Abstract
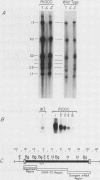
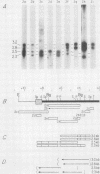
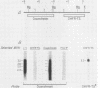
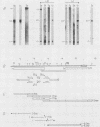
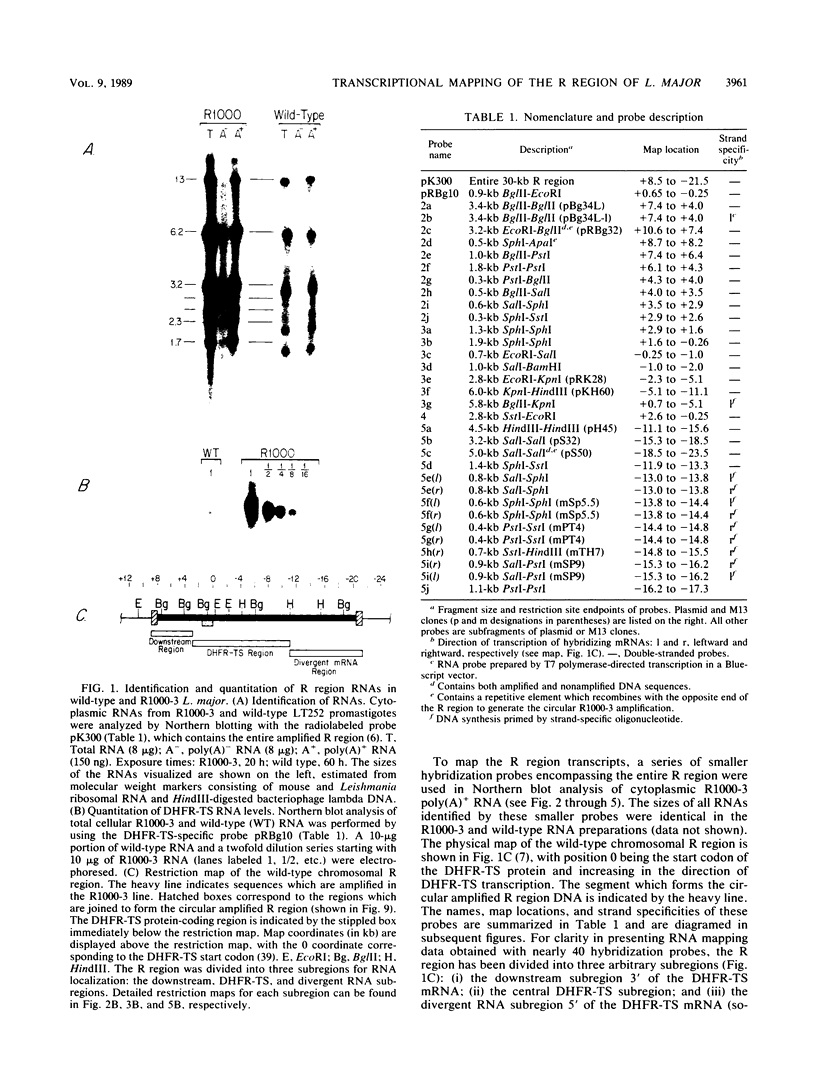
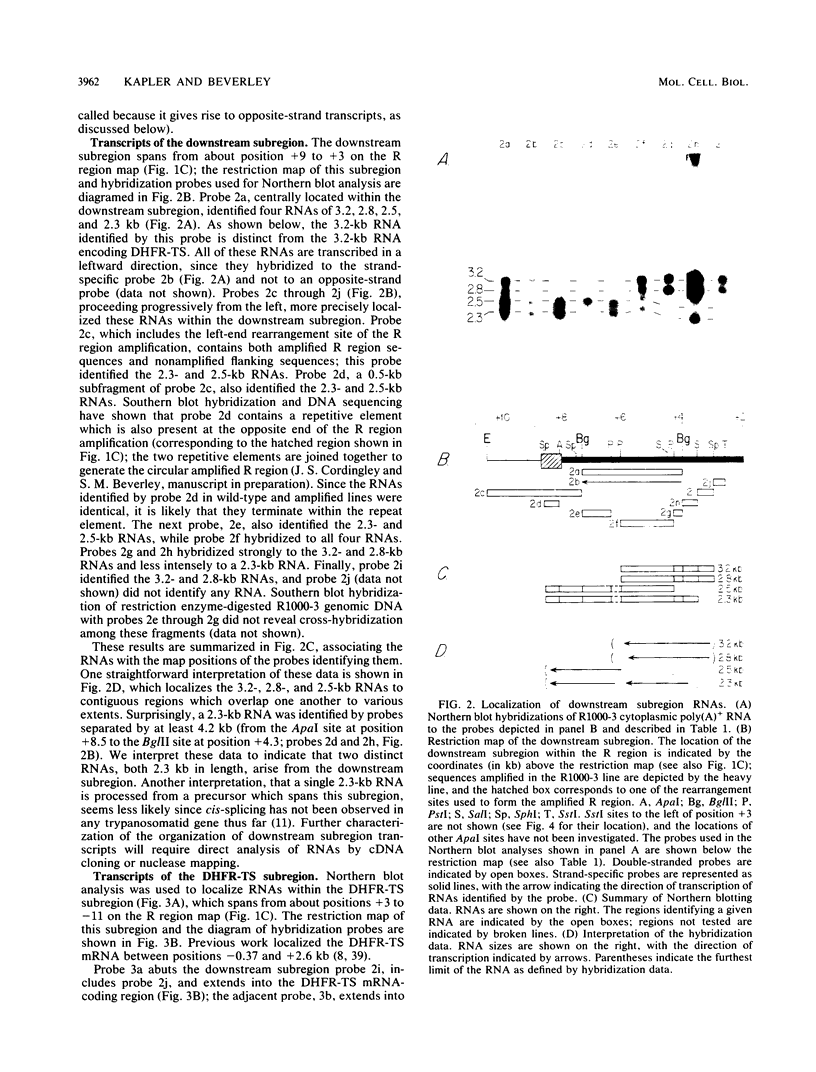
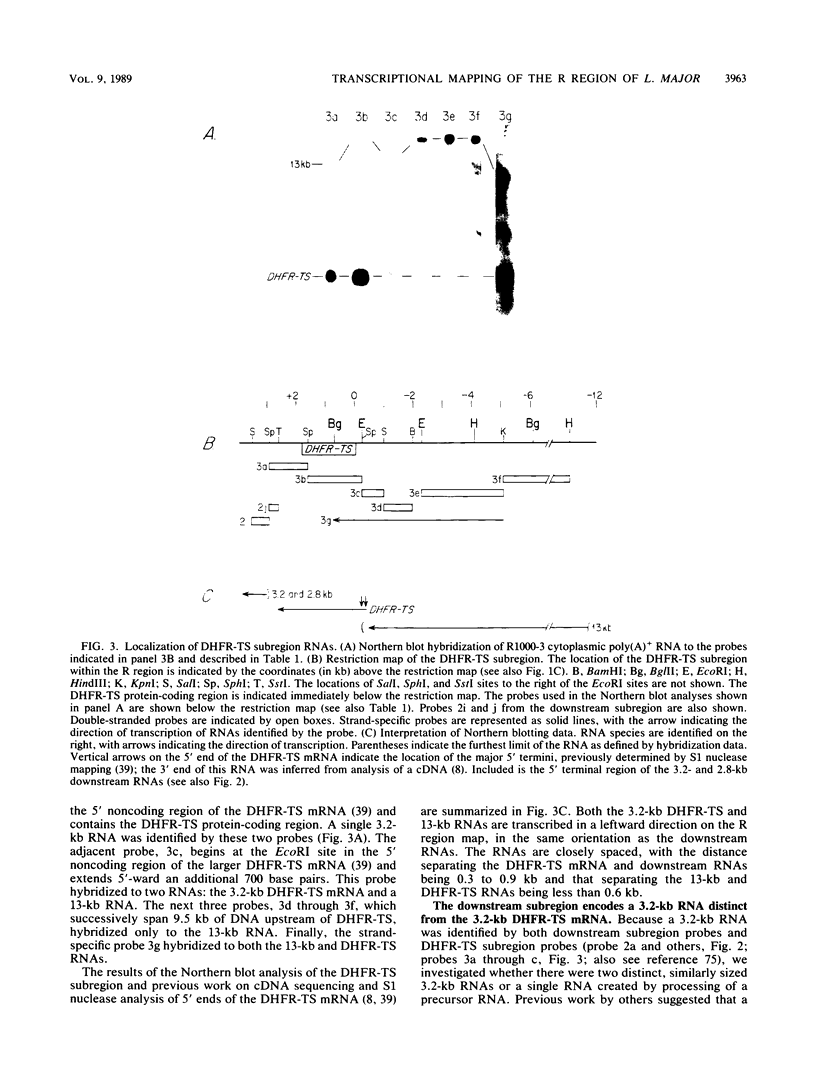
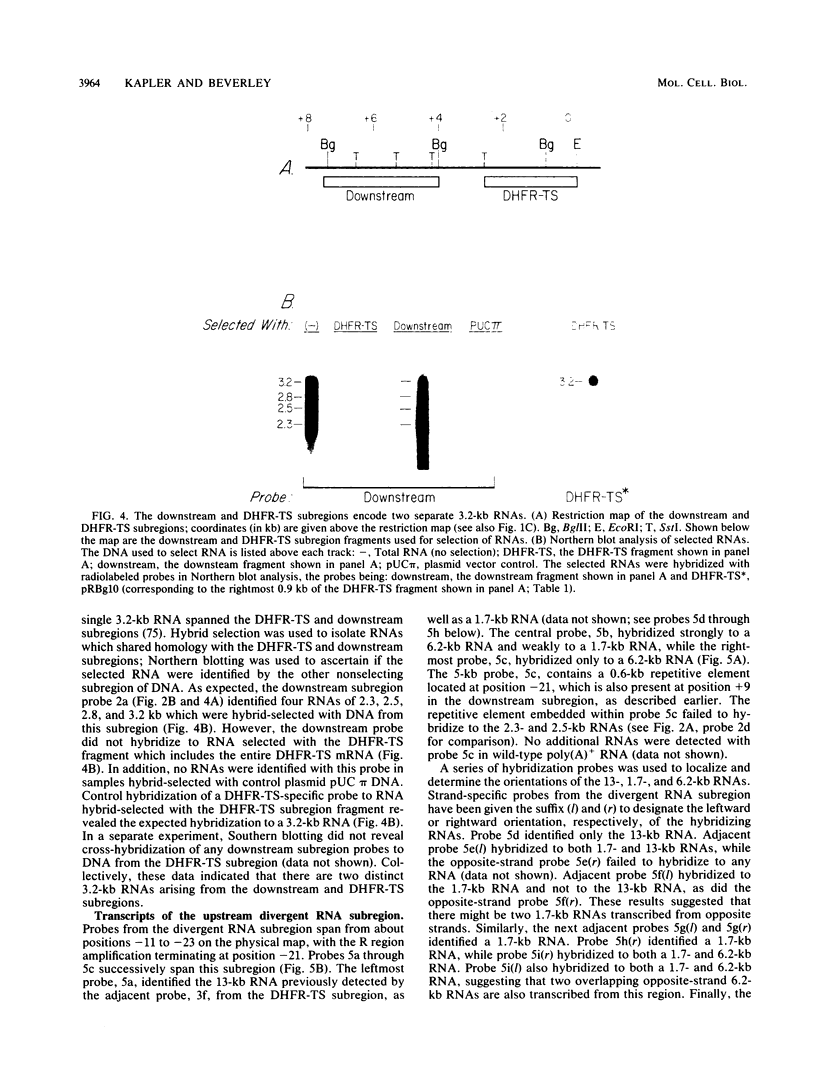
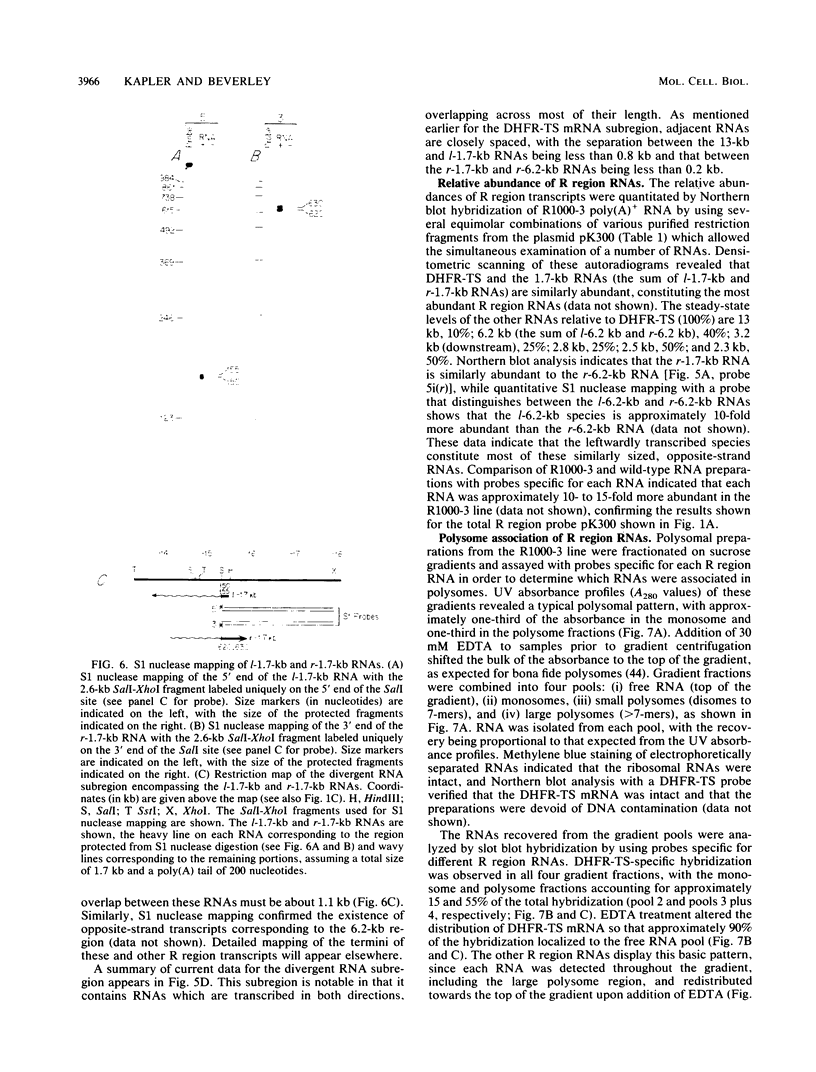
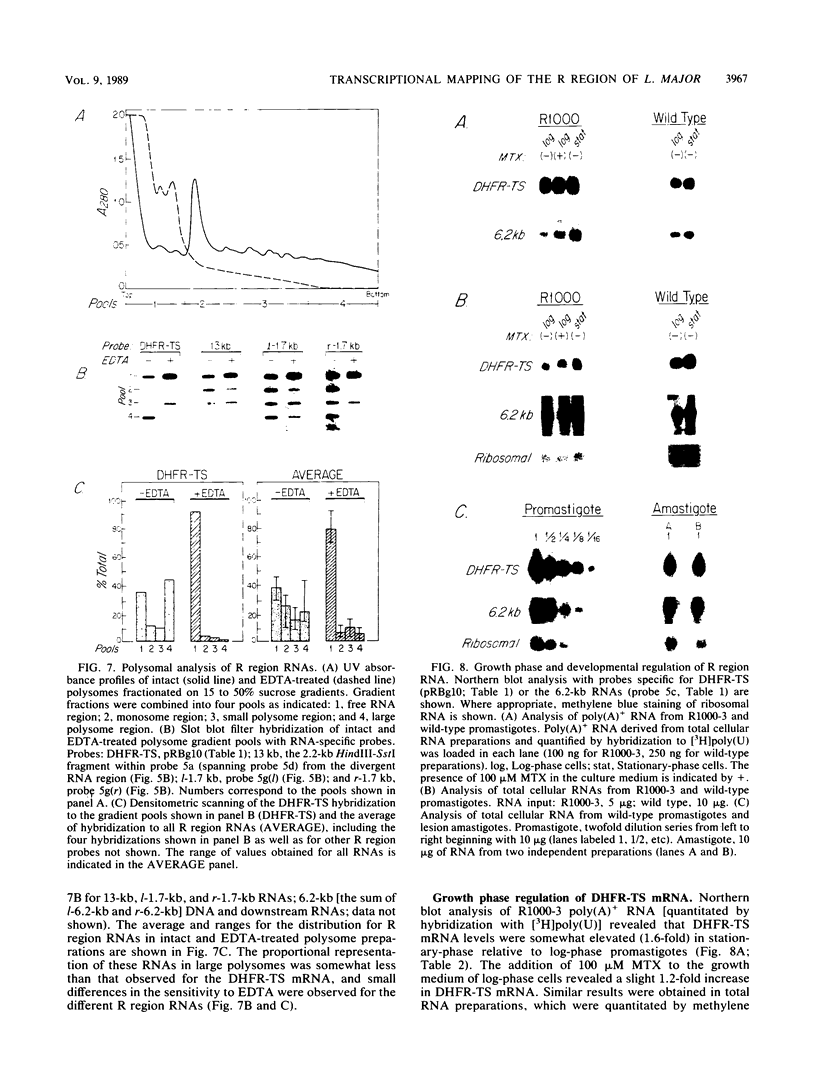
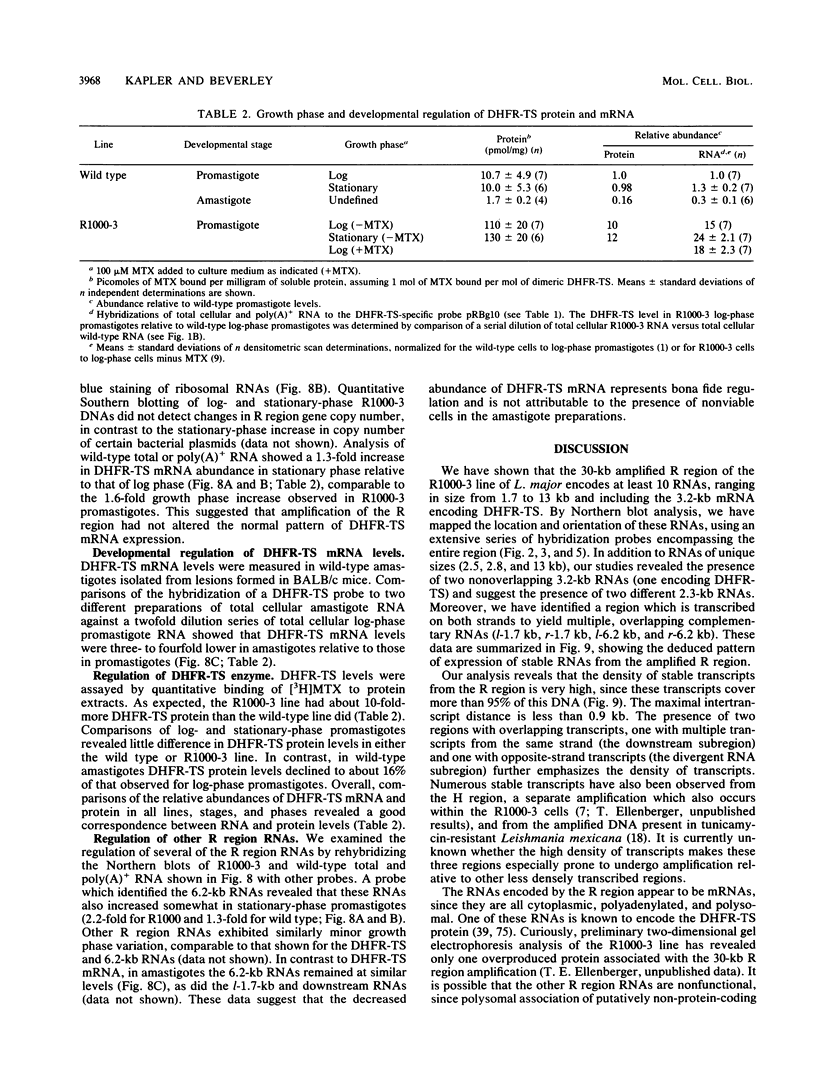
We have examined the transcriptional organization of the R region of the protozoan parasite Leishmania major. This region encodes the bifunctional enzyme dihydrofolate reductase-thymidylate synthase (DHFR-TS) and is frequently amplified as a 30-kilobase (kb) extrachromosomal circular DNA in methotrexate-resistant lines. Northern (RNA) blot analysis shows that the R region encodes at least 10 stable cytoplasmic polysomal poly(A)+ RNAs, ranging in size from 1.7 to 13 kb and including the 3.2-kb DHFR-TS mRNA. Transcriptional mapping reveals that these RNAs are closely spaced and collectively cover more than 95% of the 30-kb amplified R region. The organization is complex, including several overlapping RNAs 3' of DHFR-TS and two examples of antisense RNAs 5' of DHFR-TS. The R region RNAs can be grouped into two empirical domains, with eight contiguous RNAs transcribed in the same direction as that of DHFR-TS and two contiguous RNAs transcribed in the orientation opposite to that of DHFR-TS. The two 5'-most RNAs of the DHFR-TS-containing domain overlap the RNAs transcribed from the opposite strand. These data are relevant to models of transcription, including recent studies suggesting polycistronic transcription in trypanosomatids. The abundance of R region RNAs increases uniformly 10- to 15-fold in the amplified R1000-3 line relative to the wild type, and no new RNAs were observed. This suggests that all elements required in cis for DHFR-TS expression are contained within the 30-kb circular DNA. Quantitative analysis reveals that the steady-state DHFR-TS mRNA and protein levels are not growth phase regulated, unlike the monofunctional mouse DHFR. DHFR-TS is developmentally regulated, however, declining about fivefold in lesion amastigotes relative to promastigotes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman J. P., Bond C. T., Douglass J., Herbert E. Two mammalian genes transcribed from opposite strands of the same DNA locus. Science. 1987 Mar 20;235(4795):1514–1517. doi: 10.1126/science.3547652. [DOI] [PubMed] [Google Scholar]
- Aline R. F., Jr, Scholler J. K., Stuart K. Transcripts from the co-transposed segment of variant surface glycoprotein genes are in Trypanosoma brucei polyribosomes. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):169–178. doi: 10.1016/0166-6851(89)90068-6. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., De Vries B. F., Van den Burg J., Klaver B. The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983 Oct 25;11(20):6925–6941. doi: 10.1093/nar/11.20.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Ismach R. B., Pratt D. M. Evolution of the genus Leishmania as revealed by comparisons of nuclear DNA restriction fragment patterns. Proc Natl Acad Sci U S A. 1987 Jan;84(2):484–488. doi: 10.1073/pnas.84.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley J. S., Turner M. J. Isolation and characterization of polysomes from Trypanosoma brucei. Parasitology. 1980 Dec;81(Pt 3):537–551. doi: 10.1017/s0031182000061928. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Leys E. J., McEwan R. N., Frayne E. G., Kellems R. E. Analysis of the mouse dhfr promoter region: existence of a divergently transcribed gene. Mol Cell Biol. 1985 Aug;5(8):1847–1858. doi: 10.1128/mcb.5.8.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Michels P. A., Veerman H. J., Cornelissen A. W., Borst P. Many trypanosome messenger RNAs share a common 5' terminal sequence. Nucleic Acids Res. 1984 May 11;12(9):3777–3790. doi: 10.1093/nar/12.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke S., Chaudhuri G., Kink J. A., Chang K. P. DNA amplification in tunicamycin-resistant Leishmania mexicana. Multicopies of a single 63-kilobase supercoiled molecule and their expression. J Biol Chem. 1988 Mar 5;263(7):3418–3424. [PubMed] [Google Scholar]
- Dynan W. S., Sazer S., Tjian R., Schimke R. T. Transcription factor Sp1 recognizes a DNA sequence in the mouse dihydrofolate reductase promoter. Nature. 1986 Jan 16;319(6050):246–248. doi: 10.1038/319246a0. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh A., Jones T. C. A comparative study of different Leishmania tropica isolates from Iran: correlation between infectivity and cytochemical properties. Am J Trop Med Hyg. 1983 Jul;32(4):694–702. doi: 10.4269/ajtmh.1983.32.694. [DOI] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Biochemistry and regulation of folate and methotrexate transport in Leishmania major. J Biol Chem. 1987 Jul 25;262(21):10053–10058. [PubMed] [Google Scholar]
- Farnham P. J., Abrams J. M., Schimke R. T. Opposite-strand RNAs from the 5' flanking region of the mouse dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3978–3982. doi: 10.1073/pnas.82.12.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham P. J., Schimke R. T. In vitro transcription and delimitation of promoter elements of the murine dihydrofolate reductase gene. Mol Cell Biol. 1986 Jul;6(7):2392–2401. doi: 10.1128/mcb.6.7.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Shaw J. M., Simpson L., Stuart K. Creation of AUG initiation codons by addition of uridines within cytochrome b transcripts of kinetoplastids. Proc Natl Acad Sci U S A. 1988 Jan;85(2):539–543. doi: 10.1073/pnas.85.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garvey E. P., Coderre J. A., Santi D. V. Selection and properties of Leishmania tropica resistant to 10-propargyl-5,8-dideazafolate, an inhibitor of thymidylate synthetase. Mol Biochem Parasitol. 1985 Oct;17(1):79–91. doi: 10.1016/0166-6851(85)90129-x. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Schimke R. T. Cell cycle regulation of transfected murine dihydrofolate reductase genes. J Biol Chem. 1986 May 25;261(15):6938–6946. [PubMed] [Google Scholar]
- Gibson W. C., Swinkels B. W., Borst P. Post-transcriptional control of the differential expression of phosphoglycerate kinase genes in Trypanosoma brucei. J Mol Biol. 1988 May 20;201(2):315–325. doi: 10.1016/0022-2836(88)90140-4. [DOI] [PubMed] [Google Scholar]
- Grumont R., Washtien W. L., Caput D., Santi D. V. Bifunctional thymidylate synthase-dihydrofolate reductase from Leishmania tropica: sequence homology with the corresponding monofunctional proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5387–5391. doi: 10.1073/pnas.83.15.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Keene M. A., Fechtel K., Fristrom J. W. Gene within a gene: nested Drosophila genes encode unrelated proteins on opposite DNA strands. Cell. 1986 Jan 17;44(1):33–42. doi: 10.1016/0092-8674(86)90482-4. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Wong M. L., Ruiz-Perez L., Santi D. V. Electron microscopy of amplified DNA forms in antifolate-resistant Leishmania. J Biol Chem. 1987 Oct 25;262(30):14618–14624. [PubMed] [Google Scholar]
- Hughes D. E., Shonekan O. A., Simpson L. Structure, genomic organization and transcription of the bifunctional dihydrofolate reductase-thymidylate synthase gene from Crithidia fasciculata. Mol Biochem Parasitol. 1989 May 1;34(2):155–166. doi: 10.1016/0166-6851(89)90007-8. [DOI] [PubMed] [Google Scholar]
- Jenh C. H., Geyer P. K., Johnson L. F. Control of thymidylate synthase mRNA content and gene transcription in an overproducing mouse cell line. Mol Cell Biol. 1985 Oct;5(10):2527–2532. doi: 10.1128/mcb.5.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenh C. H., Rao L. G., Johnson L. F. Regulation of thymidylate synthase enzyme synthesis in 5-fluorodeoxyuridine-resistant mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1985 Jan;122(1):149–154. doi: 10.1002/jcp.1041220122. [DOI] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Zhang K., Beverley S. M. Sequence and S1 nuclease mapping of the 5' region of the dihydrofolate reductase-thymidylate synthase gene of Leishmania major. Nucleic Acids Res. 1987 Apr 24;15(8):3369–3383. doi: 10.1093/nar/15.8.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Kellems R. E., Alt F. W., Schimke R. T. Regulation of folate reductase synthesis in sensitive and methotrexate-resistant sarcoma 180 cells. In vitro translation and characterization of folate reductase mRNA. J Biol Chem. 1976 Nov 25;251(22):6987–6993. [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Kink J. A., Chang K. P. Tunicamycin-resistant Leishmania mexicana amazonensis: expression of virulence associated with an increased activity of N-acetylglucosaminyltransferase and amplification of its presumptive gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1253–1257. doi: 10.1073/pnas.84.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kohchi T., Yoshida T., Komano T., Ohyama K. Divergent mRNA transcription in the chloroplast psbB operon. EMBO J. 1988 Apr;7(4):885–891. doi: 10.1002/j.1460-2075.1988.tb02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., van der Spek H. J., Wagter R., d'Oliveira C. E., van der Hoeven F., Johnson P. J., Borst P. The anatomy and transcription of a telomeric expression site for variant-specific surface antigens in T. brucei. Cell. 1987 Oct 23;51(2):261–272. doi: 10.1016/0092-8674(87)90153-x. [DOI] [PubMed] [Google Scholar]
- Lazar M. A., Hodin R. A., Darling D. S., Chin W. W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989 Mar;9(3):1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys E. J., Kellems R. E. Control of dihydrofolate reductase messenger ribonucleic acid production. Mol Cell Biol. 1981 Nov;1(11):961–971. doi: 10.1128/mcb.1.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani B. D., Slate D. L., Schimke R. T. S phase-specific synthesis of dihydrofolate reductase in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4985–4989. doi: 10.1073/pnas.78.8.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller J. S., Paterson B. M., Ricciardi R. P., Cohen L., Roberts B. E. Methods utilizing cell-free protein-synthesizing systems for the identification of recombinant DNA molecules. Methods Enzymol. 1983;101:650–674. doi: 10.1016/0076-6879(83)01046-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Carothers A. M., Han J. H., Harding J. D., Kas E., Venolia L., Chasin L. A. Multiple transcription start sites, DNase I-hypersensitive sites, and an opposite-strand exon in the 5' region of the CHO dhfr gene. Mol Cell Biol. 1986 Feb;6(2):425–440. doi: 10.1128/mcb.6.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Polycistronic transcripts in trypanosomes and their accumulation during heat shock: evidence for a precursor role in mRNA synthesis. Mol Cell Biol. 1988 Sep;8(9):3837–3846. doi: 10.1128/mcb.8.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B. Intragenic pausing and anti-sense transcription within the murine c-myc locus. EMBO J. 1986 Nov;5(11):2859–2865. doi: 10.1002/j.1460-2075.1986.tb04580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Freundlich M. Mechanism for the autogenous control of the crp operon: transcriptional inhibition by a divergent RNA transcript. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5000–5004. doi: 10.1073/pnas.83.14.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Watkins K. P., Agabian N. Trypanosome mRNAs share a common 5' spliced leader sequence. Cell. 1984 Aug;38(1):309–316. doi: 10.1016/0092-8674(84)90552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto M. P., Beverley S. M. In vitro activity of sulfonamides and sulfones against Leishmania major promastigotes. Antimicrob Agents Chemother. 1987 Oct;31(10):1575–1578. doi: 10.1128/aac.31.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rosbash M. Polyadenylic acid-containing RNA in Xenopus laevis oocytes. J Mol Biol. 1974 May 5;85(1):87–101. doi: 10.1016/0022-2836(74)90131-4. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Hieny S., Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985 Jul;135(1):564–569. [PubMed] [Google Scholar]
- Scott D. A., Coombs G. H., Sanderson B. E. Folate utilisation by Leishmania species and the identification of intracellular derivatives and folate-metabolising enzymes. Mol Biochem Parasitol. 1987 Mar;23(2):139–149. doi: 10.1016/0166-6851(87)90149-6. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Williams J. G. Quantitative analysis of specific labelled RNA'S using DNA covalently linked to diazobenzyloxymethyl-paper. Nucleic Acids Res. 1979 Jan;6(1):195–203. doi: 10.1093/nar/6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Polygene transcripts are precursors to calmodulin mRNAs in trypanosomes. EMBO J. 1988 Feb;7(2):455–463. doi: 10.1002/j.1460-2075.1988.tb02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- Washtien W. L., Grumont R., Santi D. V. DNA amplification in antifolate-resistant Leishmania. The thymidylate synthase-dihydrofolate reductase gene and abundant mRNAs. J Biol Chem. 1985 Jul 5;260(13):7809–7812. [PubMed] [Google Scholar]