Abstract
A major constituent of many Staphylococcus aureus biofilms is a polysaccharide known as the polysaccharide intercellular adhesin, or poly N-acetylglucosamine (PIA/PNAG). PIA/PNAG is synthesized by the 4 gene products of the icaADBC operon, which is negatively regulated by the divergently transcribed icaR gene. We previously reported the identification of a gene, rbf, involved in the positive transcriptional regulation of icaADBC transcription by repressing icaR in S. aureus strain 8325-4. However, we were unable to show binding of Rbf to DNA upstream of icaR or icaA, suggesting that Rbf may control expression of an unknown factor(s) that, in turn, regulates ica expression. Here we report that the unknown factor is SarX protein. Results from epistasis assays and genetic complementation analyses suggest that Rbf upregulates SarX, which then downregulates IcaR, thereby activating icaADBC. Electrophoretic mobility shift assays revealed that SarX protein bound to a sequence upstream of icaR within the icaA coding region. Cross-linking and immunoprecipitation experiments further suggested that Rbf binds to the sarX promoter in S. aureus. These results demonstrate that Rbf and SarX represent a regulatory cascade that promotes PIA-dependent biofilm formation in S. aureus.
INTRODUCTION
Staphylococcus aureus is a major human pathogen causing a diverse array of nosocomial and community-acquired infections. Staphylococcal infections range from superficial infections of the skin and mucosa to highly invasive and potentially lethal infections. Some S. aureus infections, such as endocarditis, osteomyelitis, and infections associated with indwelling medical devices, are associated with the formation of bacterial biofilms. Bacterial biofilms are complex communities of organisms containing layers of bacteria within a glycocalyx composed of polysaccharides, DNA, and/or proteins. In addition to aiding bacterial colonization of surfaces, biofilms are believed to confer resistance to antibiotics and immune defenses (1–3).
The major exopolysaccharide in S. aureus biofilms is referred to as the polysaccharide intercellular adhesin (PIA), also known as poly-N-acetylglucosamine (PNAG) (4, 5). The synthesis of PIA/PNAG is accomplished by four proteins, IcaA, IcaD, IcaB, and IcaC, encoded by the ica operon (5). Production of PIA/PNAG is tightly regulated, but the signals that are responsible for induction of PIA/PNAG synthesis in vivo are unknown. A variety of environmental conditions have been shown to affect icaADBC expression under laboratory conditions. High temperature, high osmolarity, glucose, ethanol, anaerobiosis, and subinhibitory concentrations of certain antibiotics have all been found to induce PIA/PNAG production in vitro. There is, however, significant strain-to-strain variation regarding what factors affect expression.
Several different S. aureus proteins have been shown to be involved in the transcriptional regulation of icaADBC. These factors include global regulatory proteins, such as SarA and σB, as well as factors like IcaR and TcaR, which seem to regulate relatively few genes (6–11). Some factors regulate icaADBC expression directly (e.g., IcaR), whereas regulation by other proteins seems to be indirect (e.g., σB). IcaR is arguably the most important factor involved in icaADBC regulation. The icaR gene is located immediately upstream of and is divergently transcribed from icaADBC. IcaR binds to the icaADBC promoter and represses transcription (10). Deletion of icaR has been shown to dramatically increase icaADBC expression and PNAG production (10, 12). Some regulatory proteins appear to upregulate icaADBC expression by inhibiting expression of icaR. IcaR also plays an important role in icaADBC expression in Staphylococcus epidermidis (13).
We have previously described a gene, rbf, which regulates expression of icaADBC and PIA/PNAG production in S. aureus strain 8325-4 (12, 14). Rbf is a member of the AraC/XylS family of transcriptional regulators, a family in which all members bear a highly conserved 100-amino-acid region forming a dual, helix-turn-helix DNA binding motif (15, 16). Rbf is a positive regulator of biofilm (12, 14). Extensive macrocellular aggregation was observed when Rbf was expressed from a multicopy plasmid in S. aureus or S. epidermidis (14, 17). Overexpression of Rbf significantly increased icaA transcription and PIA/PNAG production in both wild-type and rbf mutant strains of S. aureus 8325-4 and UAMS-1 (12). The gene was also found to play a significant role in S. aureus virulence (18).
Microarray experiments revealed that rbf was able to reduce icaR transcription in a clinical isolate of S. aureus, strain UAMS-1 (12). This finding was confirmed by quantitative reverse transcription-PCR (qRT-PCR) experiments for both UAMS-1 and 8325-4 (12). Thus, it appears that rbf activates icaADBC expression, at least in part, by inhibiting expression of icaR. AraC/XylS proteins typically act as activators of transcription, but at least some, such as AraC, can also function as repressors (15, 16). These data suggested that Rbf might bind directly to the icaR-icaA promoter region. Experiments to test for binding of recombinant Rbf to the ica promoter yielded only negative results, however. These results suggested that Rbf may regulate ica expression through another factor (12).
Microarray experiments also revealed that Rbf regulates several genes that encode potential transcriptional regulatory proteins, including SarX, a member of the Sar family of transcriptional regulatory proteins (19). The sarX gene is positioned immediately downstream of rbf in the S. aureus chromosome (20). Overexpression of Rbf in the S. aureus clinical isolate UAMS-1 increased sarX expression by over 50-fold. SarX has also been shown to promote biofilm formation and icaADBC expression in S. epidermidis (21). Thus, sarX appeared to be a likely transcription factor through which Rbf may regulate ica expression. Here we report that SarX is an activator of icaADBC transcription and is required for biofilm formation in S. aureus. Additionally, we show that SarX binds with high affinity to icaA DNA. We also show that transcription of the sarX gene is dependent upon Rbf and provide evidence that Rbf binds to the sarX promoter in vivo.
MATERIALS AND METHODS
Bacterial strains and culture media.
The bacterial strains used in this study are listed in Table 1. Staphylococci were cultured in tryptic soy broth (TSB) (Difco Laboratories, Detroit, MI) or tryptic soy agar (TSA). In some experiments, growth medium was supplemented with glucose to a final concentration of 0.75% and NaCl to a final concentration of 3.5%, as described below. Antibiotics were added to S. aureus culture media, as appropriate, at final concentrations of 10 μg per ml chloramphenicol (Cm), 3 μg per ml tetracycline (Tc), 50 μg per ml kanamycin (Kn), and 150 ng per ml anhydrotetracycline (aTc). Escherichia coli strains DH5α and XL1-Blue were used for plasmid construction and maintenance. E. coli BL21 (λDE3) (plysS) was used for expression of recombinant Rbf and SarX. E. coli was cultivated in Luria-Bertani broth or agar (Difco) supplemented with, as appropriate, 100 μg per ml penicillin (Pen), 34 μg per ml Cm, or 50 μg per ml Kn.
Table 1.
Strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| 8325-4 | Prophage-free laboratory strain | J. Iandolo |
| RN4220 | Restriction-negative laboratory strain | J. Iandolo |
| SH1000 | rsbU-positive 8325-4 | 47 |
| S. aureus 8325-4 derivatives | ||
| CYL1112 | Wild type (pLI50) | 14 |
| CYL6968 | rbf (pLI50) | 14 |
| CYL6973 | Wild type (pYL8565) | 14 |
| CYL6974 | rbf (pYL8565) | 14 |
| CYL11688 | icaR (pLI50) | 12 |
| CYL11699 | icaR (pML3796) | This study |
| CYL11551 | sarX (pLI50) | This study |
| CYL11552 | sarX (pYL8565) | This study |
| CYL11580 | Wild type (pML3793) | This study |
| CYL11514 | rbf (pML3793) | This study |
| CYL11555 | sarX (pML3793) | This study |
| CYL11696 | icaADBC | 12 |
| CYL12512 | Wild type (pML100) | 27 |
| CYL12513 | Wild type (pAG4031) | This study |
| CYL12514 | Wild type (pAG4032) | This study |
| CYL12515 | rbf (pML100) | This study |
| CYL12516 | rbf (pAG4031) | This study |
| CYL12517 | rbf (pAG4032) | This study |
| CYL12518 | sarX (pML100) | This study |
| CYL12519 | sarX (pAG4031) | This study |
| CYL12520 | sarX (pAG4032) | This study |
| CYL12653 | sarX icaR (pLI50) | This study |
| CYL12654 | sarX icaR (pML3793) | This study |
| CYL12655 | sarX icaR (pML3796) | This study |
| CYL12656 | icaR (pML3793) | This study |
| CYL12657 | sarX (pML3796) | This study |
| CYL12642 | SH1000 (pLI50) | This study |
| CYL12646 | SH1000 rbf::Tn917 (pML4068) | This study |
| E. coli | ||
| DH5α | Host strain for plasmids | Invitrogen |
| XL1-Blue | Host strain for plasmids | Stratagene |
| BL21(λDE3)pLysS | Host for recombinant protein production | Novagen |
| Plasmids | ||
| E. coli | ||
| pET15b | Expression vector | Novagen |
| pET28a | Expression vector | Novagen |
| pAG3919 | His-SarX expression plasmid | This study |
| pTL3664 | His-Rbf expression plasmid | This study |
| S. aureus | ||
| pLI50 | E. coli-S. aureus shuttle vector | 48 |
| pML100 | aTc-inducible expression vector | 27 |
| pML3793 | pLI50 with sarX | This study |
| pYL8565 | pLI50 with rbf | 14 |
| pML3796 | pLI50 with icaR | 12 |
| pKOR1 | Vector for allele replacement | 22 |
| pAG4031 | pML100 with sarX | This study |
| pAG4032 | pML100 with sarX in antisense orientation | This study |
| pML4068 | S. aureus His-Rbf expression plasmid | This study |
Plasmid and strain construction.
To construct a sarX deletion mutant of 8325-4, PCR primer set attB1-sarX-KO1 and sarX-KO2 and primer set attB2-sarX-KO4 and sarX-KO3 (Table 2) were used to amplify the upstream (1.2-kb) and downstream (0.93-kb) fragments of the sarX gene, respectively. Advantage High-Fidelity 2 polymerase was used for amplification (Clontech, Mountain View, CA). The fragments were cloned into plasmid pKOR1 (22) using Gateway BP Clonase II enzyme mix (Invitrogen) and transformed into E. coli DH5α. The resulting plasmid, pML3792, was first transformed into S. aureus RN4220 by electroporation (23). Cm was used for selection of transformants. The plasmid was then transduced into 8325-4 using phage 52A. The sarX mutant was selected by using aTc as described by Bae and Schneewind (22). Allelic replacement was confirmed by PCR.
Table 2.
Oligonucleotide primers used in this study
| Primer purpose and name | Sequence |
|---|---|
| Plasmid and strain construction | |
| attB1-sarX-KO1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTCACTAGTATCACAAATAAAGCG |
| sarX-KO2 | CTATGCTTTCGACACTCAATTTCAATTACTAATTTCTCAGTATTCAAAATGTTGC |
| sarX-KO3 | GCAACATTTTGAATACTGAGAAATTAGTAATTGAAATTGAGTGTCGAAAGCATAG |
| attB2-sarX-KO4 | GGGGACCACTTTGTACAAGAAAGCTGGGTCTTTCAATACTTCTCGAATTGTC |
| sarXP1 | GAATTCCACCTTGATATGTATTGC |
| sarXP2 | GGATCCGCCGATAAGCAAATTCTATGC |
| Rbf-hisF | GGATCCGTCATACGCAACGTTTACCACG |
| Rbf-hisR | TTAGTGGTGATGGTGATGGTGTCCACCTTTTTTTCCTATTTTAATTATGTATAACGC |
| Pcp1rbf1 | CAGGTCGACTCTAGAGGATCCGTTTGCAAAATATACAGGGGATTATATATAATGGAAAAC |
| Pcp1rbf2 | TATACAGGGGATTATATATAATGGAAAACAAGAAAGGAAAATAGGAGGTTTATATGGC |
| pcp1rbf3 | ATATTCATTATTAGTAAGTATATGCAAGCATGATTTTGCCATATAAACCTCCTATTTTCC |
| pcp1rbf4 | GACTATGCCATCTTGGCAACGCGTTGTCGCATATTCATTATTAGTAAGTATATGCAAGC |
| rbf39 | ATACATATGGCAAAATCATGCTTGCATATAC |
| rbf41 | ATAGGATCCTTTTGCAATACATATCAAGGTG |
| sarX5 | ACGGCATATGAGAAAGACAATGGCAAAAAAATT |
| sarX6 | TACGGGATCCTCAAATATTTAAAAATTGTTCTACATCTTCAAATAAAGC |
| sarX7 | CATATGAACACCGAAAAACTGGAAACCCTG |
| sarX8 | GGATCCTTAGATGTTCAGGAACTGTTCAACGTCTTC |
| qRT-PCR | |
| sgSarx1 | TGTCCTACTTAAATCTAGCTCATCCATTGCAGTT |
| sgSarx2 | TGAATACTGAGAAATTAGAAACATTGCTTGGCTTCTATAAACA |
| sgicaA2 | GACCTCCCAATGTTTCTGGAACCAACATCC |
| icaA3 | GTCAGACGTTGGCTACTGGGATACTGATATG |
| icaRfor | TACGTTCAATTATCTAATACGCCTGAGGAATTTTCTGGAA |
| icaRrev | AGGATGCTTTCAAATACCAACTTTCAAGAAACAGCAAATATT |
| delta rbf for | ACGCGTTGCCAAGATGGCATAGTCTT |
| delta rbf rev | AGCCTAATTCCGCAAACCAATCGCTA |
| sggyrB3 | GGAATCGGTGGCGACTTTGATCTAGCGAAA |
| sggyrB4 | CGCTCCATCCACATCGGCATCAGTCATAAT |
| EMSAs and immunoprecipitation assays | |
| sgfnbAF2 | GAATATTTGCAAGGGTCAGATCAGGTTAATTTTAGAACTG |
| sgfnbAR2 | CTGTGTGGTAATCAATGTCAAGCGGTGTATTG |
| icaRP1 | CTGCAGGAATTTCTTTACCTACCTTTCGTTAG |
| icaRP2 | GGATCCAACATTTAACACTTTGTTCGTA |
| icaRP3 | GGATCCTCTTGTATTTGTCCGTAAATATTTCCAGAAAATTCC |
| icaAlong 1 | GGATCCGTGTCCCCCTTGAGCCCATC |
| icaAlong 2 | CTGCAGCTTATCCTTCAATTTTTATAACCCCCTACTG |
| icaAdc1 | CTGCAGCCATATGGCTTACAACCTAACTAACGAAAGGTAG |
| icaAdc2 | GGATCCGAAATAGTATTGACTGCGCCAGC |
| icaAdc3 | GGATCCGTGCATCTTGATCAACGATAGTATCTGCATC |
| icaAdc4 | GGATCCTTTCTTCTCGTATTTGAGTGCAAGAACATTAGACA |
| icaAdc5 | GGATCCACATTTATGTCAGGCTTCTTGTTCAATGAATATC |
| icaAdc6 | TTGCAATTTTTTAACTTTTTGCTTTTTTATCCTGTATTTATGTC |
| icaAdc7 | AGATATTCATTGAACAAGAAGCCTGACATAAATG |
| SAO0009F3 | AAGGTGCGCAATTAGAGCGTGCT |
| SAO0009R3 | TCTGCGTTCACAAGCTGTGGTACC |
| sarXdc7 | GAATTCGTTTATAGAAGCCAAGCAATGTTTCTAATTTCTCA |
| sarXdc8 | GGATCCGGAAAAAAATAACACCTTGATATGTATTGCA |
The sarX complementation plasmid pML3793 (pLI50-sarX) was constructed by PCR amplification of the 8325-4 sarX gene using primers sarXP1 and sarXP2. The amplified fragment was cut with BamHI and EcoRI and cloned into plasmid pLI50. The aTc-inducible sarX expression plasmid was similarly constructed. Primers sarX5 and sarX6 were used to PCR amplify sarX, and the resulting DNA fragment was cloned into pGEM-T Easy (Promega Corp., Madison, WI), resulting in plasmid pAG4084. Plasmid pAG4084 was digested with EcoRI, and the sarX-bearing DNA fragment was cloned into pML100 to create pAG4031 (pML100-sarX) and pAG4032 (pML100->sarX). Plasmids were transduced into the S. aureus strains listed in Table 1 using phage 80α or phage 52A.
Plasmid pTL3664 was constructed by PCR amplification of the rbf gene of 8325-4 using primers rbf39 and rbf41 (Table 2) and cloning it into the expression vector pET15b. Plasmid pAG3919 was constructed for expression of SarX in E. coli. A synthetic sarX gene, with codons that would be efficiently recognized in E. coli, was synthesized by EZBiolab Inc. (Carmel, IN). Based upon the studies of Manna and Cheung (20), we placed the sarX start codon at bp 664,327 of the 8325-4 genome (GenBank accession no. NC_007795) (24). The open reading frame of the synthetic gene was amplified using primers sarX7 and sarX8 and cloned into pET28a to form pAG3919.
Plasmid pML4068 (pLI50-His-Rbf) was constructed for constitutive expression of His-tagged Rbf in S. aureus by first ligating the PCR fragment containing the rbf gene with its promoter (primers rbf-hisF and rbf-hisR) to pLI50 at the EcoRI and BamHI sites. The rbf promoter in the resulting plasmid was then replaced with Pcap1 (25) by the sequence- and ligation-independent cloning (SLIC) method (26) using primers Pcp1rbf1, Pcp1rbf2, Pcp1rbf3, and Pcp1rbf4. The DNA insert was confirmed by sequencing.
Assays for biofilm and PIA/PNAG production.
Biofilm assays were performed in 96-well microtiter plates as described previously (14, 27). Assays for PNAG were performed as previously described (12).
RNA isolation and quantitative real-time PCR.
RNA was isolated as described previously (27). Quantitative real-time RT-PCR was performed as previously described (27) using the primers listed in Table 2. RNA from at least 2 cultures of each strain was analyzed.
Purification of recombinant SarX.
Expression of histidine-tagged SarX was done in E. coli BL21 (λDE3) (plysS). Expression was induced in log-phase cultures by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Three hours after induction, bacterial cells were harvested by centrifugation, subjected to osmotic shock (28), and then stored at −80°C. The cells were thawed, incubated with 400 μg per ml lysozyme (Sigma), and sonicated, and the resulting lysate was clarified by centrifugation. His-tagged SarX was purified from clarified lysates by metal affinity chromatography using reagents purchased from EMD Chemicals, San Diego, CA. Following chromatography, SarX protein was dialyzed against buffer containing 25 mM Tris-Cl (pH 7.5), 300 mM NaCl, and 1 mM EDTA. Dithiothreitol (DTT) (1 mM) was then added to the dialyzed protein, aliquoted, and stored at −80°C.
Electrophoretic mobility shift assays (EMSAs).
DNA fragments were generated by PCR amplification of 8325-4 DNA using the oligonucleotide primers listed in Table 2. The DNA fragments were end labeled with digoxigenin-dUTP using reagents purchased from Roche Applied Sciences, Indianapolis, IN. Binding reactions were performed in 20 μl of 25 mM Tris-Cl (pH 7.5), 1 mM EDTA, 75 mM NaCl, 1 mM DTT, 5% glycerol, and 200 ng poly-dIdC. Reaction mixtures were incubated for 15 min at room temperature and then electrophoresed through 4.0% or 5.0% polyacrylamide gels, buffered with [1/2] × Tris-borate-EDTA (44.5 mM Tris base, 44.5 mM boric acid, 1 mM EDTA, pH 8.0) at 5°C. The DNA fragments were then electroblotted onto nylon membrane (Applied Biosystems, Austin, TX). The digoxigenin-labeled DNA was detected using reagents purchased from Roche Applied Sciences. Negative-control (nonspecific) competitor DNAs were amplified with primer pair sarX7 and sarX8 or sgfnbAF2 and sgfnbAR2, with either pAG3919 or S. aureus genomic DNA, respectively, as the template.
Immunoprecipitation of His-Rbf DNA complexes.
The assay described by Benson et al. (29) was utilized to recover His-Rbf-DNA complexes from S. aureus CYL12646 and the negative-control strain CYL12642. Cultures were treated with 1% formaldehyde for 20 min at room temperature. After neutralization with 0.5 M glycine, the cells were washed twice with Tris-buffered saline (TBS) and stored at −80°C. Cells were thawed and incubated with lysostaphin in 10 mM Tris (pH 8.0), 20% sucrose, and 50 mM NaCl. Protoplasts were diluted with immunoprecipitation (IP) buffer (50 mM HEPES-KOH [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS) and sonicated. The lysates were filtered through 0.45-μm filters and then incubated with magnetic beads covalently coated with mouse monoclonal antibody against polyhistidine tags (GenScript, Piscataway, NJ). Mock incubations, containing lysates but no magnetic beads, were conducted in parallel. After incubation at room temperature for 2 h with end-over-end rotation, beads were washed three times with IP buffer, twice with wash buffer (10 mM Tris [pH 8.0], 250 mM LiCl, 0.5% Nonidet-P40, 0.5% sodium deoxycholate), and once with 10 mM Tris (pH 7.5). Bound material was then eluted by incubation at 65°C for 10 min in 50 mM Tris (pH 7.5) and 1% SDS. The eluted material was treated with RNase A and then incubated overnight at 65°C with proteinase K. DNA was recovered by extraction with phenol-chloroform and ethanol precipitation. Five or 10 ng of the recovered DNA was subjected to PCR using (separately) primer pair sarXdc7 and sarXdc8 (to amplify the sarX promoter region DNA), icaAP1 and icaAdc4 (to amplify the icaR-icaA intergenic region), or SAO0009F3 and SAO0009R3 (to amplify an irrelevant DNA region encoding seryl-tRNA synthetase [24]). PCR products were electrophoresed through 6% polyacrylamide gels. The immunoprecipitation experiment was performed 3 times with similar results.
RESULTS
Effects of SarX on biofilm formation, PIA/PNAG production, and icaADBC expression in S. aureus 8325-4.
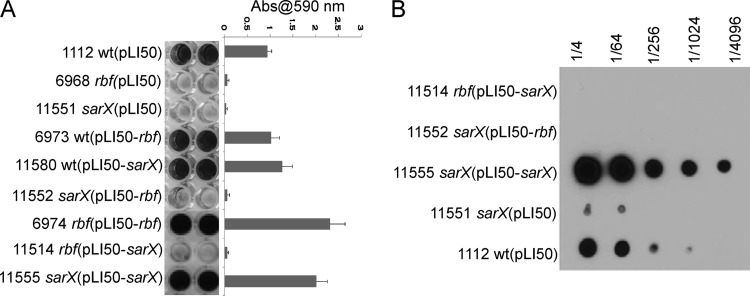
We previously reported that Rbf modulates expression of the icaR gene and the icaADBC operon in strain 8325-4 but that we were unable to detect binding of purified Rbf to the icaR or icaA promoter region (12). This suggested that the effect of Rbf on ica expression might be manifest through activation of another transcription factor. A likely candidate transcription factor is SarX. Transcription of the sarX gene is highly activated by Rbf (12), and SarX has recently been shown to affect biofilm formation by S. epidermidis (21). Therefore, we introduced an internal deletion of sarX in the 8325-4 chromosome. The resulting strain was unable to form a biofilm (Fig. 1A). The sarX mutation was able to be complemented with a multicopy plasmid (pLI50-sarX) carrying the wild-type sarX gene. Additionally, pLI50-sarX enhanced biofilm formation in the wild-type strain (Fig. 1A). Notably, a plasmid carrying the wild-type rbf gene, pLI50-rbf, suppressed the rbf mutation and enhanced biofilm formation in 8325-4 but had no significant effect on biofilm formation by the sarX mutant.
Fig 1.
Biofilm formation requires sarX. (A) Biofilm formation under static incubation conditions in 96-well plates inoculated with each of the 8325-4 derivatives listed to the left of the image. Following 24 h of incubation, wells were washed and biofilms were stained with crystal violet. Quantitation of biofilms is shown on the right side. Each assay was performed a minimum of 2 times. Error bars indicate standard deviations. (B) Regulation of PIA/PNAG production by sarX. PIA/PNAG was extracted from overnight cultures of each strain, serially diluted, and applied to a membrane. PNAG was detected by incubating the membrane, successively, with rabbit anti-PNAG serum, goat anti-rabbit horseradish peroxidase (HRP), and a chemiluminescent substrate. Numbers at the top of the figure indicate PNAG dilutions.
In order to determine whether SarX affects icaADBC expression, we measured icaA expression by qRT-PCR and PIA/PNAG production in a set of isogenic strains with mutations in sarX or rbf. The results in Table 3 and Fig. 1B suggest that the effect of SarX on biofilm formation is due largely to its effect on expression of the icaADBC genes and subsequent PIA/PNAG production. The above-described results also showed that although sarX was activated by Rbf, rbf was not activated by SarX (Table 3). These results are consistent with a model wherein Rbf affects biofilm, at least in part, by upregulating expression of sarX. SarX, in turn, activates ica and possibly other biofilm-related genes. However, pLI50-sarX was not able to suppress an rbf mutation at the level of icaADBC transcription, PIA/PNAG production, or biofilm formation, suggesting interdependency between Rbf and SarX. We reasoned either that this was due to poor expression of sarX in the rbf mutant or that Rbf affects biofilm formation by both SarX-dependent and SarX-independent mechanisms.
Table 3.
qRT-PCR assays to determine the effects of sarX on expression of icaA and icaR
| Strain [description] | Relative gene expressiona |
|||
|---|---|---|---|---|
| icaA | icaR | rbf | sarX | |
| 6973 [wt (pLI50-rbf)] | 10.65 ± 0.94 | 0.67 ± 0.07 | 6.52 ± 2.70 | 5.24 ± 0.50 |
| 11551 [sarX (pLI50)] | 0.27 ± 0.13 | 1.05 ± 0.04 | ND | <0.001 |
| 11552 [sarX (pLI50-rbf)] | 0.14 ± 0.08 | 1.01 ± 0.21 | 8.00 ± 5.3 | <0.001 |
| 11580 [wt (pLI50-sarX)] | 142 ± 23.8 | 0.39 ± 0.25 | 1.79 ± 0.13 | 9.13 ± 4.64 |
| 11514 [rbf (pLI50-sarX)] | 0.14 ± 0.03 | 1.02 ± 0.06 | <0.001 | 0.053 ± 0.01 |
| 11555 [sarX (pLI50-sarX)] | 152 ± 25.6 | 0.40 ± 0.07 | ND | 19.2 ± 1.41 |
| 11696 [icaADBC] | <0.01 | ND | ND | ND |
| 11688 [icaR (pLI50)] | ND | <0.001 | ND | ND |
| 6968 [rbf (pLI50)] | 0.26 ± 0.01 | ND | ND | 0.01 ± 0.002 |
The levels of mRNA (means and standard deviations, in arbitrary units) for each of the indicated genes are expressed relative to expression in the wild-type strain 1112 [wt (pLI50)]. ND, not done.
Suppression of an rbf mutation by sarX.
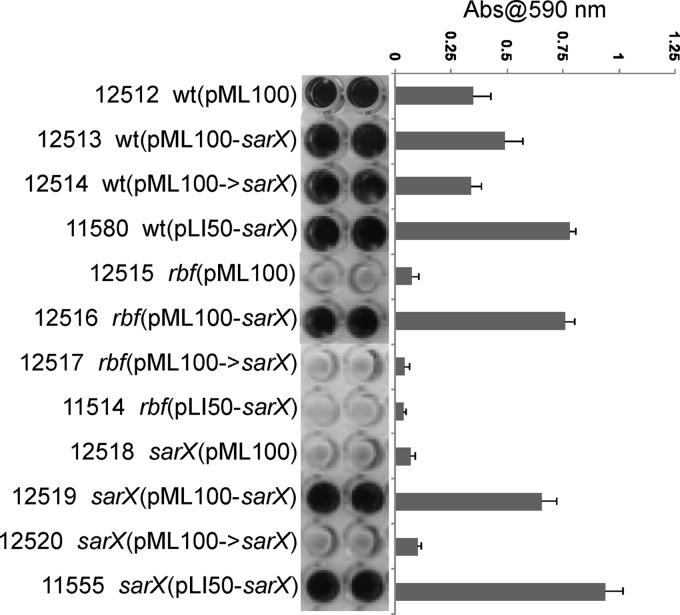
To decipher the Rbf-SarX interdependency, we tested the hypothesis that Rbf and SarX are part of a regulatory cascade wherein SarX is the primary effector molecule that acts at ica, and possibly other biofilm-related genes, and expression of sarX is highly dependent on Rbf. To this end, we placed sarX under the control of an anhydrotetracycline (aTc)-inducible promoter, Pxyl/tetO, in plasmid pML100 (27). Plasmids pML100-sarX and pML100->sarX are pML100 derivatives that carry the sarX gene in sense and antisense orientations, respectively. We found that pML100-sarX can promote biofilm formation in cultures grown with 150 ng per ml of aTc but not in cultures lacking aTc (data not shown). As shown in Fig. 2, pML100-sarX increased biofilm formation in the wild-type, rbf, and sarX strains (strains 12513, 12516, and 12519, respectively). Neither pML100 nor pML100->sarX significantly affected biofilm formation in any strain. These results indicated that the reason pLI50-sarX did not suppress the rbf mutation was due to poor expression of the plasmid-encoded, rbf-regulated sarX gene. Transcription of icaA was increased in both the rbf and sarX mutants carrying pML100-sarX, demonstrating that SarX is capable of modulating icaA expression in the complete absence of Rbf (Table 4). Thus, taken together, the above-described results suggest that Rbf promotes icaADBC expression and biofilm formation by activating sarX expression.
Fig 2.
Suppression of the rbf mutation by sarX. Biofilm formation under static incubation conditions. Assays were performed as described in the legend of Fig. 1 except that cultures contained 150 ng per ml anhydrotetracycline.
Table 4.
Expression of sarX and icaA in pML100-sarX transformants
| Strain [description] | Relative gene expressiona |
|
|---|---|---|
| sarX | icaA | |
| 12515 [rbf (pML100)] | 0.01 ± 0.0 | 0.42 ± 0.14 |
| 12516 [rbf(pML100-sarX)] | 6.55 ± 0.91 | 18.06 ± 1.62 |
| 12518 [sarX (pML100)] | 0.001 | 0.23 ± 0.03 |
| 12519 [sarX (pML100-sarX)] | 6.94 ± 0.76 | 7.19 ± 0.38 |
Numbers represent levels of mRNA (means and standard deviations, in arbitrary units), as measured by qRT-PCR assays, for each of the indicated genes, relative to expression by strain 12512 [8325-4 (pML100)]. Cultures were grown in the presence of 150 ng per ml anhydrotetracycline.
Binding of recombinant SarX protein to ica DNA.
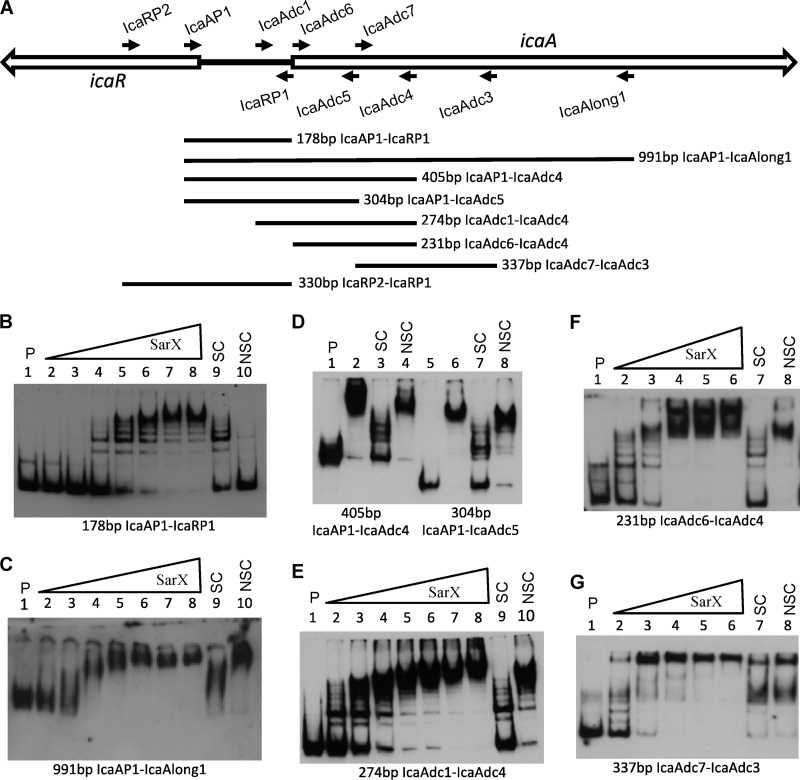
The S. epidermidis SarX protein has been shown to bind specifically to the icaA promoter and activates transcription of icaADBC (21). It seemed likely that SarX from S. aureus would display similar activity. To verify this, electromobility shift assays (EMSAs) were performed to measure binding of His-tagged SarX protein to the icaA promoter region. We first used the 178-bp PCR DNA fragment generated using oligonucleotide primers IcaAP1 and IcaRP1 (Fig. 3A), which extended from the fifth codon of icaR to the start codon of icaA and thus encompassed the entire 164-bp icaR-icaA intergenic region, including the putative icaA and icaR transcription start sites (7, 30). Surprisingly, although SarX bound the DNA fragment, binding appeared to be nonspecific, as it was able to be competed out with several different nonspecific competitor DNAs (Fig. 3B). Similar results were obtained using a labeled 330-bp DNA fragment (generated using primers icaRP2and icaRP1) that included an additional 152 bp of icaR DNA (data not shown).
Fig 3.
SarX binding to ica DNA and localization of the SarX binding region. (A) Locations of ica primers. Forward primers are listed above the genetic map, and reverse primers are listed below the map. PCR DNA fragments for use in EMSAs are shown in the lower portion of the figure. (B to G) EMSAs, with each of the different labeled PCR fragment probes indicated below the figure. P indicates labeled probe without SarX; SC and NSC indicate specific and nonspecific competitors, respectively, each at 50 molar excess. All assays were run on 5% acrylamide gels except that shown in panel C, which was on a 4% gel. The amounts of SarX in each assay are shown below. (B and C) Lanes 2 to 10 were 5.2, 15.3, 45.9, 138, 407, 814, 1,628, 407, and 407 nM, respectively. (D) Lanes 2 to 4 and 6 to 8 were all at 407 nM. (E) Lanes 2 to 10 were 25.4, 50.9, 102, 204, 407, 814, 1,628, 407, and 407 nM, respectively. (F and G) Lanes 2 to 8 were 52.0, 153, 407, 814, 1,628, 407, and 407 nM, respectively.
In further experiments, we found that SarX did bind specifically to a labeled 991-bp DNA fragment that extended 824 bp into the icaA open reading frame (ORF) (Fig. 3C). The affinity of SarX for this fragment was relatively high, with most of the labeled DNA being bound at a SarX concentration of approximately 40 nM. The SarX-DNA complex formed was able to be competed out with an unlabeled, specific competitor DNA fragment (Fig. 3C, lane 9) but not by a 50 molar excess of unlabeled, nonspecific competitor DNA (Fig. 3C, lane 10). Moreover, SarX binding occurred in the presence of 50 μg per ml of poly dIdC DNA or 200 μg per ml of herring sperm DNA (data not shown). Binding was inhibited by poly dAdT DNA at concentrations over 10 μg per ml, however, indicating that SarX may have a relatively high affinity for A-T-rich DNA sequences (data not shown). Importantly, the unlabeled ica promoter DNA fragment (used in Fig. 3B) did not significantly affect SarX binding to the 991-bp DNA fragment containing the promoter region and the icaA ORF (data not shown).
In order to further define the sarX binding region, we tested for binding to several different PCR fragments representing overlapping sequences of the ica promoter and icaA coding region. We first deleted DNA from the 3′ end of the icaA coding region. We found that SarX bound specifically to the 405-bp icaAP1-to-icaAdc4 and 304-bp icaAP1-to-icaAdc5 PCR products (Fig. 3D). We then deleted DNA from upstream of the icaA coding region. We found that SarX bound specifically to a 274-bp PCR fragment (generated using primers icaAdc1 and icaAdc4) which carried the putative icaA −10 promoter element and the icaA transcription start site but lacked a −35 promoter region (Fig. 3E). Interestingly, we found that SarX bound specifically to a 231-bp PCR fragment, generated using the icaAdc6 to icaAdc4 primers, which contained no sequence upstream of the icaA coding region (Fig. 3F). Further deletion of the icaA coding region, in the 337-bp icaAdc7-to-icaAdc3 PCR product, abolished the specific binding of SarX (Fig. 3G). Taken together, these results argue that DNA sequences 5′ to the icaA start codon are not required for SarX binding and that there is a high-affinity SarX binding site within the first 129 bp of the icaA coding region.
Binding of Rbf to the sarX promoter.
Due to the fact that Rbf increases the level of sarX transcripts, it seemed reasonable to expect that Rbf can bind to the sarX promoter region. However, we were unable to demonstrate sequence-specific binding of either His-Rbf or a maltose-binding Rbf fusion protein to the sarX promoter (data not shown). These results prompted us to see if we could detect Rbf binding to the sarX promoter in vivo. To accomplish this, we constructed an S. aureus strain (CYL12646) that expresses a His-tagged Rbf protein. The strain used in this experiment was chosen because we found a relatively high level of rbf expression in this strain (data not shown), a property we felt would increase the likelihood of detecting His-Rbf interaction with DNA. Cultures of strain 12646 were treated with formaldehyde to promote cross-linking of proteins to DNA. Cells were harvested from these cultures, incubated with lysostaphin, and lysed by sonication. The resulting cell-free lysates were incubated with magnetic beads coated with an antibody that recognizes His tags. Parallel mock incubations in which magnetic beads were omitted from the incubation were performed. The beads were washed, and bound proteins were eluted. DNA was recovered from the eluted samples and used in PCRs. As an additional negative control, cultures of CYL12642, which does not express any His-tagged protein, were subjected to the identical regimen.
To determine if Rbf-sarX promoter complexes were recovered, PCRs were performed with the recovered DNA using primers that are specific for the sarX promoter region. As shown in Fig. 4A, lane 3, the sarX promoter DNA fragment was able to be amplified from the DNA obtained from beads incubated with the strain 12646 lysate. This fragment was not amplified from the mock (i.e., without beads) incubation of the same lysate (lane 4). Moreover, the promoter fragment was not amplified from either of the 12642 strain samples (lanes 5 and 6). These results are consistent with the selective enrichment of His-Rbf-sarX promoter complexes from the strain 12646 lysate.
Fig 4.
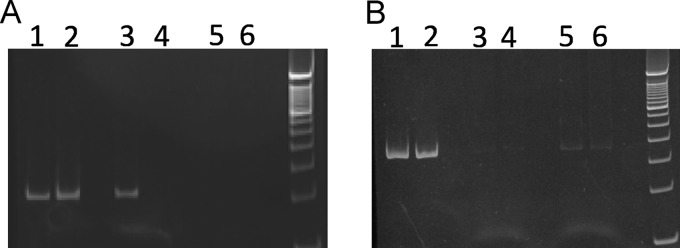
Immunoprecipitation of His-Rbf bound to the sarX promoter. Purified DNA recovered from CYL12646 (His-Rbf expression strain) and CYL12642 (negative-control strain) was PCR amplified with primers sarXdc7 and sarXdc8 (A) or SAO0009F3 and SAO0009R3 (B). Lanes 1, 3, and 4 are from 12646, and lanes 2, 5, and 6 are from 12642. DNA recovered from cell lysates prior to incubation with magnetic beads was used for the reactions shown in lanes 1 and 2. DNA for the reactions shown in lanes 3 and 5 was recovered from incubation of magnetic beads with extracts. Material recovered from mock incubations (extracts without beads) was used for reactions in lanes 4 and 6.
As an additional control, the same DNA templates used for Fig. 4A were subjected to PCR using primers specific for an irrelevant DNA sequence encoding seryl-tRNA synthetase (SAOUHSC_00009). We observed no enrichment of DNA in the experiment (Fig. 4B). Collectively, these results are consistent with the idea that Rbf binds to the sarX promoter in vivo. We were unable to detect enrichment of icaA DNA in these same experiments (data not shown).
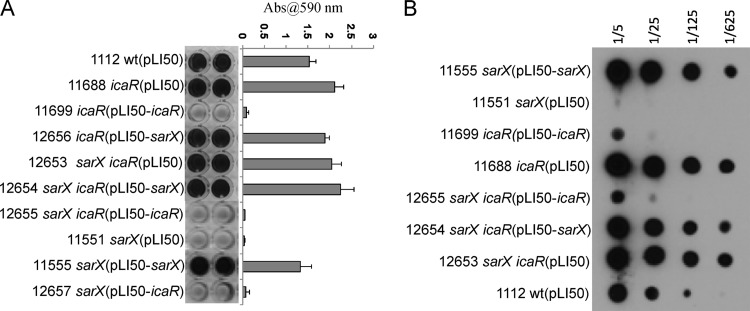
Regulation of biofilm formation by icaR and sarX.
We have shown that Rbf affects transcription of the icaADBC genes by downregulating icaR transcription and thereby upregulating transcription of icaADBC (12). These results, in conjunction with the findings reported above, strongly suggest that SarX may enhance PIA/PNAG production and biofilm formation by repressing transcription of icaR, which, in turn, would increase icaADBC transcription. To test this, we first performed biofilm assays with icaR and sarX mutants and the icaR sarX double mutant. As shown in Fig. 5A, inactivation of icaR (strain 11688) resulted in derepression of biofilm formation and repression was restored by transformation of the mutant with an icaR-bearing plasmid, pLI50-icaR (strain 11699). In the icaR sarX double mutant, biofilm formation was similar to that of the icaR mutant. These results indicate that the effect of icaR is epistatic to sarX, evidence that icaR acts downstream of sarX in biofilm regulation. We also performed complementation of the icaR sarX double mutant with either the sarX-bearing plasmid, pLI50-sarX, or the icaR-bearing plasmid, pLI50-icaR (strains 12654 and 12655, respectively). We found that transformation of the double mutant with pLI50-icaR repressed biofilm formation. In contrast, pLI50-sarX appeared not to affect biofilm formation relative to the double mutant strain.
Fig 5.
icaR is epistatic to sarX. (A) Biofilm assays and quantitation were performed as described in the legend of Fig. 1. (B) PIA/PNAG production. Assays were performed as described in the legend of Fig. 1.
To confirm that increased biofilm formation was associated with increased PNAG levels, immunoassays for PNAG were performed (Fig. 5B). The results showed that the icaR sarX double mutant produced approximately the same amount of PIA/PNAG as the icaR mutant. Transformation of the icaR or icaR sarX mutant with pLI50-icaR (carrying icaR) decreased PNAG synthesis to less than the wild-type level. Carriage of pLI50-sarX had no significant effect on PIA/PNAG production in the double mutant. These results confirm that icaR functions downstream of sarX.
To test whether SarX affects icaR expression, we performed qRT-PCR in the sarX mutant and complemented strains. The results in Table 3 indicate that the sarX mutation did not appreciably affect icaR expression (strain 11551). However, overexpression of sarX resulted in repression of icaR (see strains 11580 and 11555). These results indicate that SarX significantly represses icaR transcription only when SarX is overproduced.
DISCUSSION
The production of PIA/PNAG is an important contributing factor to biofilm formation by staphylococci. The genes encoding PIA/PNAG biosynthetic proteins, icaADBC, are subject to regulation by numerous factors. One factor that plays an important role in activating icaADBC expression is Rbf (12). Because Rbf is a member of the AraC/XylS family of proteins, many of which are known transcriptional activators, we anticipated that Rbf would bind directly to the icaA-icaR intergenic region. This appears not to be the case, however, as we have been unable to detect specific binding of recombinant Rbf protein to ica DNA. Instead, Rbf seems to increase icaADBC expression by upregulating transcription of sarX. SarX, in turn, activates icaADBC expression. In support of this proposal, a mutation in rbf reduced transcription of sarX by approximately 4-fold and overexpression of Rbf enhanced sarX expression by 5- to-6-fold. Although we have been able to demonstrate binding of recombinant Rbf to a DNA fragment encompassing the putative sarX promoter in vitro, several different “nonspecific” DNA fragments readily compete for Rbf binding. These results suggest that the in vitro interaction of Rbf with the sarX promoter is nonspecific. In immunoprecipitation experiments using antibody against histidine-tagged proteins, we were able to enrich for sarX promoter DNA cross-linked to His-Rbf. On the other hand, no amplification was detected in negative-control experiments in which either antibody was omitted from the immunoprecipitation reaction or when the immunoprecipitation was performed with a strain that did not express His-Rbf. In addition, neither an irrelevant DNA sequence encoding seryl-tRNA synthetase nor even the icaA promoter region was enriched. Collectively, these results support the argument that Rbf selectively binds the sarX promoter in vivo.
The failure to detect specific binding of Rbf in vitro may be due to a number of factors. First, some AraC-like proteins, including AraC (15, 16, 31, 32), have been implicated in DNA looping, which requires protein interaction with multiple binding sites. DNA binding by some members of the AraC/XylS family involves protein binding to sites up to several hundred bp upstream or downstream of a regulated promoter. Thus, it is possible that Rbf binds to DNA sites distal to the sarX promoter or requires binding to multiple sites to form a stable complex with DNA. Second, protein solubility is another factor that may be relevant to binding of recombinant Rbf to DNA. AraC-like proteins are notoriously insoluble. We have been able to isolate relatively small amounts of recombinant Rbf from E. coli, but the vast amount of the protein is insoluble. We cannot be certain that the soluble fraction of recombinant Rbf, which was used in our experiments, is in its native conformation. Third, it is possible that Rbf undergoes some form of posttranslational modification in vivo that affects its interaction with DNA. Alternatively, Rbf binding may be influenced by a cofactor, such as a low-molecular-weight molecule, that is present in S. aureus. In this regard, we have isolated His-tagged Rbf directly from S. aureus and showed that the protein bound to sarX promoter but nonspecifically (data not shown), resembling the results using recombinant protein isolated from E. coli. These results suggest that a cofactor(s) that may be required for specific binding is (are) absent in our in vitro EMSA experiments.
The Sar family of proteins is composed of at least 11 different proteins, some of which are found in both S. aureus and S. epidermidis. The various Sar proteins have been categorized as fitting into one of three subfamilies (19). The proteins in one subfamily, which includes SarA and SarX, are generally small, about 15-kDa, basic proteins with a single DNA binding domain that probably bind DNA as homodimers. SarA has a central core region comprised of a winged-helix DNA binding domain in which the helix-turn-helix domain recognizes the major groove and the winged region interacts with the minor groove. SarA has a conserved α-helical region near the N terminus of the protein that mediates protein dimerization (19, 33–35). These structural elements appear to be conserved in SarX, suggesting that SarA and SarX may affect transcription by a similar mechanism. Although SarA has been characterized as a DNA binding protein, no true consensus sequence of a SarA binding site, other than that the protein has a high propensity for binding A-T-rich DNA, has emerged. SarX binding to DNA is inhibited by poly-dAdT, suggesting that SarX also has a propensity for binding A-T-rich DNA. Recently, Morrison et al. (36) reported that SarA binds a variety of mRNA molecules and protects them from degradation. It is possible that SarX may affect the steady-state level of icaADBC RNA by a similar mechanism. To date, we have not determined the effect of SarX on the half-life of icaADBC RNA. In S. epidermidis CSF41498, SarX was found to upregulate biofilm formation in an ica-dependent manner (21). In that study, expression of S. epidermidis sarX on a multicopy plasmid not only complemented a sarX mutation but also enhanced biofilm formation by the wild-type strain. S. epidermidis SarX has also been shown to bind ica DNA (21). Thus, SarX appears to function similarly in S. aureus and S. epidermidis. However, Manna and Cheung (20) reported that sarX did not affect biofilm formation in S. aureus RN6390. It is unclear why the difference exists between RN6390 and 8325-4, since they are closely related strains derived from the same parent strain, NCTC8325.
We have clearly shown that SarX can bind specifically to ica DNA with high affinity in vitro. Surprisingly, however, we found that the SarX binding site was within the icaA coding region. While this is an unusual finding, it is not without precedent. The E. coli Rns protein is an activator of its own transcription that has a binding site within the rns open reading frame (31), and the S. aureus SarA protein has a binding site located downstream of its own promoter (34). However, our epistasis assays showed that SarX activation of icaADBC is through icaR, suggesting that the binding of SarX interferes with IcaR repression (Fig. 5). It is still unknown how IcaR represses icaADBC transcription, but it is most likely by blocking RNA polymerase binding to the icaA promoter. It has been argued that SarA is a histone-like architectural protein that modifies DNA topology. In fact, it has been shown that SarA can partially substitute for the bacteriophage λ Xis protein in integrase-mediated excision of λ from an att site (37). SarA binding has also been shown to cause DNA bending (38). Thus, it is possible that SarX binding within the icaA coding region may alter DNA topology such that the affinity of IcaR for the icaADBC promoter is decreased and/or the affinity of RNA polymerase is increased. Alternatively, effective repression of icaR may require binding to an additional site within the icaA coding region, exemplified by lacI repression of the lac operon in E. coli (39).
Our qRT-PCR results showed that deletion of sarX did not affect icaR transcription but profoundly affected icaA transcription and PIA/PNAG production (Table 3 and Fig. 5). The qRT-PCR data also showed that repression of icaR transcription was observed only with a multiple-copy plasmid carrying the sarX gene. These results suggest that a relatively low level of SarX does not affect icaR expression but can partially block IcaR repression of icaADBC whereas a high level of SarX not only affects icaR repression of icaADBC but also inhibits transcription of icaR. How can SarX function differently depending on its concentration? One clue may come from the EMSA results (Fig. 3), which showed that SarX bound specifically to the icaA coding region upstream of the icaR binding site and may also bind, nonspecifically, to the intergenic region containing the icaR and icaA promoters. These results suggest that when the concentration of SarX is low, the protein binds to the specific site within the icaA coding region. At a high concentration, SarX also binds to the intergenic region containing the icaR promoter. Binding to the icaA coding region may interfere with IcaR repression as discussed above, but binding may be too far upstream to affect icaR transcription whereas binding to the intergenic region would most likely inhibit icaR transcription. SarX binding to multiple sites in ica DNA probably accounts for the ladder of SarX-DNA complexes observed in EMSAs (Fig. 3) (20). We have recently started to map the transcription initiation sites of icaR. Our preliminary results suggest that the icaR promoter is located close to the icaADBC promoter in the central portion of the 164-bp intergenic region. Thus, binding of SarX in this region, though nonspecific, would be expected to block icaR transcription. Additionally, SarX binding in the intergenic region may promote RNA polymerase binding to the icaA promoter, which may sterically hinder polymerase binding to the icaR promoter.
Based on the above-described interpretation of our results, we propose the following model. At a low level of expression, SarX binds to the specific SarX binding site within the icaA coding region with high affinity, which interferes with IcaR repression of icaADBC but does not significantly affect icaR transcription. Under this condition, IcaR can still repress icaADBC but not fully. At a higher level of expression, in addition to binding in the icaA coding region, SarX binds to the icaR/icaA promoter region. This would stabilize RNA polymerase binding to the icaADBC promoter and effectively reduce icaR transcription. Under this condition, icaADBC is fully expressed. We also hypothesize that the level of SarX is likely to be controlled by Rbf, whose activity can be modulated in response to stimuli, such as a cofactor discussed above.
It has been demonstrated that the SarX protein of S. aureus RN6390 binds to the agr promoter, repressing synthesis of RNAII and RNAIII and thereby indirectly repressing exoprotein synthesis (20). SarX from S. epidermidis strain CSF41498 also binds its cognate agr promoter and represses agr transcription (21). Agr is a negative regulator of biofilm formation; thus, agr repression by SarX would be predicted to enhance biofilm formation (40–42). The agr effect on biofilm is ica independent, so agr repression is a second mechanism by which sarX can regulate biofilm production.
It is important to note that there are some apparent discrepancies regarding biofilm formation by 8325-4 and related strains. Although several laboratories have found that 8325-4 and its parent strain RN1 can form biofilms under laboratory conditions (12, 17, 43, 44), others report that this strain is a poor former of biofilms. For example, it has been reported that 8325-4 does not form a biofilm whereas strain SA113, also of the 8325 lineage, forms an ica-dependent biofilm (1, 5). S. aureus SH1000, a SigB-positive (SigB+) derivative of 8325-4, has been reported to form a PIA/PNAG-independent biofilm, but rsbU mutants of SH1000 (which have a SigB-negative [SigB−] phenotype) do not form biofilms (45). These results are at odds with our findings, as the major genetic difference between 8325-4 and SH1000 is a functional rsbU gene in the latter strain (5). 8325-4 and SH1000 also differ by 3 single nucleotide changes and a deletion upstream of spa in SH1000 (46). It is possible that these mutations account for the difference in biofilm formation by 8325-4 and SH1000 rsbU. Alternatively, strains derived from NCTC8325 may have acquired mutations after they were segregated into different laboratories. In this regard, we have checked our laboratory 8325-4 strain by a blood agar method and found that the Agr activity was reduced compared to that of RN6390, a strain derived from 8325-4 (43), suggesting that agr expression in our 8325-4 isolate may be reduced relative to that in some other closely related strains, an alteration that may affect biofilm formation.
The findings presented here have increased our understanding of how Rbf increases expression of icaADBC. Many questions remain, however, as we do not completely understand what signals induce rbf expression in vivo, how Rbf upregulates sarX expression, or precisely how SarX promotes icaADBC transcription. Further study of the interplay between Rbf, SarX, and ica will undoubtedly uncover novel methods of gene regulation.
ACKNOWLEDGMENTS
We thank J. P. O'Gara and S. Rowe for providing the maltose-binding Rbf recombinant protein, T. Maira-Litran and G. B. Pier for anti-PNAG antisera, and M. A. Benson and V. J. Torres for providing a detailed protocol for the immunoprecipitation experiments.
This work was supported by grant AI067857 from the National Institute of Allergy and Infectious Diseases.
DNA sequencing was performed at the UAMS Sequencing Core Facility.
Footnotes
Published ahead of print 25 January 2013
REFERENCES
- 1. Gotz F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43: 1367–1378 [DOI] [PubMed] [Google Scholar]
- 2. O'Gara JP. 2007. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol. Lett. 270: 179–188 [DOI] [PubMed] [Google Scholar]
- 3. Otto M. 2008. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322: 207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178: 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67: 5427–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cerca N, Brooks JL, Jefferson KK. 2008. Regulation of the intercellular adhesin locus regulator (icaR) by SarA, σB, and IcaR in Staphylococcus aureus. J. Bacteriol. 190: 6530–6533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cue D, Lei MG, Lee CY. 2012. Genetic regulation of the intercellular adhesion locus in staphylococci. Front. Cell. Infect. Microbiol. 2: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182: 6824–6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jefferson KK, Cramton SE, Gotz F, Pier GB. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 48: 889–899 [DOI] [PubMed] [Google Scholar]
- 10. Jefferson KK, Pier DB, Goldmann DA, Pier GB. 2004. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J. Bacteriol. 186: 2449–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penades JR, Lasa I. 2003. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 48: 1075–1087 [DOI] [PubMed] [Google Scholar]
- 12. Cue D, Lei MG, Luong TT, Kuechenmeister L, Dunman PM, O'Donnell S, Rowe S, O'Gara JP, Lee CY. 2009. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J. Bacteriol. 191: 6363–6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conlon KM, Humphreys H, O'Gara JP. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J. Bacteriol. 184: 4400–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim Y, Jana M, Luong TT, Lee CY. 2004. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J. Bacteriol. 186: 722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. 1997. Arac/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61: 393–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schleif R. 2010. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol. Rev. 34: 779–796 [DOI] [PubMed] [Google Scholar]
- 17. Rowe S. 2010. Contribution of Rbf and SarX to biofilm regulation in Staphylococcus epidermidis. PhD thesis. University College, Dublin, Ireland [Google Scholar]
- 18. Luong TT, Lei MG, Lee CY. 2009. Staphylococcus aureus Rbf activates biofilm formation in vitro and promotes virulence in a murine foreign body infection model. Infect. Immun. 77: 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheung AL, Nishina KA, Trotonda MP, Tamber S. 2008. The SarA protein family of Staphylococcus aureus. Int. J. Biochem. Cell Biol. 40: 355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manna AC, Cheung AL. 2006. Expression of SarX, a negative regulator of agr and exoprotein synthesis, is activated by MgrA in Staphylococcus aureus. J. Bacteriol. 188: 4288–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rowe SE, Mahon V, Smith SG, O'Gara JP. 2011. A novel role for SarX in Staphylococcus epidermidis biofilm regulation. Microbiology 157: 1042–1049 [DOI] [PubMed] [Google Scholar]
- 22. Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55: 58–63 [DOI] [PubMed] [Google Scholar]
- 23. Kraemer GR, Iandolo JJ. 1990. High-frequency transformation of Staphylococcus aureus by electroporation. Curr. Microbiol. 21: 373–376 [Google Scholar]
- 24. Glllaspy AF, Worrel V, Orvis J, Roe BA, Dyer DW, Iandolo JJ. 2006. The Staphylococcus aureus NCTC 8325 genome, p 381–412 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood JI. (ed), Gram-positive pathogens, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 25. Ouyang S, Lee CY. 1997. Transcriptional analysis of type 1 capsule genes in Staphylococcus aureus. Mol. Microbiol. 23: 473–482 [DOI] [PubMed] [Google Scholar]
- 26. Li MZ, Elledge SJ. 2007. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 4: 251–256 [DOI] [PubMed] [Google Scholar]
- 27. Lei MG, Cue D, Roux CM, Dunman PM, Lee CY. 2011. Rsp inhibits attachment and biofilm formation by repressing fnbA in Staphylococcus aureus MW2. J. Bacteriol. 193: 5231–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magnusdottir A, Johansson I, Dahlgren LG, Nordlund P, Berglund H. 2009. Enabling IMAC purification of low abundance recombinant proteins from E. coli lysates. Nat. Methods 6: 477–478 [DOI] [PubMed] [Google Scholar]
- 29. Benson MA, Lilo S, Nygaard T, Voyich JM, Torres VJ. 2012. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J. Bacteriol. 194: 4355–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20: 1083–1091 [DOI] [PubMed] [Google Scholar]
- 31. Munson GP, Scott JR. 2000. Rns, a virulence regulator within the AraC family, requires binding sites upstream and downstream of its own promoter to function as an activator. Mol. Microbiol. 36: 1391–1402 [DOI] [PubMed] [Google Scholar]
- 32. Wade JT, Belyaeva TA, Hyde EI, Busby SJ. 2000. Repression of the Escherichia coli melR promoter by MelR: evidence that efficient repression requires the formation of a repression loop. Mol. Microbiol. 36: 223–229 [DOI] [PubMed] [Google Scholar]
- 33. Liu Y, Manna AC, Pan CH, Kriksunov IA, Thiel DJ, Cheung AL, Zhang G. 2006. Structural and function analyses of the global regulatory protein SarA from Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 103: 2392–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheung AL, Nishina K, Manna AC. 2008. SarA of Staphylococcus aureus binds to the sarA promoter to regulate gene expression. J. Bacteriol. 190: 2239–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schumacher MA, Hurlburt BK, Brennan RG. 2001. Crystal structures of SarA, a pleiotropic regulator of virulence genes in S. aureus. Nature 409: 215–219. [DOI] [PubMed] [Google Scholar]
- 36. Morrison JM, Anderson KL, Beenken KE, Smeltzer MS, Dunman PM. 2012. The staphylococcal accessory regulator, SarA, is an RNA-binding protein that modulates the mRNA turnover properties of late-exponential and stationary phase Staphylococcus aureus cells. Front. Cell. Infect. Microbiol. 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujimoto DF, Higginbotham RH, Sterba KM, Maleki SJ, Segall AM, Smeltzer MS, Hurlburt BK. 2009. Staphylococcus aureus SarA is a regulatory protein responsive to redox and pH that can support bacteriophage lambda integrase-mediated excision/recombination. Mol. Microbiol. 74: 1445–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reyes D, Andrey DO, Monod A, Kelley WL, Zhang G, Cheung AL. 2011. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J. Bacteriol. 193: 6020–6031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oehler S, Eismann ER, Kramer H, Muller-Hill B. 1990. The three operators of the lac operon cooperate in repression. EMBO J. 9: 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beenken KE, Mrak LN, Griffin LM, Zielinska AK, Shaw LN, Rice KC, Horswill AR, Bayles KW, Smeltzer MS. 2010. Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PLoS One 5: e10790 doi:10.1371/journal.pone.0010790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. 2009. Interconnections between sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect. Immun. 77: 1623–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vuong C, Saenz HL, Gotz F, Otto M. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182: 1688–1693 [DOI] [PubMed] [Google Scholar]
- 43. Herbert S, Ziebandt AK, Ohlsen K, Schafer T, Hecker M, Albrecht D, Novick R, Gotz F. 2010. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 78: 2877–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. 2012. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 8: e1002626 doi:10.1371/journal.ppat.1002626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boles BR, Thoendel M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5: e10146 doi:10.1371/journal.pone.0010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Neill AJ. 2010. Staphylococcus aureus SH1000 and 8325-4: comparative genome sequences of key laboratory strains in staphylococcal research. Lett. Appl. Microbiol. 51: 358–361 [DOI] [PubMed] [Google Scholar]
- 47. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184: 5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee CY, Buranen SL, Ye ZH. 1991. Construction of single-copy integration vectors for Staphylococcus aureus. Gene 103: 101–105 [DOI] [PubMed] [Google Scholar]