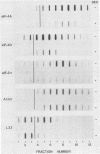
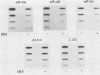
Abstract
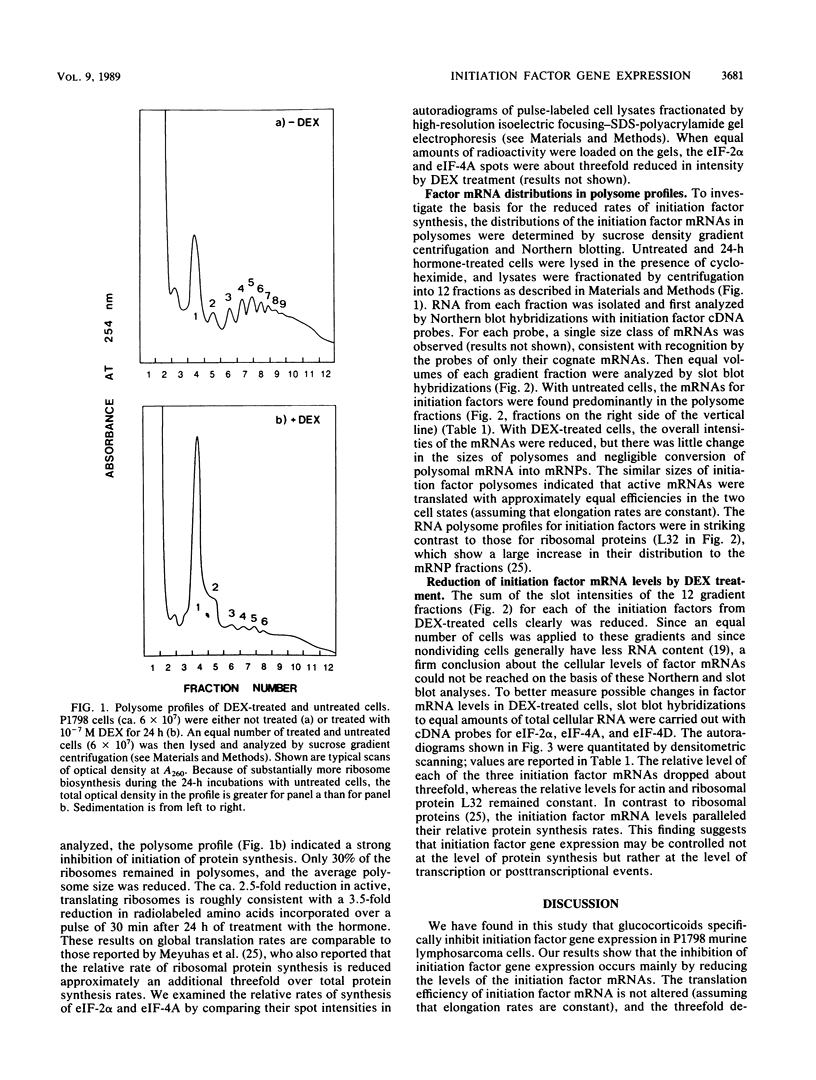
P1798 murine lymphosarcoma cells cease to proliferate upon exposure to 10(-7) M dexamethasone and exhibit a dramatic inhibition of rRNA and ribosomal protein synthesis (O. Meyuhas, E. Thompson, Jr., and R. P. Perry, Mol. Cell Biol. 7:2691-2699, 1987). These workers demonstrated that ribosomal protein synthesis is regulated primarily at the level of translation, since dexamethasone did not alter mRNA levels but shifted the mRNAs from active polysomes into inactive messenger ribonucleoproteins. We have examined the effects of dexamethasone on the biosynthesis of initiation factor proteins in the same cell line. The relative protein synthesis rates of eIF-4A and eIF-2 alpha were inhibited by about 70% by the hormone, a reduction comparable to that for ribosomal proteins. The mRNA levels of eIF-4A, eIF-4D, and eIF-2 alpha also were reduced by 60 to 70%, indicating that synthesis rates are proportional to mRNA concentrations. Analysis of polysome profiles showed that the average number of ribosomes per initiation factor polysome was only slightly reduced by dexamethasone, and little or no mRNA was present in messenger ribonucleoproteins. The results indicate that initiation factor gene expression is coordinately regulated with ribosomal protein synthesis but is controlled primarily by modulating mRNA levels rather than mRNA efficiency.
Full text
PDF

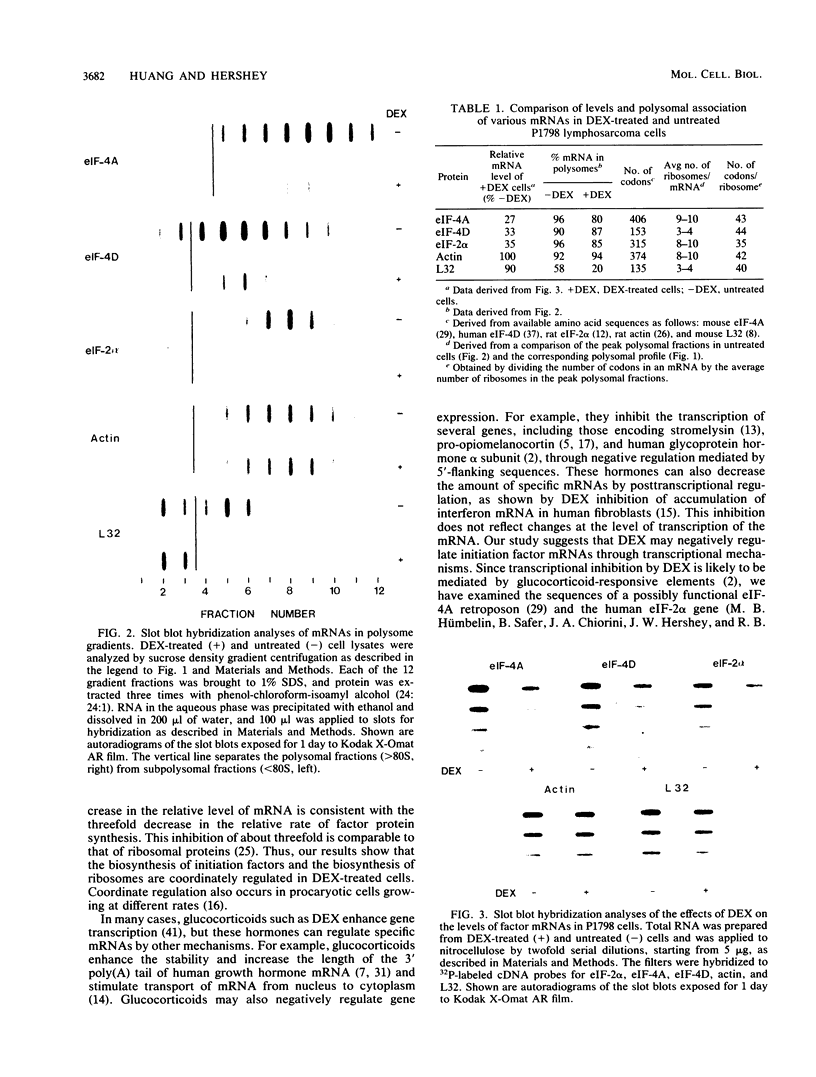


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal M. G., Bowman L. H. Transcriptional and translational regulation of ribosomal protein formation during mouse myoblast differentiation. J Biol Chem. 1987 Apr 5;262(10):4868–4875. [PubMed] [Google Scholar]
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Cavannaugh A. H., Thompson E. A., Jr Hormonal regulation of transcription of rDNA. Inhibition of transcription during glucocorticoid-mediated inhibition of proliferation of lymphosarcoma P1798 cells in culture. J Biol Chem. 1983 Aug 25;258(16):9768–9773. [PubMed] [Google Scholar]
- Charron J., Drouin J. Glucocorticoid inhibition of transcription from episomal proopiomelanocortin gene promoter. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8903–8907. doi: 10.1073/pnas.83.23.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig N., Perry R. P. Persistent cytoplasmic synthesis of ribosomal proteins during the selective inhibition of ribosomal RNA synthesis. Nat New Biol. 1971 Jan 20;229(3):75–80. doi: 10.1038/newbio229075a0. [DOI] [PubMed] [Google Scholar]
- Diamond D. J., Goodman H. M. Regulation of growth hormone messenger RNA synthesis by dexamethasone and triiodothyronine. Transcriptional rate and mRNA stability changes in pituitary tumor cells. J Mol Biol. 1985 Jan 5;181(1):41–62. doi: 10.1016/0022-2836(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Identification and quantitation of levels of protein synthesis initiation factors in crude HeLa cell lysates by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem. 1983 Jun 10;258(11):7228–7235. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Abundance, synthesis, and turnover rates. J Biol Chem. 1985 May 10;260(9):5486–5492. [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Rapid alterations in initiation rate and recruitment of inactive RNA are temporally correlated with S6 phosphorylation. Eur J Biochem. 1982 Apr;123(3):539–544. doi: 10.1111/j.1432-1033.1982.tb06565.x. [DOI] [PubMed] [Google Scholar]
- Ernst H., Duncan R. F., Hershey J. W. Cloning and sequencing of complementary DNAs encoding the alpha-subunit of translational initiation factor eIF-2. Characterization of the protein and its messenger RNA. J Biol Chem. 1987 Jan 25;262(3):1206–1212. [PubMed] [Google Scholar]
- Frisch S. M., Ruley H. E. Transcription from the stromelysin promoter is induced by interleukin-1 and repressed by dexamethasone. J Biol Chem. 1987 Dec 5;262(34):16300–16304. [PubMed] [Google Scholar]
- Fulton R., Birnie G. D., Knowler J. T. Post-transcriptional regulation of rat liver gene expression by glucocorticoids. Nucleic Acids Res. 1985 Sep 25;13(18):6467–6482. doi: 10.1093/nar/13.18.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessani S., McCandless S., Baglioni C. The glucocorticoid dexamethasone inhibits synthesis of interferon by decreasing the level of its mRNA. J Biol Chem. 1988 Jun 5;263(16):7454–7457. [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Initiation factor and ribosome levels are coordinately controlled in Escherichia coli growing at different rates. J Biol Chem. 1983 Feb 10;258(3):1954–1959. [PubMed] [Google Scholar]
- Israel A., Cohen S. N. Hormonally mediated negative regulation of human pro-opiomelanocortin gene expression after transfection into mouse L cells. Mol Cell Biol. 1985 Sep;5(9):2443–2453. doi: 10.1128/mcb.5.9.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs F. A., Bird R. C., Sells B. H. Differentiation of rat myoblasts. Regulation of turnover of ribosomal proteins and their mRNAs. Eur J Biochem. 1985 Jul 15;150(2):255–263. doi: 10.1111/j.1432-1033.1985.tb09015.x. [DOI] [PubMed] [Google Scholar]
- Krauter K. S., Soeiro R., Nadal-Ginard B. Unco-ordinate regulation of ribosomal RNA and ribosomal protein synthesis during L6E9 myoblast differentiation. J Mol Biol. 1980 Sep 15;142(2):145–159. doi: 10.1016/0022-2836(80)90042-x. [DOI] [PubMed] [Google Scholar]
- LaMarca M. J., Wassarman P. M. Relationship between rates of synthesis and intracellular distribution of ribosomal proteins during oogenesis in the mouse. Dev Biol. 1984 Apr;102(2):525–530. doi: 10.1016/0012-1606(84)90221-5. [DOI] [PubMed] [Google Scholar]
- Lawson T. G., Ray B. K., Dodds J. T., Grifo J. A., Abramson R. D., Merrick W. C., Betsch D. F., Weith H. L., Thach R. E. Influence of 5' proximal secondary structure on the translational efficiency of eukaryotic mRNAs and on their interaction with initiation factors. J Biol Chem. 1986 Oct 25;261(30):13979–13989. [PubMed] [Google Scholar]
- Meyer L. J., Milburn S. C., Hershey J. W. Immunochemical characterization of mammalian protein synthesis initiation factors. Biochemistry. 1982 Aug 31;21(18):4206–4212. doi: 10.1021/bi00261a003. [DOI] [PubMed] [Google Scholar]
- Meyuhas O., Thompson E. A., Jr, Perry R. P. Glucocorticoids selectively inhibit translation of ribosomal protein mRNAs in P1798 lymphosarcoma cells. Mol Cell Biol. 1987 Aug;7(8):2691–2699. doi: 10.1128/mcb.7.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Nielsen P. J., McConkey E. H. Evidence for control of protein synthesis in HeLa cells via the elongation rate. J Cell Physiol. 1980 Sep;104(3):269–281. doi: 10.1002/jcp.1041040302. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., McMaster G. K., Trachsel H. Cloning of eukaryotic protein synthesis initiation factor genes: isolation and characterization of cDNA clones encoding factor eIF-4A. Nucleic Acids Res. 1985 Oct 11;13(19):6867–6880. doi: 10.1093/nar/13.19.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. J., Trachsel H. The mouse protein synthesis initiation factor 4A gene family includes two related functional genes which are differentially expressed. EMBO J. 1988 Jul;7(7):2097–2105. doi: 10.1002/j.1460-2075.1988.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Paek I., Axel R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol Cell Biol. 1987 Apr;7(4):1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Raghow R., Gossage D., Kang A. H. Pretranslational regulation of type I collagen, fibronectin, and a 50-kilodalton noncollagenous extracellular protein by dexamethasone in rat fibroblasts. J Biol Chem. 1986 Apr 5;261(10):4677–4684. [PubMed] [Google Scholar]
- Rao T. R., Slobin L. I. Regulation of the utilization of mRNA for eucaryotic elongation factor Tu in Friend erythroleukemia cells. Mol Cell Biol. 1987 Feb;7(2):687–697. doi: 10.1128/mcb.7.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao T. R., Slobin L. I. The stability of mRNA for eucaryotic elongation factor Tu in Friend erythroleukemia cells varies with growth rate. Mol Cell Biol. 1988 Mar;8(3):1085–1092. doi: 10.1128/mcb.8.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smit-McBride Z., Dever T. E., Hershey J. W., Merrick W. C. Sequence determination and cDNA cloning of eukaryotic initiation factor 4D, the hypusine-containing protein. J Biol Chem. 1989 Jan 25;264(3):1578–1583. [PubMed] [Google Scholar]
- Sterling K. M., Jr, Harris M. J., Mitchell J. J., DiPetrillo T. A., Delaney G. L., Cutroneo K. R. Dexamethasone decreases the amounts of type I procollagen mRNAs in vivo and in fibroblast cell cultures. J Biol Chem. 1983 Jun 25;258(12):7644–7647. [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. In the absence of ribosomal RNA synthesis, the ribosomal proteins of HeLa cells are synthesized normally and degraded rapidly. J Mol Biol. 1977 Sep 25;115(3):315–333. doi: 10.1016/0022-2836(77)90157-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]