Abstract
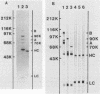
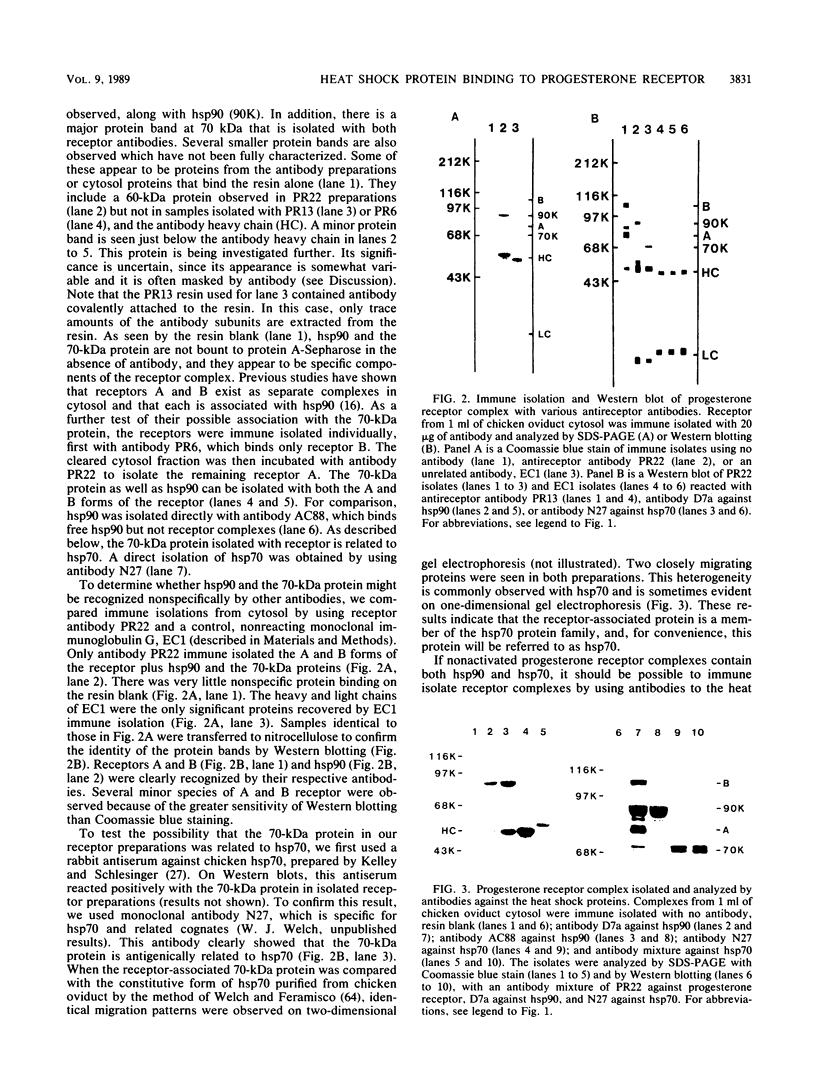
The protein composition of the avian progesterone receptor was analyzed by immune isolation of receptor complexes and gel electrophoresis of the isolated proteins. Nonactivated cytosol receptor was isolated in association with the 90-kilodalton (kDa) heat shock protein, hsp90, as has been described previously. A 70-kDa protein was also observed and was shown by Western immunoblotting to react with an antibody specific to the 70-kDa heat shock protein. Thus, two progesterone receptor-associated proteins are identical, or closely related, to heat shock proteins. When the two progesterone receptor species, A and B, were isolated separately in the absence of hormone, both were obtained in association with hsp90 and the 70-kDa protein. However, activated receptor isolated from oviduct nuclear extracts was associated with the 70-kDa protein, but not with hsp90. A hormone-dependent dissociation of hsp90 from the cytosolic form of the receptor complex was observed within the first hour of in vivo progesterone treatment, which could explain the lack of hsp90 in nuclear receptor complexes. In a cell-free system, hsp90 binding to receptor was stabilized by molybdate but disrupted by high salt. These treatments, however, did not alter the binding of the 70-kDa protein to receptor. Association of the 70-kDa protein with the receptor could be disrupted by the addition of ATP at elevated temperatures (23 degrees C). The receptor-associated 70-kDa protein is an ATP-binding protein, as demonstrated by its affinity labeling with azido[32P]ATP. These results indicate that the two receptor-associated proteins interact with the progesterone receptor by different mechanisms and that they are likely to affect the structure or function of the receptor in different ways.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham K. I., Haley B., Modak M. J. Biochemistry of terminal deoxynucleotidyltransferase: characterization and properties of photoaffinity labeling with 8-azidoadenosine 5'-triphosphate. Biochemistry. 1983 Aug 30;22(18):4197–4203. doi: 10.1021/bi00287a006. [DOI] [PubMed] [Google Scholar]
- Ali M., Vedeckis W. V. Interaction of RNA with transformed glucocorticoid receptor. I. Isolation and purification of the RNA. J Biol Chem. 1987 May 15;262(14):6771–6777. [PubMed] [Google Scholar]
- Brugge J. S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt-Duke J., Gustafsson J. A. Structure and function of the glucocorticoid receptor. J Steroid Biochem. 1987;27(1-3):99–104. doi: 10.1016/0022-4731(87)90299-8. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Clarke C. F., Cheng K., Frey A. B., Stein R., Hinds P. W., Levine A. J. Purification of complexes of nuclear oncogene p53 with rat and Escherichia coli heat shock proteins: in vitro dissociation of hsc70 and dnaK from murine p53 by ATP. Mol Cell Biol. 1988 Mar;8(3):1206–1215. doi: 10.1128/mcb.8.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvard D. S., Wilson E. M. Identification of an 8S androgen receptor-promoting factor that converts the 4.5S form of the androgen receptor to 8S. Endocrinology. 1981 Aug;109(2):496–504. doi: 10.1210/endo-109-2-496. [DOI] [PubMed] [Google Scholar]
- Denis M., Wikström A. C., Gustafsson J. A. The molybdate-stabilized nonactivated glucocorticoid receptor contains a dimer of Mr 90,000 non-hormone-binding protein. J Biol Chem. 1987 Aug 25;262(24):11803–11806. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Diehl E. E., Schmidt T. J. ATP-induced activation of purified rat hepatic glucocorticoid receptors. J Steroid Biochem. 1987 Nov;28(5):485–491. doi: 10.1016/0022-4731(87)90506-1. [DOI] [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Polypeptide components of two 8 S forms of chicken oviduct progesterone receptor. J Biol Chem. 1984 Jun 25;259(12):8004–8009. [PubMed] [Google Scholar]
- Economidis I. V., Rousseau G. G. Association of the glucocorticoid hormone receptor with ribonucleic acid. FEBS Lett. 1985 Feb 11;181(1):47–52. doi: 10.1016/0014-5793(85)81111-x. [DOI] [PubMed] [Google Scholar]
- Ennis B. W., Stumpf W. E., Gasc J. M., Baulieu E. E. Nuclear localization of progesterone receptor before and after exposure to progestin at low and high temperatures: autoradiographic and immunohistochemical studies of chick oviduct. Endocrinology. 1986 Nov;119(5):2066–2075. doi: 10.1210/endo-119-5-2066. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Suba E. J., Lawler-Heavner J., Elashry-Stowers D., Wei L. L., Toft D. O., Sullivan W. P., Horwitz K. B., Edwards D. P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987 Sep 22;26(19):6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Frey A. B., Levine A. J. Immunological evidence for the association of p53 with a heat shock protein, hsc70, in p53-plus-ras-transformed cell lines. Mol Cell Biol. 1987 Aug;7(8):2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Bodwell J. E., Jeffries M., Munck A. Characterization of nonactivated and activated glucocorticoid-receptor complexes from intact rat thymus cells. J Biol Chem. 1983 May 25;258(10):6477–6485. [PubMed] [Google Scholar]
- Howard K. J., Distelhorst C. W. Evidence for intracellular association of the glucocorticoid receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Mar 5;263(7):3474–3481. [PubMed] [Google Scholar]
- Iannotti A. M., Rabideau D. A., Dougherty J. J. Characterization of purified avian 90,000-Da heat shock protein. Arch Biochem Biophys. 1988 Jul;264(1):54–60. doi: 10.1016/0003-9861(88)90569-3. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. Involvement of ATP in the nuclear and nucleolar functions of the 70 kd heat shock protein. EMBO J. 1985 Dec 1;4(12):3137–3143. doi: 10.1002/j.1460-2075.1985.tb04056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K., Georgopoulos C., Zylicz M. Role of the Escherichia coli DnaK and DnaJ heat shock proteins in the initiation of bacteriophage lambda DNA replication. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6632–6636. doi: 10.1073/pnas.85.18.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Pamphile R., Milgrom E. The nuclear-bound form of the progesterone receptor is generated through a hormone-dependent phosphorylation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):421–427. doi: 10.1016/0006-291x(85)91819-4. [DOI] [PubMed] [Google Scholar]
- McBlain W. A., Toft D. O. Interaction of chick oviduct progesterone receptor with the 2',3'-dialdehyde derivative of adenosine 5'-triphosphate. Biochemistry. 1983 Apr 26;22(9):2262–2270. doi: 10.1021/bi00278a032. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Bodwell J. E., Gametchu B., Harrison R. W., Munck A. Molybdate-stabilized nonactivated glucocorticoid-receptor complexes contain a 90-kDa non-steroid-binding phosphoprotein that is lost on activation. J Biol Chem. 1986 Mar 15;261(8):3758–3763. [PubMed] [Google Scholar]
- Miller J. B., Toft D. O. Requirement for activation in the binding of progesterone receptor to ATP-Sepharose. Biochemistry. 1978 Jan 10;17(1):173–177. doi: 10.1021/bi00594a025. [DOI] [PubMed] [Google Scholar]
- Moudgil V. K., Hurd C. Transformation of calf uterine progesterone receptor: analysis of the process when receptor is bound to progesterone and RU38486. Biochemistry. 1987 Aug 11;26(16):4993–5001. doi: 10.1021/bi00390a017. [DOI] [PubMed] [Google Scholar]
- Moudgil V., Kruczak V., Eessalu T., Paulose C. S., Taylor M., Hansen J. Activation of progesterone receptor by ATP. Eur J Biochem. 1981 Sep 1;118(3):547–555. doi: 10.1111/j.1432-1033.1981.tb05554.x. [DOI] [PubMed] [Google Scholar]
- Murayama A., Fukai F., Yamamoto T. Progesterone receptor system of hen oviduct. J Biochem. 1980 Nov;88(5):1305–1315. doi: 10.1093/oxfordjournals.jbchem.a133099. [DOI] [PubMed] [Google Scholar]
- Nishigori H., Toft D. Inhibition of progesterone receptor activation by sodium molybdate. Biochemistry. 1980 Jan 8;19(1):77–83. doi: 10.1021/bi00542a012. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Picard D., Salser S. J., Yamamoto K. R. A movable and regulable inactivation function within the steroid binding domain of the glucocorticoid receptor. Cell. 1988 Sep 23;54(7):1073–1080. doi: 10.1016/0092-8674(88)90122-5. [DOI] [PubMed] [Google Scholar]
- Pinhasi-Kimhi O., Michalovitz D., Ben-Zeev A., Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986 Mar 13;320(6058):182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Jolly D. J., Pratt D. V., Hollenberg S. M., Giguere V., Cadepond F. M., Schweizer-Groyer G., Catelli M. G., Evans R. M., Baulieu E. E. A region in the steroid binding domain determines formation of the non-DNA-binding, 9 S glucocorticoid receptor complex. J Biol Chem. 1988 Jan 5;263(1):267–273. [PubMed] [Google Scholar]
- Raaka B. M., Finnerty M., Sun E., Samuels H. H. Effects of molybdate on steroid receptors in intact GH1 cells. Evidence for dissociation of an intracellular 10 S receptor oligomer prior to nuclear accumulation. J Biol Chem. 1985 Nov 15;260(26):14009–14015. [PubMed] [Google Scholar]
- Redeuilh G., Moncharmont B., Secco C., Baulieu E. E. Subunit composition of the molybdate-stabilized "8-9 S" nontransformed estradiol receptor purified from calf uterus. J Biol Chem. 1987 May 25;262(15):6969–6975. [PubMed] [Google Scholar]
- Renoir J. M., Buchou T., Baulieu E. E. Involvement of a non-hormone-binding 90-kilodalton protein in the nontransformed 8S form of the rabbit uterus progesterone receptor. Biochemistry. 1986 Oct 21;25(21):6405–6413. doi: 10.1021/bi00369a010. [DOI] [PubMed] [Google Scholar]
- Rose D. W., Wettenhall R. E., Kudlicki W., Kramer G., Hardesty B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry. 1987 Oct 20;26(21):6583–6587. doi: 10.1021/bi00395a003. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Meshinchi S., Tienrungroj W., Schlesinger M. J., Toft D. O., Pratt W. B. Relationship of the 90-kDa murine heat shock protein to the untransformed and transformed states of the L cell glucocorticoid receptor. J Biol Chem. 1987 May 25;262(15):6986–6991. [PubMed] [Google Scholar]
- Sanchez E. R., Redmond T., Scherrer L. C., Bresnick E. H., Welsh M. J., Pratt W. B. Evidence that the 90-kilodalton heat shock protein is associated with tubulin-containing complexes in L cell cytosol and in intact PtK cells. Mol Endocrinol. 1988 Aug;2(8):756–760. doi: 10.1210/mend-2-8-756. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Sando J. J., Hammond N. D., Stratford C. A., Pratt W. B. Activation of thymocyte glucocorticoid receptors to the steroid binding form. The roles of reduction agents, ATP, and heat-stable factors. J Biol Chem. 1979 Jun 10;254(11):4779–4789. [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins: the search for functions. J Cell Biol. 1986 Aug;103(2):321–325. doi: 10.1083/jcb.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Stürzbecher H. W., Chumakov P., Welch W. J., Jenkins J. R. Mutant p53 proteins bind hsp 72/73 cellular heat shock-related proteins in SV40-transformed monkey cells. Oncogene. 1987 May;1(2):201–211. [PubMed] [Google Scholar]
- Sullivan W. P., Beito T. G., Proper J., Krco C. J., Toft D. O. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology. 1986 Oct;119(4):1549–1557. doi: 10.1210/endo-119-4-1549. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Madden B. J., McCormick D. J., Toft D. O. Hormone-dependent phosphorylation of the avian progesterone receptor. J Biol Chem. 1988 Oct 15;263(29):14717–14723. [PubMed] [Google Scholar]
- Sullivan W. P., Smith D. F., Beito T. G., Krco C. J., Toft D. O. Hormone-dependent processing of the avian progesterone receptor. J Cell Biochem. 1988 Feb;36(2):103–119. doi: 10.1002/jcb.240360202. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Vroman B. T., Bauer V. J., Puri R. K., Riehl R. M., Pearson G. R., Toft D. O. Isolation of steroid receptor binding protein from chicken oviduct and production of monoclonal antibodies. Biochemistry. 1985 Jul 16;24(15):4214–4222. doi: 10.1021/bi00336a060. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Maeda Y., Nakao K., Wakim N. G., Duhring J. L., Faber L. E. A 59-kilodalton protein associated with progestin, estrogen, androgen, and glucocorticoid receptors. Biochemistry. 1986 Sep 9;25(18):5269–5275. doi: 10.1021/bi00366a043. [DOI] [PubMed] [Google Scholar]
- Thampan R. V. A 62 kDa protein functions as estrogen receptor activation factor (E-RAF) in the goat uterus. Mol Cell Endocrinol. 1987 Sep;53(1-2):119–130. doi: 10.1016/0303-7207(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Tymoczko J. L., Anderson E. E., Lee K. A., Unger A. L. The ability to convert the 4 S glucocorticoid receptor to the 7-8 S form is dependent on both RNA and protein factors. Biochim Biophys Acta. 1987 Aug 19;930(1):114–121. doi: 10.1016/0167-4889(87)90163-7. [DOI] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L. L., Sheridan P. L., Krett N. L., Francis M. D., Toft D. O., Edwards D. P., Horwitz K. B. Immunologic analysis of human breast cancer progesterone receptors. 2. Structure, phosphorylation, and processing. Biochemistry. 1987 Sep 22;26(19):6262–6272. doi: 10.1021/bi00393a046. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Spector D., Welch W. Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells. J Virol. 1988 Nov;62(11):4153–4166. doi: 10.1128/jvi.62.11.4153-4166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrange O., Carlstedt-Duke J., Gustafsson J. A. Stoichiometric analysis of the specific interaction of the glucocorticoid receptor with DNA. J Biol Chem. 1986 Sep 5;261(25):11770–11778. [PubMed] [Google Scholar]
- Ziemiecki A., Catelli M. G., Joab I., Moncharmont B. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1298–1307. doi: 10.1016/s0006-291x(86)80424-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Sagstetter M., Lewis M. J., Pelham H. R. Seventy-kilodalton heat shock proteins and an additional component from reticulocyte lysate stimulate import of M13 procoat protein into microsomes. EMBO J. 1988 Sep;7(9):2875–2880. doi: 10.1002/j.1460-2075.1988.tb03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]