic osteolysis and demonstrate that physiologic osteocytic remodeling can occur. However, the engagement of these cells during lactation also raises the possibility that they may coordinate changes in osteoclast and osteoblast activity during and after lactation. Additionally, it is reasonable to speculate that osteocytes actively regulate phosphate metabolism during these periods. Understanding the functions of osteocytes during reproductive cycles will be necessary to fully understand the changes in bone and mineral metabolism that support milk production.
Acknowledgements
This work was supported by NIH Grants DK55501, DK077565 and CA153702. The author thanks Drs. Lynda Bonewald and Laleh Ardeshirpour for helpful conversations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kogianni G, Noble BS. The biology of osteocytes. Curr Osteoporos Rep. 2007;5:81–86. doi: 10.1007/s11914-007-0007-z. [DOI] [PubMed] [Google Scholar]
- 3.Bonewald LF. Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 2007;1116:281–290. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 4.Cheng F, Hulley P. The osteocyte--a novel endocrine regulator of body phosphate homeostasis. Maturitas. 2010;67:327–338. doi: 10.1016/j.maturitas.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marotti G, Ferretti M, Remaggi F, Palumbo C. Quantitative evaluation on osteocyte canalicular density in human secondary osteons. Bone. 1995;16:125–128. doi: 10.1016/s8756-3282(94)00019-0. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10:105–118. doi: 10.1007/s10911-005-5394-0. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 9.Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann N Y Acad Sci. 2010;1192:161–169. doi: 10.1111/j.1749-6632.2009.05249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belanger LF. Osteocytic osteolysis. Calcif Tissue Res. 1969;4:1–12. doi: 10.1007/BF02279101. [DOI] [PubMed] [Google Scholar]
- 11.Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009;1:59–65. doi: 10.4248/ijos.09019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–16. doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Parfitt AM. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption--bone flow theory. Clin Orthop Relat Res. 1977:236–247. [PubMed] [Google Scholar]
- 14.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanHouten J. Maternal calcium and bone metabolism during lactation. Current Opinion in Endocrinology and Diabetes. 2005;12:477–482. [Google Scholar]
- 16.Ajibade DV, Dhawan P, Fechner AJ, Meyer MB, Pike JW, Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151:2974–2984. doi: 10.1210/en.2010-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charoenphandhu N, Nakkrasae LI, Kraidith K, Teerapornpuntakit J, Thongchote K, Thongon N, Krishnamra N. Two-step stimulation of intestinal Ca(2+) absorption during lactation by long-term prolactin exposure and suckling-induced prolactin surge. Am J Physiol Endocrinol Metab. 2009;297:E609–E619. doi: 10.1152/ajpendo.00347.2009. [DOI] [PubMed] [Google Scholar]
- 18.Smith MS, Grove KL. Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol. 2002;23:225–256. doi: 10.1016/s0091-3022(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 19.Laskey MA, Prentice A, Hanratty LA, Jarjou LM, Dibba B, Beavan SR, Cole TJ. Bone changes after 3 mo of lactation: influence of calcium intake, breast-milk output, and vitamin D-receptor genotype. Am J Clin Nutr. 1998;67:685–692. doi: 10.1093/ajcn/67.4.685. [DOI] [PubMed] [Google Scholar]
- 20.Peng TC, Garner SC, Kusy RP, Hirsch PF. Effect of number of suckling pups and dietary calcium on bone mineral content and mechanical properties of femurs of lactating rats. Bone Miner. 1988;3:293–304. [PubMed] [Google Scholar]
- 21.VanHouten JN, Wysolmerski JJ. Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology. 2003;144:5521–5529. doi: 10.1210/en.2003-0892. [DOI] [PubMed] [Google Scholar]
- 22.Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, Wysolmerski JJ. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology. 2007;148:3875–3886. doi: 10.1210/en.2006-1467. [DOI] [PubMed] [Google Scholar]
- 23.Liu XS, Ardeshirpour L, VanHouten JN, Shane E, Wysolmerski JJ. Site-specific changes in bone microarchitecture, mineralization, and stiffness during lactation and after weaning in mice. J Bone Miner Res. 2012;27:865–875. doi: 10.1002/jbmr.1503. [DOI] [PubMed] [Google Scholar]
- 24.McNeilly AS, Tay CC, Glasier A. Physiological mechanisms underlying lactational amenorrhea. Ann N Y Acad Sci. 1994;709:145–155. doi: 10.1111/j.1749-6632.1994.tb30394.x. [DOI] [PubMed] [Google Scholar]
- 25.Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203–1204. doi: 10.1172/JCI11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalkwarf HJ, Specker BL. Bone mineral loss during lactation and recovery after weaning. Obstet Gynecol. 1995;86:26–32. doi: 10.1016/0029-7844(95)00083-4. [DOI] [PubMed] [Google Scholar]
- 27.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112:1429–1436. doi: 10.1172/JCI19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. Jama. 1996;276:549–554. [PubMed] [Google Scholar]
- 29.Ardeshirpour L, Brian S, Dann P, VanHouten J, Wysolmerski J. Increased PTHrP and decreased estrogens alter bone turnover but do not reproduce the full effects of lactation on the skeleton. Endocrinology. 2010;151:5591–5601. doi: 10.1210/en.2010-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int. 2001;12:828–834. doi: 10.1007/s001980170033. [DOI] [PubMed] [Google Scholar]
- 31.Laskey MA, Prentice A. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94:608–615. doi: 10.1016/s0029-7844(99)00369-5. [DOI] [PubMed] [Google Scholar]
- 32.Polatti F, Capuzzo E, Viazzo F, Colleoni R, Klersy C. Bone mineral changes during and after lactation. Obstet Gynecol. 1999;94:52–56. doi: 10.1016/s0029-7844(99)00236-7. [DOI] [PubMed] [Google Scholar]
- 33.Miller SC, Bowman BM. Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat Rec (Hoboken) 2007;290:65–73. doi: 10.1002/ar.20403. [DOI] [PubMed] [Google Scholar]
- 34.Bowman BM, Siska CC, Miller SC. Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J Bone Miner Res. 2002;17:1954–1960. doi: 10.1359/jbmr.2002.17.11.1954. [DOI] [PubMed] [Google Scholar]
- 35.Miller SC, Bowman BM. Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:143–149. doi: 10.1002/ar.a.10138. [DOI] [PubMed] [Google Scholar]
- 36.Recklinghausen F. Untersurchungen uber Rachitis and Osteomalacia. Jena: Gustav Fischer; 1910. [Google Scholar]
- 37.Rigal A, Vignal W. Recherches experimentales sur la formation du cal et sur les modifications des tissue dans les pseudoarthroses. Arch Physiol, Ser II. 1881;8:419–458. [Google Scholar]
- 38.Baud CA. [Morphology and inframicroscopic structure of osteocytes] Acta Anat (Basel) 1962;51:209–225. [PubMed] [Google Scholar]
- 39.Krempien B, Friedrich E, Ritz E. Effect of PTH on osteocyte ultrastructure. Adv Exp Med Biol. 1978;103:437–450. doi: 10.1007/978-1-4684-7758-0_45. [DOI] [PubMed] [Google Scholar]
- 40.Belanger L, Migicovsky B. Histochemical evidence of proteolysis in bone: the influence of parathormone. J Histochem Cytochem. 1963;11:734–737. [Google Scholar]
- 41.Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. J Bone Miner Metab. 2004;22:524–529. doi: 10.1007/s00774-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 42.Mosekilde L, Melsen F. A tetracycline-based histomorphometric evaluation of bone resorption and bone turnover in hyperthyroidism and hyperparathyroidism. Acta Med Scand. 1978;204:97–102. doi: 10.1111/j.0954-6820.1978.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 43.Bonucci E, Gherardi G. Osteocyte ultrastructure in renal osteodystrophy. Virchows Arch A Pathol Anat Histol. 1977;373:213–231. doi: 10.1007/BF00432238. [DOI] [PubMed] [Google Scholar]
- 44.Bonucci E, Gherardi G, Faraggiana T. Bone changes in hemodialyzed uremic subjects. Comparative light and electron microscope investigations. Virchows Arch A Pathol Anat Histol. 1976;371:183–198. doi: 10.1007/BF00433067. [DOI] [PubMed] [Google Scholar]
- 45.Iagodovskii VS, Triftanidi LA, Gorokhova GP. [Effect of space flight on rat skeletal bones (an optical light and electron microscopic study)] Kosm Biol Aviakosm Med. 1977;11:14–20. [PubMed] [Google Scholar]
- 46.Rodionova NV, Oganov VS, Zolotova NV. Ultrastructural changes in osteocytes in microgravity conditions. Adv Space Res. 2002;30:765–770. doi: 10.1016/s0273-1177(02)00393-9. [DOI] [PubMed] [Google Scholar]
- 47.Cramer SF, Fried L, Carter KJ. The cellular basis of metastatic bone disease in patients with lung cancer. Cancer. 1981;48:2649–2660. doi: 10.1002/1097-0142(19811215)48:12<2649::aid-cncr2820481217>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 48.Kwiecinski GG, Krook L, Wimsatt WA. Annual skeletal changes in the little brown bat, Myotis lucifugus lucifugus, with particular reference to pregnancy and lactation. Am J Anat. 1987;178:410–420. doi: 10.1002/aja.1001780410. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg B, Singh IJ, Mitchell OG. The effects of cold-stress. Hibernation, and prolonged inactivity on bone dynamics in the golden hamster, Mesocricetus auratus. J Morphol. 1981;167:43–51. doi: 10.1002/jmor.1051670105. [DOI] [PubMed] [Google Scholar]
- 50.Haller AC, Zimny ML. Effects of hibernation on interradicular alveolar bone. J Dent Res. 1977;56:1552–1557. doi: 10.1177/00220345770560122601. [DOI] [PubMed] [Google Scholar]
- 51.Alcobendas M, Baud CA, Castanet J. Structural changes of the periosteocytic area in Vipera aspis (L.) (Ophidia, Viperidae) bone tissue in various physiological conditions. Calcif Tissue Int. 1991;49:53–57. doi: 10.1007/BF02555903. [DOI] [PubMed] [Google Scholar]
- 52.Baylink DJ, Wergedal JE. Bone formation by osteocytes. Am J Physiol. 1971;221:669–678. doi: 10.1152/ajplegacy.1971.221.3.669. [DOI] [PubMed] [Google Scholar]
- 53.McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 54.Zambonin Zallone AZ, Teti A, Nico B, Primavera MV. Osteoplastic activity of mature osteocytes evaluated by H-proline incorporation. Basic Appl Histochem. 1982;26:65–67. [PubMed] [Google Scholar]
- 55.Zambonin Zallone A, Teti A, Primavera MV, Pace G. Mature osteocytes behaviour in a repletion period: the occurrence of osteoplastic activity. Basic Appl Histochem. 1983;27:191–204. [PubMed] [Google Scholar]
- 56.Weisbrode SE, Capen CC, Nagode LA. Effects of parathyroid hormone on bone of thyroparathyroidectomized rats: an ultrastructural and enzymatic study. Am J Pathol. 1974;75:529–541. [PMC free article] [PubMed] [Google Scholar]
- 57.Sissons HA, Kelman GJ, Marotti G. Mechanisms of bone resorption in calcium-deficient rats. Calcif Tissue Int. 1984;36:711–721. doi: 10.1007/BF02405394. [DOI] [PubMed] [Google Scholar]
- 58.Krook L, Belanger LF, Henrikson PA, Lutwak L, Sheffy BE. Bone flow. Rev Can Biol. 1970;29:157–167. [PubMed] [Google Scholar]
- 59.van der Plas A, Aarden EM, Feijen JH, de Boer AH, Wiltink A, Alblas MJ, de Leij L, Nijweide PJ. Characteristics and properties of osteocytes in culture. J Bone Miner Res. 1994;9:1697–1704. doi: 10.1002/jbmr.5650091105. [DOI] [PubMed] [Google Scholar]
- 60.Rasmussen P. Calcium deficiency, pregnancy, and lactation in rats. Microscopic and microradiographic observations on bones. Calcif Tissue Res. 1977;23:95–102. doi: 10.1007/BF02012772. [DOI] [PubMed] [Google Scholar]
- 61.Tang S, Herber RP, Ho S, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong J, O'Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res. 2012;27:499–505. doi: 10.1002/jbmr.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 65.O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]